Abstract
Decreasing the partition coefficient (LogP) by the introduction of a hydrophilic group is the conventional approach for improving the aqueous solubility of drug candidates, but is not always effective. Since melting point is related to aqueous solubility, we and other groups have developed alternative strategies to improve solubility by means of chemical modification to weaken intermolecular interaction in the solid state, thereby lowering the melting point and increasing the solubility. Here, we show that converting the symmetrical molecular structure of the clinically used estrogen receptor (ER) antagonist cyclofenil (1) into asymmetrical form by introducing an alkyl group enhances the aqueous solubility. Among the synthesized analogs, the chiral methylated analog (R)-4c shows the highest solubility, being 3.6-fold more soluble than 1 even though its hydrophobicity is increased by the methylation. Furthermore, (R)-4c also showed higher membrane permeability than 1, while retaining a comparable metabolic rate, and equivalent biological activity of the active forms (R)-13a to 2. Further validation of this strategy using lead compounds having symmetric structures is expected.
Similar content being viewed by others
Introduction
Aqueous solubility is an important physiochemical property of organic molecules, influencing ease of synthesis and purification, chemical and biological properties, functionality, effect on the environment, and so on1,2. From the viewpoint of drug discovery, the absorption of a drug by passive diffusion depends on the concentration gradient between the intestinal lumen and the blood, which is also influenced by solubility. Furthermore, efficacy evaluation and risk assessment of poorly soluble compounds are difficult. Thus, the aqueous solubility of drug candidates is regarded as a key physicochemical property3.
Thermodynamic solubility of a solid is defined as the concentration in solution when it is at equilibrium with the most stable crystal form4,5. In contrast, formulation strategies including crystal modification, particle size reduction can produce an increase in dissolution rate and a temporary increase of solubility4,5. However, it cannot produce a permanent alteration of solubility. Given sufficient time, the undissolved solute will revert to its most stable crystal form, and the solubility will approach the true thermodynamic solubility6. As a temporary increase of solubility by formulation strategies is not always achieved, poor solubility of drug candidates has been identified as the cause of numerous drug development failures. Thus, it would be better to generate drug candidates with sufficient thermodynamic solubility at the drug discovery stage.
The conventional and general method to increase aqueous solubility by chemical modification is introduction of hydrophilic group(s) into molecules to decrease the hydrophobicity (the common logarithm of partition coefficient, LogPow). However, decreasing LogP is not necessarily effective to improve the properties of drug candidates. Oral drugs have to permeate the lipid bilayer membrane when they are absorbed from the intestinal lumen, so ideal drug candidates would have high hydrophobicity in addition to high aqueous solubility. Therefore, strategies to increase aqueous solubility without lowering hydrophobicity are required.
The solubility of a compound in water is also influenced by the molecular packing in the solid state7,8. Early studies to elucidate how the intermolecular interaction of organic compounds in the solid state affects their solubility in water were quite limited, although there is an old rule of thumb that organic compounds possessing weaker intermolecular interaction tend to show higher solubility in organic solvents. However, during the past decade, various strategies to improve the aqueous solubility of pharmaceutical compounds by means of chemical modification to disrupt intermolecular interactions have been developed5,9,10,11,12. Examples include disruption of intermolecular hydrogen bonds13,14, disruption of molecular planarity by ortho-substitution of biaryl groups15,16,17, bending molecular structure by changing the position of substituents18,19,20 (we had referred to this as disruption of molecular symmetry9,12, but “bending” is more appropriate because the examples shown in refs 9 and 12 possess the same point group after chemical modification), increasing the number of sp3-hybridized carbons21,22 (so-called “escape from flatland”), and introduction of a non-flat substituent at the meta position of a phenyl group23. We have shown that these strategies for disrupting intermolecular interactions can increase the aqueous solubility of molecules even if the hydrophobicity is concomitantly increased9,12. However, we sometimes encounter the cases that above molecular design strategies to increase the aqueous solubility are restricted to other parameters including permeability, biological activity, metabolism, promiscuous binding, safety, and so on. Therefore, the more choices of concrete molecular design strategies to increase the aqueous solubility, the possibility to the rational design of compounds satisfying all the parameters increases.
Among structurally simple compounds, compounds with higher symmetry tend to possess higher melting points24,25,26,27. Yalkowsky et al. reported that the higher the molecular symmetry number (σ), the higher the melting point tends to be, based on an analysis of 1200 structurally simple compounds28. In addition, in a cycloalkane having 3 to 10 carbon atoms, the boiling point increases with increase in the number of carbon atoms, but the melting point is relatively high for compounds having high symmetry at carbon numbers 3, 4 and 629. Also, monosubstitution of unsubstituted benzene often leads to a decrease in melting point due to a decrease in symmetry. However, the relationship between symmetry and melting point does not generally hold for compounds with complex structures, such as many pharmaceutical compounds, and the relationship between structural symmetry and aqueous solubility remains to be established. Here, we report a new approach for improving aqueous solubility by chemical modification to change a symmetric molecular structure to an asymmetric molecular structure, illustrated by application to the estrogen receptor (ER) antagonist cyclofenil (1).
Methods
Physiochemical properties
Determination of melting point
Each compound was recrystallized twice from an appropriate solvent. The melting points of the crystals obtained in the two crystallizations were compared. If both crystals melted at the same temperature, this temperature was taken as the melting point. Recrystallization and melting point comparison was repeated until a consistent melting point was obtained.
Thermodynamic aqueous solubility of the most stable crystals
We assumed that crystals for which the melting point remained the same after repeated recrystallizations were the most stable crystal forms of the compounds, and we used them to evaluate aqueous solubility. The crystals were ground with an agate mortar and suspended in a mixture of 0.067 M phosphate buffer (pH 6.8) and EtOH (6:4). The suspension was shaken for 48 h at 4 °C, then filtered through a Millipore Millex-LG filter (0.20 μm). The filtrate was diluted in DMF and subjected to HPLC. The solubility was calculated by the absolute calibration curve method. We used a mixed solvent because the solubility in 0.067 M phosphate buffer (pH 6.8) was too low to allow quantification. EtOH is used clinically as a solubilizer for insoluble drugs such as taxol and pacritaxel. When the solutions were shaken at 37 °C, hydrolysis of the ester moiety occurred, and therefore the suspensions were shaken at 4 °C.
Membrane permeability
Membrane permeability was determined by HPLC on an IAM.PC.DD2 column (Regis Technologies, Inc., 10 μm, 300 Å, 30 mm × 4.6 mm) eluted with mobile phase consisting of 0.1 M phosphate buffer (pH 6.8) and CH3CN (4:6) at a flow rate of 1.0 mL/min, with UV monitoring at 254 nm, 37 °C.
Results and discussion
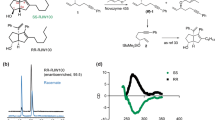
Cyclofenil (1) is an antagonist of estrogen receptors (ER) α and β that is clinically used to treat menstrual disturbances, anovulatory infertility and menopausal symptoms (Fig. 1). However, 1 has poor aqueous solubility (< 1 μg/mL in 0.067 M phosphate buffer pH 7.4). We considered that this low solubility might be due to the symmetric structure (σ = 2, point group: C2v), and we hypothesized that chemical modification of 1 to an asymmetric structure (point group: Cs and C1) would improve the aqueous solubility. To investigate the relationship between molecular shape (symmetry) and aqueous solubility, we focused on a small chemical modification, that is, introduction of a hydrophobic alkyl group. Cyclofenil (1) is a prodrug that is hydrolyzed by esterases after administration, and the active form is diol 2. Therefore, we initially considered that replacement of an acetyl group on 1 with a different acyl group (3a–d) might improve the aqueous solubility without loss of biological activity. As a second approach, we designed methylated analogs 4a–c substituted at the 2-/3-position of the phenyl group or the 3-position of the cyclohexyl group, respectively. We also designed symmetrical analogs 5a–c and 6 for comparison.
Analogs of 1 were synthesized as shown in Fig. 2. Acetylation of one hydroxyl group of 230 with 1 equivalent of acetic anhydride afforded 7 along with 1. Acylation of 7 and 2 with corresponding acyl chlorides gave asymmetric 3a-d and symmetric 5a–c, respectively. Friedel Crafts acylation between compounds 8a and o-cresol, and 8b and m-cresol gave 9a–b, respectively. McMurry coupling reaction between 9a/9b/12 and cyclohexanones, including chiral (R)-3-hydroxyhexan-1-one, gave 10a, 11, (R)-13a, and 13b, respectively. Diacetylation of 10a-b, (R)-13a and 13b gave 4a-b, and (R)-4c, 6, respectively.
Reagents and conditions: (a) acetic anhydride (1.0 eq.), pyridine, DCM, 52% b.r.s.m.; (b) acid chlorides, pyridine, DCM or DCE, 80–87%; (c) o-cresol, ZnCl2, POCl3, 70 °C, 69%; (d) SOCl2, reflux, then m-cresol, AlCl3, DCM, 0 °C to rt, 70%; (e) cyclohexanones, Zn, TiCl4, THF, reflux, 75–91%; (f) acetic anhydride, pyridine, DCM, 75–95%; (g) BBr3, DCM, − 78 °C to rt, 66%.
We prepared the most stable crystals of 1, 3a–d, 4a–c, 5a–c and 6, and measured their melting points, thermodynamic aqueous solubility (i.e., the solubility of the most stable crystal form to afford a saturated solution at equilibrium) and LogP (Table 1). Notably, asymmetric structural analogs 3b, 4a, 4b and (R)-4c were more soluble than 1. The melting points of these analogs were significantly lower than those of 1 and the other analogs. LogP values of all analogs were higher than that of 1, as expected. These results indicate that the improvement in the aqueous solubility of 3b, 4a, 4b and (R)-4c was not due to a decrease in hydrophobicity, but rather, was due to a decrease of intermolecular interaction in the crystals. The observation that the measured crystal densities of 3b, 4a and (R)-4c were lower than that of 1 supports this idea. On the other hand, 3a, 3c and 3d showed decreased aqueous solubility compared to 1, suggesting that an increase in hydrophobicity affects solubility more strongly than a decrease in intermolecular interaction.
The solubility of 4b was higher than that of not only 1 but also 4a, even though 4b has almost the same hydrophobicity as 4a. A plausible explanation for this is the difference of molecular planarity9. The melting point of 4b was lower than that of 4a, and the calculated dihedral angle between the cresol moiety and vinyl group in 4b was larger than that of 4a and 1. These results indicate that introduction of a methyl group at the 2-position of the phenyl group of 1 disrupts the molecular planarity by increasing the dihedral angle, owing to steric hinderance between the methyl group and cyclohexyl group. Thus, not only introduction of asymmetry into the molecular structure, but also the disruption of planarity, appears to contribute to the increased aqueous solubility of 4b.
The best result was obtained by introduction of a chiral methyl group as in (R)-4c, which showed the highest solubility among the compounds examined here, being 3.6 times soluble than 1, together with the lowest crystal density. The reason for this is presumably the introduction of the chiral center, which results in greater molecular asymmetry. Considering conformational isomerization of the cyclohexyl group, the symmetry point groups of cyclofenil (1), 4a/4b and (R)-4c are C2v, Cs and C1, respectively.
To exclude the possibility that the improvement in aqueous solubility was caused by increased molecular flexibility, which is known to be associated with a decrease of melting point24, we next compared the symmetrical and asymmetrical analogs. The solubility of each symmetrical compound 5a-c was lower than that of the corresponding asymmetric compound 3a–c. The symmetrical analog 6 also had lower solubility than the asymmetric isomer (R)-4c. Furthermore, comparison of the isomers 3b/5a and 4a/4b/(R)-4c/6 clearly showed that asymmetric analogs 3b (17.3-fold more soluble)/4a (2.9-fold)/4b (6.5-fold)/(R)-4c (7.7-fold) possess higher solubility and lower melting points than symmetric isomers 5a and 6. These results indicate that asymmetry contributes to the decrease in intermolecular interaction and the improvement of aqueous solubility.
Next, we investigated whether the synthesized compounds act as prodrugs, i.e., we measured their membrane permeability as prodrugs, the metabolic conversion of prodrugs into the active forms, and the biological activity of the active forms.
Firstly, we evaluated membrane permeability by using an Immobilized Artificial Membrane (IAM) column, which contains silica gel bearing phosphatidylcholine and widely utilized to predict membrane permeability31,32. As expected, the retention time of all asymmetric structural analogs was longer than that of 1 (Table 2), suggesting that the asymmetric structural analogs show higher membrane permeability due to increased hydrophobicity.
The metabolic rate of prodrugs was evaluated using human cryopreserved hepatocytes (Table 3). Although the metabolic rate of 3d with a bulky acyl group was decreased, most of the asymmetric structural analogs, including those with improved aqueous solubility (3b, 4a, (R)-4c), were metabolized to the active form as quickly as 1. Thus, we concluded that the chemical modifications applied here had little effect on the metabolic rate.
Finally, we evaluated the ERα and ERβ antagonistic activities of the active forms 2, 10a and (R)-13a by means of luciferase reporter gene assay (Table 4). Under our assay conditions, 2, which is the active form of not only 1 but also 3a-d, showed antagonistic activities with IC50 values of 13 nM against ERα and 4.5 nM against ERβ. Compound 10a showed decreased antagonistic activities, with IC50 values of 120 nM against ERα and 51 nM against ERβ. On the other hand, (R)-13a showed similar antagonistic activities to 2 against both ERα and ERβ. This results is consistent with the reported ERs antagonistic activity of racemic-13a33.
Conclusion
The aqueous solubility of drug candidates is regarded as a key physicochemical property, and a greater number of concrete molecular design strategies to improve the aqueous solubility is required. This paper describes relationships between structural feature and solubility of a small molecular drug. And we developed a strategy for improving aqueous solubility by chemical modification to convert a symmetric molecular structure into an asymmetric molecular structure, focusing on cyclofenil (1). We found that a tiny molecular change, i.e. the introduction of an alkyl group to generate asymmetric analogs 3b, 4a, 4b and (R)-4c increased the aqueous solubility in spite of the associated increase in hydrophobicity. Indeed, asymmetric analog 3b was 17.3-fold more soluble than the corresponding symmetric isomer 5a. The melting point and crystal density of 3b and (R)-4c were lower than those of 1, supporting the view that the improvement in aqueous solubility was due to the disruption of intermolecular interaction.
In this report, we carried out chemical modifications that intentionally increase hydrophobicity. This is because (1) to clarify the mechanisms of improvement of aqueous solubility, and (2) increase of both aqueous solubility and hydrophobicity is obviously tough. When the balance between hydrophobicity and aqueous solubility is important for compounds, we believe that this strategy would open up one possibility for molecular design. To enhance the utility of this strategy in the real medicinal chemistry, multiple strategies to improve aqueous solubility are likely to be superior to the single strategy. In fact, not only introduction of asymmetry into the molecular structure, but also the disruption of planarity appears to contribute to the further increased aqueous solubility of 4b. In addition, introducing substituents would be selected depending on the Log P of lead compounds in the purpose of not only improving aqueous solubility but also optimizing hydrophobicity of drug candidates. Further validation of this strategy using lead compounds having symmetric structures is expected.
Simple methylation of 1 afforded the most soluble compound, (R)-4c, which was 3.6-fold more soluble than 1 even though its hydrophobicity is concomitantly increased. Furthermore, asymmetric (R)-4c is 7.7-fold more soluble than the corresponding symmetric isomer 6, and (R)-4c showed better membrane permeability, a similar metabolic rate, and equivalent biological activity of the active forms (R)-13a to 2. It is noteworthy that (R)-4c possesses a chiral center, which disrupts symmetry. Chiral pharmaceutical compounds are not necessarily desirable because of the difficulties of asymmetric synthesis and the need for additional quality control. However, it has been reported that the presence of chiral centers correlates with success in translation from discovery through clinical testing to drug approval21. In addition, it was recently reported that a benzene mimetic bearing a chiral center showed improved aqueous solubility, though its hydrophobicity was also decreased35. Our finding that a chiral compound showed better drug-like properties (aqueous solubility and membrane permeability) than achiral analogs highlights the importance of chiral compounds and asymmetric synthesis for medicinal chemistry36,37.
Medicinal chemists knows that methylation rarely leads to improved aqueous solubility34. And the mechanism of improved solubility by methylation is not fully understood. Our continuous works including this paper at least partially unveil the mechanism of improved aqueous solubility by methylation. And this work will provide one rational strategy for improvement of aqueous solubility by methylation into the specific position.
Supporting Information
Methods including preparation of compounds, computational chemistry, and biology.
Abbreviations
- ER:
-
Estrogen receptor
- HPLC:
-
High-performance liquid chromatography
References
Chanda, A. & Fokin, V. V. Organic synthesis “on water”. Chem. Rev. 109, 725–748 (2009).
Bisballe, N. & Laursen, B. W. What is best strategy for water soluble fluorescence dyes? A case study using long fluorescence lifetime DAOTA dyes. Chem. A Eur. J. 26, 15969-15976 (2020).
Borchardt, R. et al. (eds) Pharmaceutical Profiling in Drug Discovery for Lead Selection (Springer, Berlin, 2004).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 64, 3–26 (2001).
Walker, M. A. Improvement in aqueous solubility achieved via small molecular changes. Bioorg. Med. Chem. Lett. 27, 5100–5108 (2017).
Babu, N. J. & Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 11, 2662–2679 (2011).
Franc, I., Lipinski, A. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Jain, N. & Yalkowsky, S. H. Estimation of the aqueous solubility I: Application to organic nonelectrolytes. J. Pharm. Sci. 90, 234–252 (2001).
Ishikawa, M. & Hashimoto, Y. Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J. Med. Chem. 54, 1539–1554 (2011).
Walker, M. A. Novel tactics for designing water-soluble molecules in drug discovery. Expert Opin. Drug Discov. 9, 1421–1433 (2014).
Tehler, U. et al. Optimizing solubility and permeability of a biopharmaceutics classification system (BCS) class 4 antibiotic drug using lipophilic fragments disturbing the crystal lattice. J. Med. Chem. 56, 2690–2694 (2013).
Ishikawa, M. & Hashimoto, Y. In The Practice of Medicinal Chemistry 4th edn (eds Wermuth, C. G. et al.) 747–765 (Academic Press, Cambridge, 2015).
Lange, J. H. M. et al. Synthesis, biological properties, and molecular modeling investigations of novel 3, 4-diarylpyrazolines as potent and selective CB1 cannabinoid receptor antagonistsSynthesis, biological properties, and molecular modeling investigations of novel 3, 4-diarylpyrazolines as potent and selective CB1 cannabinoid receptor antagonists. J. Med. Chem. 47, 627–643 (2004).
Scott, J. S. et al. Use of small-molecule crystal structures to address solubility in a novel series of G protein coupled receptor 119 agonists: Optimization of a lead and in vivo evaluation. J. Med. Chem. 55, 5361–5379 (2012).
Ishikawa, M. et al. Tricyclic pharmacophore-based molecules as novel integrin αvβ3 antagonists. Part 2: Synthesis of potent αvβ3/αIIbβ3 dual antagonists. Bioorg. Med. Chem. 14, 2109–2130 (2006).
Fujita, Y. et al. β-Naphthoflavone analogs as potent and soluble aryl hydrocarbon receptor agonists: Improvement of solubility by disruption of molecular planarity. Bioorg. Med. Chem. 18, 1194–1203 (2010).
Kasuga, J. et al. Improvement of water-solubility of biarylcarboxylic acid peroxisome proliferator-activated receptor (PPAR) δ-selective partial agonists by disruption of molecular planarity/symmetry. Bioorg. Med. Chem. 18, 7164–7173 (2010).
Ishikawa, M. et al. Tricyclic pharmacophore-based molecules as novel integrin αvβ3 antagonists. Part III: Synthesis of potent antagonists with αvβ3/αIIbβ3 dual activity and improved water solubility. Bioorg. Med. Chem. 14, 2131–2150 (2006).
Hiramatsu, M. et al. Improvement in aqueous solubility of retinoic acid receptor (RAR) agonists by bending the molecular structure. Chem. Asian J. 11, 2210–2217 (2016).
Ishikawa, M., Ohzono, T., Yamaguchi, T. & Norikane, Y. Photo-enhanced aqueous solubilization of an azo-compound. Sci. Rep. 7, 6909 (2017).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Locke, G. M., Bernhard, S. S. R. & Senge, M. O.. Nonconjugated hydrocarbons as rigid‐linear motifs: Isosteres for material sciences and bioorganic and medicinal chemistry. Chem. A Eur. J. 25, 4590–4647 (2019).
Ichikawa, Y. et al. meta-Non-flat substituents: A novel molecular design to improve aqueous solubility in small molecule drug discovery. Org. Biomol. Chem. 19, 446–456 (2021).
Brown, R. J. C. & Brown, R. F. C. Melting point and molecular symmetry. J. Chem. Educ. 77, 724–731 (2000).
Slovokhotov, Y. L., Neretin, I. S. & Howard, J. A. K. Symmetry of van der Waals molecular shape and melting points of organic compounds. New J. Chem. 28, 967–979 (2004).
Gavezzotti, A. Molecular symmetry, melting temperatures and melting enthalpies of substituted benzenes and naphthalenes. J. Chem. Soc. Perkin Trans. 2, 1399–1404 (1995).
Yalkowsky, S. H. Carnelley's rule and the prediction of melting point. J. Pharm. Sci. 103, 2629–2634 (2014).
Dannenfelser, R.-M. & Yalkowsky, S. H. Estimation of entropy of melting from molecular structure: A non-group contribution method. Ind. Eng. Chem. Res. 35, 1483–1486 (1996).
Wei, J. Molecular symmetry, rotational entropy, and elevated melting pointsMolecular symmetry, rotational entropy, and elevated melting points. Ind. Eng. Chem. Res. 38, 5019–5027 (1999).
Seo, J. W. et al. Fluorine-substituted cyclofenil derivatives as estrogen receptor ligands: Synthesis and structure− affinity relationship study of potential positron emission tomography agents for imaging estrogen receptors in breast cancer. J. Med. Chem. 49, 2496–2511 (2006).
Pidgeon, C. et al. IAM chromatography: An in vitro screen for predicting drug membrane permeability. J. Med. Chem. 38, 590–594 (1995).
Ong, S., Liu, H. & Pidgeon, C. Immobilized-artificial-membrane chromatography: Measurements of membrane partition coefficient and predicting drug membrane permeability. J. Chromatogr. A 728, 113–128 (1996).
Zhu, H., Yang, Z., Lin, J.-G., Luo, S.-N. & Shen, Y.-M. Synthesis and evaluation of fluoroethyl cyclofenil analogs: Models for potential estrogen receptor imaging agent. J. Fluor. Chem. 139, 46–52 (2012).
Garcia, S. N., Yang, X., Bereczki, L. & Kónya, D. Aqueous solubility of organic compounds for flow battery applications: Symmetry and counter ion design to avoid low-solubility polymorphs. Molecules 26, 1203 (2021).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water‐soluble non‐classical benzene mimeticsWater‐soluble non‐classical benzene mimetics. Angew. Chemie Int. Ed. 59, 7161–7167 (2020).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Acknowledgements
The work described in this paper was partially supported by Grants-in Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Japan Society for the Promotion of Science, Platform for Drug Discovery, Informatics, and Structural Life Science, and The Tokyo Biochemical Research Foundation (KAKENHI Grant Nos. 17H06173 (M.U.) and 18H02551 (M.I.), and 25293027 (M.I.)).
Author information
Authors and Affiliations
Contributions
J.M. performed organic synthesis, recrystallization of compounds, measurements of physicochemical properties of compounds, biological experiments, and computational experiments. K.M. and M.U. performed X-ray crystal analyses. M.M. prepared reagents for ERs assay. All authors discussed the results. M.U., Y.H. and Y. I. developed the concept, and supervised the experiments and the manuscript. M.I. conceptualized, designed and supervised the experiments. J.M. and M.I. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morimoto, J., Miyamoto, K., Ichikawa, Y. et al. Improvement in aqueous solubility of achiral symmetric cyclofenil by modification to a chiral asymmetric analog. Sci Rep 11, 12697 (2021). https://doi.org/10.1038/s41598-021-92028-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92028-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.