Abstract
Various non-intraocular pressure factors have been identified as possible risk factors for open-angle glaucoma (OAG). However, there is still controversy around the association between OAG and chronic kidney disease (CKD). In this study, we used a nationwide cohort to investigate the risk of OAG in the 12 years following a diagnosis of CKD. This retrospective cohort study included 1,103,302 subjects from the Korean National Health Insurance Service National Sample Cohort database. The CKD group (n = 1318) included patients who were initially diagnosed with CKD between 2003 and 2008. The subjects in the comparison group were matched at a 1:5 ratio using propensity scores. In multivariate Cox regression analysis, a diagnosis of CKD was significantly associated with an increased incidence of OAG (hazard ratio [HR] = 1.546, 95% confidence interval [CI] 1.363–1.754, p < 0.001). Further analysis revealed that the risk of OAG increased with the severity of CKD (mild to moderate CKD [CKD stage 1–3]: HR = 1.280, 95% CI 1.077–1.521, p = 0.005; advanced CKD [CKD stage 4–5]: HR = 1.861, 95% CI 1.589–2.180, p < 0.001). In subgroup analysis, female CKD patients had a greater risk of developing OAG than males, and subjects with CKD aged ≥ 40 years were more likely to develop OAG compared with those aged < 40 years. Our study demonstrates that CKD is a significant risk factor for OAG and that severe CKD is associated with an increased risk of developing OAG.
Similar content being viewed by others
Introduction
Open-angle glaucoma (OAG), one of the leading causes of irreversible blindness, is characterized by chronic progressive glaucomatous optic neuropathy with corresponding visual field defects1,2. While intraocular pressure (IOP) is known to be the main cause of OAG development and progression, several non-IOP factors are risk factors for OAG3,4,5,6. Vascular risk factors are among those suggested as a major cause of glaucomatous damage, which would support vascular and ischemic mechanisms of OAG6,7,8. However, there is still controversy around the role of these vascular factors. Studies of vascular diseases have had contradictory results.
Chronic kidney disease (CKD) is a major microvascular disease involving renal function impairment and is associated with various cardiovascular and metabolic comorbidities9,10. Its global prevalence is increasing, and it has become a serious public health problem worldwide11,12. There have been reports of associations between CKD and vision-threatening ocular conditions, including diabetic retinopathy13,14, cataracts13,15, age-related macular degeneration16,17,18, and OAG. Especially, CKD and OAG have been reported to have common risk factors, including hypertension19, diabetes mellitus9, hyperlipidemia20, and old age19, and may share similar pathogenetic mechanisms such as atherosclerosis21,22, oxidative stress23,24, and renin-angiotensin system (RAS) dysfunction25. Moreover, several population-based studies have shown a positive association between CKD and OAG26,27,28,29,30, indicating both an increased risk of OAG in CKD patients as well as an increased risk of CKD in OAG patients. On the other hands, other studies have not found a significant association between the two conditions13,31,32. This leaves a room for an opportunity to explore the nature of the causal relationship between the two conditions further using longitudinal study design.
In light of above, in this study, we investigated the risk of developing OAG after CKD diagnosis using a representative sample of approximately 1.1 million South Koreans from the National Health Insurance Service-National Sample Cohort 2002–2015 (NHIS-NSC 2002–2015). In addition, we analyzed the risk of developing OAG according to CKD severity.
Results
Table 1 shows the baseline characteristics of the study population and the differences between the OAG group and the control group. There was a significant difference in the prevalence of CKD in the OAG group and the control group (p < 0.001). Subjects in the OAG group were older (p < 0.001), had lower income (p < 0.001), were more likely to live in rural areas (p < 0.001), and had lower CCI scores (p < 0.001). Hypertension (p < 0.001), diabetes mellitus (p < 0.001), and hyperlipidemia (p < 0.001) were more prevalent in the OAG group than in the control group. There were no significant differences in sex or ischemic stroke.
The risk of a CKD patient developing OAG during the 12-year follow-up period was analyzed using multivariate Cox hazard regression analysis. The risk of developing OAG during the 12-year follow-up period was significantly higher in the CKD group than the control group (CKD group: adjusted HR = 1.546, 95% CI 1.363–1.754, p < 0.001) (Table 2). Higher CKD stages were associated with a higher risk of OAG (CKD group 1: adjusted HR = 1.280, 95% CI 1.077–1.521, p = 0.005; CKD group 2: adjusted HR = 1.861, 95% CI 1.589–2.180, p < 0.001) (Table 3). Older age, low income, rural residency, hypertension, diabetes mellitus, and hyperlipidemia were also associated with an increased risk of OAG.
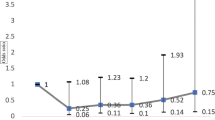
In subgroup analyses based on multivariate Cox regression, the adjusted HR of OAG in the female CKD patients was higher (adjusted HR = 2.989) than that in the male CKD patients (adjusted HR = 2.041, Fig. 1). Moreover, the risk of developing AD in patients with OAG was higher in the subgroup of age ≥ 40 years (HR = 2.861) than that in the subgroup of age < 40 years (HR = 0.279).
As shown in the Kaplan–Meier survival curves in Fig. 1, the risk of OAG was higher for patients at CKD stages 1–3 (mild to moderate CKD) and CKD stages 4–5 (advanced CKD) than in the non-CKD comparison group (log-rank test, < 0.001) (Fig. 2). In addition, the cumulative incidence of OAG during the follow-up period was significantly higher in advanced CKD than mild to moderate CKD (log-rank test, p < 0.001). Furthermore, the risk of developing OAG was higher for CKD patients in both male and female subgroups, as well as in the subgroup of age ≥ 40 years (log-rank test, < 0.001, Fig. 3A–C). However, no significant difference in the cumulative OAG incidence was observed between the CKD and comparison group in the subgroup of age < 40 years (log-rank test, p = 0.3022, Fig. 3D).
Discussion
In this population-based cohort study, we evaluated the risk of OAG in CKD patients using propensity score matching. We used multivariate Cox proportional hazard regression analysis and found that CKD was a significant risk factor for OAG. In a subgroup analysis in which CKD patients were divided into two groups based on CKD severity, there was an increased risk of OAG in CKD patients with more advanced disease. To our knowledge, this is the first study using a large longitudinal cohort from a nationwide database to evaluate the risk of OAG in CKD patients by disease stage and to show a significant increase in OAG risk with worsening kidney function.
Several population-based studies have explored the relationship between CKD and OAG, with conflicting results. A study using National Health and Nutrition Examination Survey data found no significant association between CKD and glaucoma33. Another report on a large pool of Asian population-based studies showed that neither lower estimated glomerular filtration rate (eGFR) nor CKD were associated with primary OAG (POAG) prevalence31. However, subgroup analysis revealed a significant association between lower eGFR and POAG prevalence in East Asians, including Korean and Chinese individuals31. This association was supported by a recent population-based cross-sectional study from South Korea demonstrating a positive association between lower eGFR and POAG34, suggesting that kidney function decline is a risk factor for POAG. The discrepancies in study results may be due to ethnic differences and the higher prevalence of normal-tension glaucoma (NTG) in East Asian countries. Vascular risk factors, including hypertension, diabetes mellitus, and hyperlipidemia, are considered more important in NTG than POAG6. Our findings, which are based on a longitudinal cohort study, are consistent with previous results demonstrating a causal relationship between CKD and OAG.
It has been suggested that CKD and OAG share several pathophysiological mechanisms. The most frequently mentioned mechanism is the RAS. The RAS is a systemic mechanism that is essential for maintaining blood pressure and electrolyte homeostasis, and has been reported to be upregulated in CKD patients35. In the eye, the RAS has a role in the production of aqueous humor from the ciliary body and its outflow through the trabecular meshwork36,37. Especially, Angiotensin II (AngII), a major active component of RAS system affected by the release of renin, has been reported to increase production and decrease outflow of AH by acting on non-pigmented epithelium of ciliary body as well as trabecular meshwork38,39,40. Another in vivo study using rabbits showed that intracamerally intected AngII diminished uveoscleral outflow41, one of main AH outflow pathway other than trabecular meshwork. Such changes in the eye may result in increased IOP, which is a well-known risk factor for the pathogenesis of glaucoma. Moreover, AngII also has been reported to play a significant role in the regulation of ophthalmic microcirculation by controlling contraction and relaxation of vascular endothelium42,43. Disruption in blood–brain barrier of the optic nerve head in glaucoma, as previously reported44, may result in local passage of systemic RAS component to the optic nerve and retina, leading to dysregulation of the retinal microcirculation. In addition, an animal study using a rodent model of glaucoma found that angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists may have a beneficial effect on RGC survival45, suggesting that RAS may modulate RGC survival through exerting a neuroprotective effect.
Other possible common pathologic mechanisms between CKD and OAG may include atherosclerosis and oxidative stress. It has been previously reported that decreased renal function may induce atherosclerosis21,46, and another study by Shim et al22. showed that POAG was significantly associated with increased systemic arterial stiffness, suggesting possible connection between the two conditions. Moreover, oxidative stress has been considered as one of well-known pathogenic mechanisms of OAG23, which also has been suggested as important underlying mechanism in CKD pathogenesis24. Also, low eGFR may reflect accumulation of reactive oxygen species47, and this may lead to increased susceptibility of glaucoma development by stimulating RGC apoptosis and glial activation in posterior segment of eye48. Such hypothesis may be supported by our findings that subjects suffering from more severe CKD condition showed increased HRs of developing OAG. Furthermore, previous studies showed significant association between elevated serum uric acid level and open-angle glaucoma49,50,51. Interestingly, serum uric acid level was reported to be commonly elevated in patients with CKD52, suggesting possible connection between the two diseases. However, further large scale prospective studies are needed to exactly evaluate these relationships.
Several previous cross-sectional population-based studies have demonstrated an association between lower eGFR and POAG31,34, but whether a causal relationship was present is uncertain. Our study, using a longitudinal population-based cohort design, showed an increased risk of OAG in CKD patients with advanced disease. We found that patients in advanced CKD group (CKD stages 3–5) had a significantly increased risk of OAG compared to mild to moderate CKD group (CKD stages 1–2). Although eGFR is the standard for staging CKD, the results from this study suggests that lower eGFR is an independent risk factor for OAG in CKD patients. Further studies should identify the pathogenetic mechanism underlying this association.
Our study has several limitations that should be considered when interpreting the results. First, the diagnoses of OAG, CKD, and other comorbidities were based entirely on KCD codes, which may be less accurate than the results of standard diagnostic tests performed in a real clinic. In this study, however, we included only OAG patients who met all three inclusion criteria: having the KCD code for an OAG diagnosis; having the code for visual field tests; and having a prescription for anti-glaucoma eye drops. As a result, the selection of OAG subjects used in the statistical analyses may have had high specificity. Second, the data used in our study were based only on patients who had visited a hospital or clinic during the study period. Therefore, the data excluded asymptomatic patients and those who had not visited hospitals for personal or economic reasons. This may have caused selection bias, leading to an underestimation of the true prevalence or incidence of OAG and CKD. However, the effects of any such bias or underestimation is thought to be limited; our government-run healthcare system includes almost the entire population of the country and is reasonably low-cost, making healthcare highly accessible.
In conclusion, our 12-year nationwide cohort study demonstrated that CKD patients have a higher risk of OAG than those without CKD. Moreover, more severe disease is associated with a significant increase in the risk of OAG. This indicates that lower eGFR is associated with OAG. The results of this study further support eye screening in CKD patients for early detection and treatment of OAG.
Methods and materials
Database and study sample
Our study used data from the NHIS-NSC 2002–2015. The Korean National Health Insurance Service (KNHIS) is a mandatory single-payer health insurance system in which all Koreans are enrolled. The National Health Information Database (NHID) developed by the KNHIS contains socioeconomic data (sex, age, income (insurance premium), etc.) and medical records. The NHIS-NSC sampled 1,103,302 of 46,605,433 individuals in the NHID in 2002 and followed them until 2015. The study design was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital, Gyeonggi-do, Korea (IRB No. 2019-12-001–001), and the need for written informed consent was waived due to its minimal risk involved in the retrospective analysis. This study adhered to the tenets of the Declaration of Helsinki.
Selection of patients and controls
The CKD cases were selected using the Korean Classification of Diseases (KCD) code N18. The OAG cases were defined as patients diagnosed with OAG (KCD code H401) and had one or more visit to an ophthalmologist after the index date. We included only newly diagnosed CKD patients by excluding those who had been diagnosed with CKD and had used medical services for the condition in 2002. Patients diagnosed with CKD from 2003 to 2008 were included. Patients who developed OAG before a diagnosis of CKD were excluded.
To analyze the effect of CKD on the risk of OAG, a control group was selected using propensity scores with nearest neighbor matching at a 1:5 ratio of CKD to non-CKD subjects. Ultimately, 25,560 subjects were included: 4260 in the CKD group and 21,300 in the non-CKD comparison group. These groups included 1318 OAG cases. Propensity scores were calculated using socioeconomic parameters, including age group (≤ 49, 50–59, 60–69, 70–79, ≥ 80), sex, household income percentile (≤ 30th, 31st–70th, ≥ 71st percentile according to insurance premium), residential area (urban included cities including metropolitan cities and rural included all other areas), and Charlson comorbidity index (CCI) scores (< 3, ≥ 3).
Outcomes and comorbidities
We compared the incidence of OAG in the matched populations. CKD was the main outcome in the analysis. Subjects with CKD were divided into two groups based on CKD severity (mild to moderate CKD: CKD stages 1–3; advanced CKD: CKD stages 4–5). Socioeconomic variables and comorbid conditions were selected as confounding factors. Socioeconomic variables were age, sex, household income, residential area, and CCI. Comorbid conditions included hypertension (KCD codes I10–I15), diabetes mellitus (KCD codes E10–E14), hyperlipidemia (KCD codes E78.0–E78.5), and ischemic stroke (KCD code I61).
Statistical analysis
The chi-square test was used to compare the groups. Multivariate Cox proportional hazard regression analysis was performed to calculate HRs with 95% confidence intervals (CI) for estimating the incidence of OAG in CKD patients. Survival curves for OAG were generated. All statistical analyses were conducted using SAS 7.3 and STATA 16.
References
Quigley, H. A. & Broman, A. T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267. https://doi.org/10.1136/bjo.2005.081224 (2006).
Resnikoff, S. et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 82, 844–851 (2004).
Suzuki, Y. et al. Risk factors for open-angle glaucoma in a Japanese population: The Tajimi Study. Ophthalmology 113, 1613–1617. https://doi.org/10.1016/j.ophtha.2006.03.059 (2006).
Kim, C. S., Seong, G. J., Lee, N. H. & Song, K. C. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 118, 1024–1030. https://doi.org/10.1016/j.ophtha.2010.10.016 (2011).
Yamamoto, S. et al. Primary open-angle glaucoma in a population associated with high prevalence of primary angle-closure glaucoma: The Kumejima Study. Ophthalmology 121, 1558–1565. https://doi.org/10.1016/j.ophtha.2014.03.003 (2014).
Lee, S. H. et al. Vascular and metabolic comorbidities in open-angle glaucoma with low- and high-teen intraocular pressure: A cross-sectional study from South Korea. Acta Ophthalmol. 95, e564–e574. https://doi.org/10.1111/aos.13487 (2017).
Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog. Retin Eye Res. 21, 359–393. https://doi.org/10.1016/s1350-9462(02)00008-3 (2002).
Gherghel, D., Orgül, S., Gugleta, K., Gekkieva, M. & Flammer, J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am. J. Ophthalmol. 130, 597–605. https://doi.org/10.1016/s0002-9394(00)00766-2 (2000).
Fox, C. S. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 291, 844–850. https://doi.org/10.1001/jama.291.7.844 (2004).
Zhang, L. et al. Prevalence and factors associated with CKD: A population study from Beijing. Am. J. Kidney Dis. 51, 373–384. https://doi.org/10.1053/j.ajkd.2007.11.009 (2008).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management: A review. JAMA 322, 1294–1304. https://doi.org/10.1001/jama.2019.14745 (2019).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. https://doi.org/10.1016/s0140-6736(12)61728-0 (2012).
Wong, C. W. et al. Increased burden of vision impairment and eye diseases in persons with chronic kidney disease: A population-based study. EBioMed. 5, 193–197. https://doi.org/10.1016/j.ebiom.2016.01.023 (2016).
Rodríguez-Poncelas, A. et al. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS ONE 11, e0149448. https://doi.org/10.1371/journal.pone.0149448 (2016).
Liu, Y. T. et al. Association between chronic kidney disease and risk of cataract: A nationwide retrospective cohort study. Am. J. Nephrol. 45, 524–531. https://doi.org/10.1159/000475555 (2017).
Klein, R., Knudtson, M. D., Lee, K. E. & Klein, B. E. Serum cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: The Beaver Dam Eye Study. Arch. Ophthalmol. 127, 193–199. https://doi.org/10.1001/archophthalmol.2008.551 (2009).
Liew, G., Mitchell, P., Wong, T. Y., Iyengar, S. K. & Wang, J. J. CKD increases the risk of age-related macular degeneration. J. Am. Soc. Nephrol. 19, 806–811. https://doi.org/10.1681/asn.2007080844 (2008).
Weiner, D. E., Tighiouart, H., Reynolds, R. & Seddon, J. M. Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol. Dial. Transplant. 26, 3159–3165. https://doi.org/10.1093/ndt/gfr022 (2011).
Zhang, L. et al. Prevalence and factors associated with CKD: A population study from Beijing. Am. J. Kidney Dis. 51, 373–384. https://doi.org/10.1053/j.ajkd.2007.11.009 (2008).
Gai, Z. et al. Lipid accumulation and chronic kidney disease. Nutrients. https://doi.org/10.3390/nu11040722 (2019).
Bostom, A. G. et al. Hyperhomocysteinemia, hyperfibrinogenemia, and lipoprotein (a) excess in maintenance dialysis patients: A matched case-control study. Atherosclerosis 125, 91–101. https://doi.org/10.1016/0021-9150(96)05865-0 (1996).
Shim, S. H. et al. The role of systemic arterial stiffness in open-angle glaucoma with diabetes mellitus. Biomed. Res. Int. 2015, 425835. https://doi.org/10.1155/2015/425835 (2015).
Izzotti, A., Bagnis, A. & Saccà, S. C. The role of oxidative stress in glaucoma. Mutat. Res. 612, 105–114. https://doi.org/10.1016/j.mrrev.2005.11.001 (2006).
Small, D. M., Coombes, J. S., Bennett, N., Johnson, D. W. & Gobe, G. C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17, 311–321. https://doi.org/10.1111/j.1440-1797.2012.01572.x (2012).
Vaajanen, A. & Vapaatalo, H. Local ocular renin-angiotensin system: A target for glaucoma therapy?. Basic Clin. Pharmacol. Toxicol. 109, 217–224. https://doi.org/10.1111/j.1742-7843.2011.00729.x (2011).
Wang, T. J., Wu, C. K., Hu, C. C., Keller, J. J. & Lin, H. C. Increased risk of co-morbid eye disease in patients with chronic renal failure: A population-based study. Ophthal. Epidemiol. 19, 137–143. https://doi.org/10.3109/09286586.2012.680531 (2012).
Chou, C. L., Hsieh, T. C., Chen, J. S. & Fang, T. C. Risks of all-cause mortality and major kidney events in patients with new-onset primary open-angle glaucoma: A nationwide long-term cohort study in Taiwan. BMJ Open 8, e021270. https://doi.org/10.1136/bmjopen-2017-021270 (2018).
Tham, Y. C. et al. Is kidney function associated with primary open-angle glaucoma? Findings from the Asian Eye Epidemiology Consortium. Br. J. Ophthalmol. 104, 1298–1303. https://doi.org/10.1136/bjophthalmol-2019-314890 (2020).
Shim, S. H. et al. Association between renal function and open-angle glaucoma: The Korea National Health and Nutrition Examination Survey 2010–2011. Ophthalmology 123, 1981–1988. https://doi.org/10.1016/j.ophtha.2016.06.022 (2016).
Park, S. J., Byun, S. J., Park, J. Y. & Kim, M. Primary open-angle glaucoma and increased risk of chronic kidney disease. J. Glaucoma 28, 1067–1073. https://doi.org/10.1097/ijg.0000000000001390 (2019).
Tham, Y.-C. et al. Is kidney function associated with primary open-angle glaucoma? Findings from the Asian Eye Epidemiology Consortium. Br. J. Ophthalmol. 104, 1298. https://doi.org/10.1136/bjophthalmol-2019-314890 (2020).
Zhu, Z. et al. Visual impairment and major eye diseases in chronic kidney disease: The National Health and Nutrition Examination Survey, 2005–2008. Am. J. Ophthalmol. 213, 24–33. https://doi.org/10.1016/j.ajo.2020.01.002 (2020).
Zhu, Z. et al. Visual impairment and major eye diseases in chronic kidney disease: The National Health and Nutrition Examination Survey, 2005–2008. Am. J. Ophthalmol. 213, 24–33. https://doi.org/10.1016/j.ajo.2020.01.002 (2020).
Shim, S. H. et al. Association between renal function and open-angle glaucoma: The Korea National Health and Nutrition Examination Survey 2010–2011. Ophthalmology 123, 1981–1988. https://doi.org/10.1016/j.ophtha.2016.06.022 (2016).
Nangaku, M. & Fujita, T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens. Res. 31, 175–184. https://doi.org/10.1291/hypres.31.175 (2008).
Fletcher, E. L., Phipps Ja Fau-Ward, M. M., Ward Mm Fau-Vessey, K. A., Vessey Ka Fau-Wilkinson-Berka, J. L. & Wilkinson-Berka, J. L. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature.
Giese, M. J. & Speth, R. C. The ocular renin-angiotensin system: a therapeutic target for the treatment of ocular disease.
Cullinane, A. B., Leung, P. S., Ortego, J., Coca-Prados, M. & Harvey, B. J. Renin–angiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br. J. Ophthalmol. 86, 676–683. https://doi.org/10.1136/bjo.86.6.676 (2002).
Hou, Y. & Delamere, N. A. Influence of ANG II on cytoplasmic sodium in cultured rabbit nonpigmented ciliary epithelium. Am. J. Physiol. Cell Physiol. 283, C552-559. https://doi.org/10.1152/ajpcell.00459.2001 (2002).
Agarwal, P. & Agarwal, R. Trabecular meshwork ECM remodeling in glaucoma: Could RAS be a target?. Expert Opin. Ther. Targets 22, 629–638. https://doi.org/10.1080/14728222.2018.1486822 (2018).
Inoue, T., Yokoyoma, T. & Koike, H. The effect of angiotensin II on uveoscleral outflow in rabbits. Curr. Eye Res. 23, 139–143. https://doi.org/10.1076/ceyr.23.2.139.5470 (2001).
Lesk, M. R., Wajszilber, M. & Deschenes, M. C. The effects of systemic medications on ocular blood flow. Can. J. Ophthalmol. 43, 351–355. https://doi.org/10.3129/i08-057 (2008).
Meyer, P., Flammer, J. & Lüscher, T. F. Local action of the renin angiotensin system in the porcine ophthalmic circulation: Effects of ACE-inhibitors and angiotensin receptor antagonists. Invest. Ophthalmol. Vis. Sci. 36, 555–562 (1995).
Grieshaber, M. C. & Flammer, J. Does the blood–brain barrier play a role in glaucoma?. Surv. Ophthalmol. 52, S115–S121. https://doi.org/10.1016/j.survophthal.2007.08.005 (2007).
Tu, X. K., Yang Wz Fau-Shi, S.-S., Shi S. Fau-Wang, C.-H., Wang Ch Fau-Chen, C.-M. & Chen, C. M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia.
Robinson, K. et al. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation 94, 2743–2748. https://doi.org/10.1161/01.cir.94.11.2743 (1996).
Ratliff, B. B., Abdulmahdi, W., Pawar, R. & Wolin, M. S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal 25, 119–146. https://doi.org/10.1089/ars.2016.6665 (2016).
Nita, M. & Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016, 3164734. https://doi.org/10.1155/2016/3164734 (2016).
Perng, W. T. et al. Increased risk of glaucoma amongst new-onset gout patients aged 20–39 years: A nationwide population-based cohort study in Taiwan. Int. J. Clin. Pract. 75, e14169. https://doi.org/10.1111/ijcp.14169 (2021).
Biggerstaff, K. S. et al. Gout and open-angle glaucoma risk in a veteran population. Graefes Arch. Clin. Exp. Ophthalmol. 259, 3371–3379. https://doi.org/10.1007/s00417-021-05273-2 (2021).
Li, S. et al. Association of serum uric acid levels with primary open-angle glaucoma: A 5-year case-control study. Acta Ophthalmol. 97, e356–e363. https://doi.org/10.1111/aos.13789 (2019).
Johnson, R. J. et al. Uric acid and chronic kidney disease: Which is chasing which?. Nephrol. Dial Transplant. 28, 2221–2228. https://doi.org/10.1093/ndt/gft029 (2013).
Acknowledgements
This research was supported by the Busan Sungmo eye hospital Sodam scholarship committee and the Soonchunhyang University research fund.
Author information
Authors and Affiliations
Contributions
J.S.R. and S.H.L contributed to data acquisition, data analysis, and writing of the manuscript. T.K.P. and J.Y.M. contributed to the design of the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ro, JS., Moon, J.Y., Park, T.K. et al. Association between chronic kidney disease and open-angle glaucoma in South Korea: a 12-year nationwide retrospective cohort study. Sci Rep 12, 3423 (2022). https://doi.org/10.1038/s41598-022-07190-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07190-8
This article is cited by
-
A comprehensive review of artificial intelligence models for screening major retinal diseases
Artificial Intelligence Review (2024)
-
Association of glaucoma and lifestyle with incident cardiovascular disease: a longitudinal prospective study from UK Biobank
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.