Abstract
Fatigue is increasingly recognized as a major complaint in patients with chronic inflammatory and autoimmune diseases. Although fatigue is assumed to represent a significant problem in celiac disease, existing knowledge is scarce, and opinions are conflicting. This study aimed to investigate the prevalence and severity of fatigue in patients with newly diagnosed celiac disease and compare it with healthy control subjects. Ninety patients with newly diagnosed celiac disease were compared with 90 age- and sex-matched healthy subjects. The primary endpoints were fatigue severity as measured by: the fatigue Visual Analog Scale (fVAS), the Fatigue Severity Scale (FSS), and the inverted Vitality subscale of the MOS36 (SF-36vs). Higher scores indicate more severe fatigue. Clinically relevant fatigue was determined using predefined cut-off values. Secondary endpoints were the associations between fatigue, and sex, age, depression, pain, and selected biochemical variables. The median (IQR) fVAS-scores were 43.0 (18.0–64.5) in patients, and 9.0 (2.0–16.0) in the control group (p < 0.001); and the FSS scores 3.8 (2.0–4.8) in patients, and 1.4 (1.0–1.9) in control subjects (p < 0.001). Inverted SF-36vs scores had a mean (SD) value of 58.8 (23.6) in patients, and 29.7 (14.3) in healthy subjects (p < 0.001). The presence of clinically relevant fatigue ranged from 41 to 50% in patients. Increased fatigue severity was associated with female sex, younger age, and elevated pain and depression scores, but not with levels of selected biochemical variables, including hemoglobin. Fatigue is a severe and frequent phenomenon in patients with untreated celiac disease.
Similar content being viewed by others
Introduction
Fatigue may be defined as “an overwhelming sense of tiredness, lack of energy, and feeling of exhaustion”1. It is frequently observed in patients with chronic inflammatory and autoimmune diseases, cancer, and neurodegenerative diseases2,3,4,5. Many patients rate fatigue as one of their most severe and troublesome afflictions, as it affects emotional, physical, and social aspects of life, and is often dismissed by their treating physician6,7,8,9. Although fatigue as a phenomenon has gained increased attention in recent years, the mechanisms behind its generation and regulation are only beginning to be revealed.
A conceptual model for understanding fatigue is the “sickness behavior response”, an evolutionarily conserved survival enhancing strategy observed in animals and humans during states of infection or bodily harm10,11. The sickness behavior response encompasses several phenomena such as weariness; fatigue; lack of thirst, hunger, and grooming; as well as social withdrawal and depression11.
Sickness behavior is triggered by the activation of innate immunity and neuroinflammation. The proinflammatory interleukin (IL)-1β is produced and released by macrophages in inflammatory states and transported to the brain, inducing sickness behavior after binding to specific IL-1 receptors7. In both animal and human studies, infusion of inflammatory cytokines results in sickness behavior12,13; fatigue is a dominant phenomenon in this complex response. Accumulating evidence points to a genetic basis and complex molecular pathways that generate fatigue in the brain through neuronal signaling14,15,16. In addition, psychosocial factors such as depression, disturbed sleep, and pain all influence fatigue severity17.
Celiac disease is an autoimmune inflammatory disease of the small intestine, driven by an immune reaction to dietary gluten in genetically susceptible individuals18 and affecting approximately 1% of the population worldwide19. Traditionally, it was believed that celiac disease is confined to the intestine, with classical symptoms mainly related to malabsorption, such as diarrhea, stomach pain, and weight loss18. However, it has become evident that extraintestinal manifestations such as arthralgia, osteoporosis, anemia, and headaches are frequent, and may dominate or represent the only manifestation of the disease18,20,21,22. These extraintestinal manifestations may result from both aberrant immune responses and malabsorption22; extreme tiredness, and fatigue have been among the most commonly reported extraintestinal complaints20,21,23. This contrasts with the traditional concept of the disease, possibly explaining why current literature regarding fatigue in celiac disease is sparse and inconsistent24,25.
Previously only a few studies have been dedicated to explore fatigue in celiac disease, and in general the more phenomenologic issues have been focused on. We believe there is a knowledge gap regarding the more objective and biologically based factors of fatigue in celiac disease, and to obtain a better understanding of these issues, we investigated patients newly diagnosed with the disease, and compared them with healthy age- and sex-matched subjects.
Methods
Patient recruitment and diagnostic procedures
This study was performed at the Department of Gastroenterology, Stavanger University Hospital, and was designed as a single-center, cross-sectional, controlled study. Eligible patients were consecutively identified based on referral letters to the outpatient clinic. The inclusion criteria were: positive tests for anti-tissue transglutaminase-IgA antibodies (tTG-IgA) at time of referral, defined as levels ≥ 7 U/mL (normal: < 7); age ≥ 18 years; and being on a gluten-containing diet. Patients fulfilling these criteria were considered for participation, and eligible patients were included at the study visit. An upper endoscopy, four mucosal biopsies from the descending part of the duodenum, and two biopsies from the proximal duodenal bulb were performed for histopathological verification of celiac disease. Exclusion criteria were histopathological findings not consistent with celiac disease, inability to consent, and inability to adhere to the treatment protocol. Patients with findings consistent with celiac disease without villous atrophy in the biopsies (Marsh I and II histology) were also included, as guidelines at the time of inclusion used the term “probably celiac disease” for these cases19.
Patient handling
Eligible patients were informed regarding the study and procedure, and provided written, informed consent before inclusion in the study. During the visit, baseline data, including demographics and clinical data, were registered; blood samples were taken before gastroscopy was performed. All data were electronically registered on an iPad case report form using the FileMaker Pro (Claris International) software.
Healthy subjects
Ninety self reportedly healthy control subjects were included in the study. They were predominantly recruited from nonfamily acquaintances of patients with inflammatory bowel disease or psoriasis; approximately 1/3 were recruited from employees of the hospital and their acquaintances. The healthy control subjects were without any chronic illness. We did not perform any physical examination, blood tests or review of their medical records to verify that they were healthy.
Matching procedure
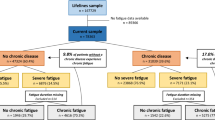
During the inclusion period between December 1, 2016, and September 30, 2018, 125 subjects were screened for participation; celiac disease was confirmed in 101 cases (80.8%). Eleven patients were excluded, as no control subjects fulfilled the matching criteria, i.e., sex and age +/− 5 years; thus, a total of 90 matching pairs were included for further study (Fig. 1).
Demographic and clinical data
Age, sex, and body mass index (BMI) were registered for all study participants. The initial symptoms and signs that led to contact with the health service, later resulting in a diagnosis of celiac disease, were registered upon study visit based on the following predefined items: abdominal discomfort, diarrhea, stomach pain, weight loss, and weariness, there was also a possibility to add free text to further specify.
Concurrent autoimmune disease was registered based on patients’ reporting and medical files. Patients were dichotomized into “any other autoimmune disease” or “only celiac disease” based on the presence of one or more concomittant autoimmune disease.
Blood tests
C-reactive protein (CRP), hemoglobin, folic acid, cobalamin, 25-OH vitamin D, tTG-IgA, and total IgA were measured in all patients at study inclusion and analyzed at the hospital’s routine laboratory. tTG-IgA analysis was performed with a fluorescence enzyme immunoassay, using EliATM CelikeyTM assays on a Phadia® 250 analyzer (Thermo Fischer Scientific). Ferritin levels measured within the last 3 months before inclusion were recorded. As ferritin is an acute phase protein, iron deficiency was defined as < 30 µg/L ferritin if CRP was < 5 mg/L, or < 100 µg/L ferritin if CRP was ≥ 5 mg/L, as recommended26. Anemia was defined as an Hb ≤ 13.4 g/dL for men, and ≤ 11.7 g/dL for women; cobalamin deficiency was defined as < 167 pmol/L, 25-OH vitamin D deficiency as < 50 nmol/L, and folic acid deficiency as < 6.3 nmol/L.
Histopathological evaluation
Duodenal biopsies were obtained during upper endoscopy and were further prepared and examined at the hospital’s Department of Pathology. All biopsies were formalin-fixed, paraffin-embedded, stained with hematoxylin and eosin (H&E), and graded according to the modified Marsh-Oberhuber classification27.
Fatigue assessment
Three generic and unidimensional fatigue instruments were used to grade fatigue severity: the fatigue Visual Analog Scale (fVAS), Fatigue Severity Scale (FSS), and Vitality subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36vs)28,29,30; these were completed by both patients and healthy control subjects under the instruction and supervision of a study nurse.
The fVAS is a single-item scale consisting of a 100 mm horizontal line with vertical anchors; the wording on the left end of the line (0 mm) is “no fatigue,” while the right end (100 mm) is “fatigue as bad as it can be”; the patient places a mark on the horizontal line to describe the degree of fatigue during the last week29. The cut-off for clinically relevant fatigue was previously defined as fVAS scores ≥ 50 mm; this was also applied in our study3,5.
The FSS is a questionnaire including nine statements regarding fatigue over the last two weeks; subjects are asked to rate their responses from 1 (completely disagree) to 7 (completely agree). The FSS score is reported as the mean score of the nine questions, ranging from 1 to 7; a score of ≥ 4 was previously used as a cut-off for fatigue, and was used in this study30,31.
The SF-36vs consists of four questions addressing energy and fatigue within the last 4 weeks, yielding score values between 0 and 100, where a higher score indicates less fatigue (and “higher vitality”)32; clinically relevant fatigue was defined by a score ≤ 355,33. The registered SF-36vs scores were inverted to make data presentation more comparable with the other scales, as well as to improve visual presentations in the figures; an inverted SF-36vs score ≥ 65 thus represents clinically relevant fatigue.
Depression and pain
The Hospital Anxiety and Depression Scale, Depression Subscale (HADS-D) was used to detect depressive mood in patients and healthy control subjects. The HADS-D consists of seven items, each answered with a number from 0 to 334. An HADS-D score of ≥ 8 was previously used as a cut-off for depression35.
Pain was assessed using the bodily pain subscale (SF-36 pain) of the SF-36 questionnaire. The SF-36 pain consists of two items, assessing body pain intensity and the interference of pain with normal activities within the last 4 weeks. The scores range between 0 and 100, where higher scores indicate less pain. The pain scores were inverted to make data presentation more comparable with the other scales, as well as to improve visual presentation in the figures.
Statistical analysis
The sample size was determined based on the assumption that fatigue severity and the ratio of patients experiencing clinically significant fatigue were similar to other inflammatory conditions, such as rheumatoid arthritis and primary Sjögren’s syndrome4,36. To detect a difference of 25 in the fVAS-scores between patients with celiac disease and healthy subjects, with a power of 0.8 and a significance level of 0.05, a minimum of 33 subjects would be required in each group. We chose to include more patients than the minimum number required as the study visits did not cause significant inconvenience to patients, and to ensure we had sufficient data for follow up studies. Continuous data normality was evaluated using the Shapiro–Wilk test. When pairwise comparing celiac disease patients with healthy subjects, normally distributed data were analyzed using the paired t-test, and non-normally distributed data using the Wilcoxon paired samples rank test.
Univariable linear regression analysis using fVAS, FSS, and inverted SF-36vs as the dependent variables was performed using the following independent variables: sex, age, body mass index, hemoglobin, folic acid, cobalamin, 25-OH vitamin D, tTG-IgA, ferritin, HADS-D, inverted SF-36 pain and “any other autoimmune disease”.
Independent variables from univariable regression analyses with a p value < 0.2 were used to develop multivariable regression models; we also included tTG-IgA due to its clinical significance. Backward and forward model selection was used to reach a final multivariable regression model. Analyses were performed using IBM SPSS statistical software (Version 24.0. IBM Corp.); a p value < 0.05 was considered statistically significant. For all regression models we report standardized beta coefficients, whose interpretation is the impact of a one standard deviation increase in the corresponding independent variable.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Ethics approval
The study was approved by the regional ethics committee (REC West, Norway 2011/2631) and conducted in compliance with the principles expressed in the Declaration of Helsinki. The study is registered on ClinicalTrials.gov (NCT01551563).
Results
Demographics and clinical data
Characteristics of the study participants are given in Table 1. Two patients had anti-tTG-IgA values below 7 at V0, but these two patients had elevated anti-tTG-IgA at the time of referral and were therefore included. The two patients with Marsh 1 histological findings at diagnosis had normalization of anti-tTG-IgA and histology after one year of a gluten free diet, finally confirming the diagnosis.
There were nine missing ferritin values, one missing hemoglobin value, one missing SF-36vs value. According to the definitions used, 27 (33%) patients were iron-deficient, 5 (6%) were anemic, 21 (23%) had low vitamin D levels, 13 (14%) had a folic acid deficiency, and 3 (3%) had a cobalamin deficiency.
Fifteen (17%) of the patients had one or more concurrent autoimmune disease. The registered concurrent autoimmune diseases were; thyroid disease (n = 7), diabetes type I (n = 4), psoriasis (n = 2), rheumatoid arthritis (n = 1), primary biliary cholangitis (n = 1) and dermatitis herpetiformis (n = 1).
Symptoms and signs causing contact with the health service
The most frequently reported symptoms were abdominal discomfort, including nausea, borborygmi, and abdominal distention (Table 2); weariness was reported in 48% of patients. The classic characteristics of celiac disease were diarrhea in 40%, stomach pain in 33%, and weight loss in 10% of patients.
Severity and prevalence of clinically significant fatigue
For all three fatigue instruments used, fatigue scores were significantly higher in patients than in healthy subjects. The median (IQR) fVAS-score was 43.0 (18.0–64.5) in patients, and 9.0 (2.0–16.0) in the control group (p < 0.001). Similarly, the FSS score was 3.8 (2.0–4.8) in patients, and 1.4 (1.0–1.9) in control subjects (p < 0.001); the inverted SF-36vs had a mean (SD) value of 58.8 (23.6) in patients, and 29.7 (14.3) in healthy subjects (p < 0.001); Fig. 2.
Fatigue measures using three different instruments in 90 patients with celiac disease and 90 healthy age- and sex-matched subjects. Box plots indicate median (line), interquartile range (box), and 5th to 95th percentiles (whiskers); dots represent outliers. To the right in the figure SF-36vs scores are inverted to ease visual interpretation. fVAS Fatigue visual analog scale, FSS Fatigue severity scale, SF-36vs Vitality subscale of the MOS 36-item short-form health survey, HCS Healthy control subjects.
Based on the predefined cut-off values for clinically relevant fatigue as recorded by the fVAS, FSS, and SF-36vs, the prevalence of fatigue in patients with celiac disease was 41.1%, 42.2%, and 50.0%, respectively, versus 0%, 1.1%, and 2.2% in healthy control subjects (all p < 0.001); Fig. 3.
Prevalence of clinically relevant fatigue in 90 patients with newly diagnosed celiac disease vs. 90 healthy control subjects using defined cut-off values for fVAS, FSS and SF-36vs. Clinically relevant fatigue defined as: fVAS ≥ 50, FSS ≥ 4, and inverted SF-36vs ≥ 65. fVAS Fatigue visual analog scale, FSS Fatigue severity scale, SF-36vs Vitality subscale of the MOS 36-item short-form health survey.
There was no difference in fatigue severity when comparing patients with concurrent autoimmune disease to patients with only celiac disease (Supplementary Table 1).
Factors associated with fatigue
In univariable regression analysis, age, female sex, inverted SF-36 pain, and HADS-D scores were significantly associated with fVAS, FSS, and inverted SF-36vs scores. Age was negatively associated with fVAS: β-0.318 (p = 0.002), FSS: β-0.311 (p = 0.003), and inverted SF-36vs scores: β-0.378 (p < 0.001). Sex (0 = female, 1 = male) was negatively associated with fVAS: β-0.323 (p = 0.002), FSS: β-0.371 (p < 0.001), and inverted SF-36vs: β-0.273 (p = 0.009), meaning that female sex was associated with fatigue. Pain and depression scores were positively associated. The associations between pain (inverted SF-36 pain) and fatigue were: fVAS: β 0.335 (p = 0.001), FSS: β 0.351 (p = 0.001), and inverted SF-36vs scores: β 0.334 (p = 0.001). The β-values reflecting the association between depression and fVAS, FSS, and inverted SF-36vs were 0.504 (p < 0.001), 0.501 (p < 0.001), and 0.334 (p = 0.001), respectively. BMI, cobalamin, folic acid, 25-OH vitamin D, hemoglobin, ferritin, tTG-IgA, and “any other autoimmune disease” showed no associations with fVAS, FSS, or inverted SF-36vs (Supplementary Table 2).
In multivariable regression analysis, both forward and backward selections resulted in the same model; this demonstrated a positive association between female sex, higher HADS-D-scores, higher inverted SF-36 pain scores, and fVAS, FSS, and inverted SF-36vs. Conversely, a negative association was shown between age and fVAS, FSS, and inverted SF-36vs, indicating that younger patients, females, and patients with more pain and/or more depression experienced more fatigue (Table 3).
With regard to other independent values included in the multiple regression model selection, tTG-IgA did not contribute significantly to the fVAS score; BMI, hemoglobin, and tTG-IgA did not contribute significantly to the FSS score; and folic acid and tTG-IgA did not contribute significantly to the inverted SF-36vs scores. These variables were thus excluded from the final multiple regression models. The final model explained 44%, 45%, and 47% of the variance in the fVAS, SF-36vs, and FSS scores, respectively.
Discussion
This study clearly demonstrates that fatigue is a common and substantial complaint in patients with newly diagnosed and untreated celiac disease. The findings were consistent across three different and widely used fatigue instruments, revealing that the prevalence of clinically relevant (meaningful) fatigue ranges between 41 and 50% in these patients. These results are in accordance with previous studies that describe more fatigue in patients with celiac disease, compared with healthy control subjects37,38.
The fatigue prevalence rates are similar to data reported in other chronic inflammatory conditions such as inflammatory bowel disease, primary Sjögren’s syndrome, and psoriasis3,5,36,39. This supports the hypothesis that fatigue represents a common mechanism across different conditions, and is triggered by chronic inflammation and immunological danger signals. Data regarding fatigue in celiac disease is scarce, and previous studies reporting prevalence rates of fatigue in celiac disease patients have not used validated fatigue instruments or defined cut-off values. Most previous studies reporting the prevalence of fatigue have used a simple question asking patients whether or not they suffered from fatigue21,40,41,42,43,44,45,46,47; only one study applied a disease-specific fatigue instrument (Celiac Disease Patient Reported Outcome, Non-gastrointestinal domain)48. The inconsistencies in rating and reporting could thus be a reason for the large variations and discrepancy in previous studies, ranging from 8 to 82%24,25.
Measuring fatigue is a challenge. To the best of our knowledge, our study is the first to apply multiple generic and unidimensional fatigue instruments, as well as use defined cut-off values, to rate clinically relevant fatigue in newly diagnosed patients with celiac disease. Generic fatigue instruments were chosen, as they are not influenced by disease-related factors; disease-specific fatigue instruments tend to comprise elements of disease activity or other disease features, not solely those related to fatigue. Generic instruments are also of value when aiming to compare fatigue between sick and healthy individuals, as well as between diseases.
Whether fatigue can be considered a unidimensional phenomenon, or whether there are several dimensions, such as mental fatigue, physical fatigue, and muscular fatigue, remains under debate. This is reflected in the numerous different fatigue questionnaires available. According to the latter comprehension, fatigue is a phenomenon that specifically influences cognition, muscles, mood, physical activity, etc., therefore representing separate features. However, we hypothesize that fatigue, in the context of sickness behavior, should be considered a universal phenomenon that influences different aspects of human life, including traits or attributes associated with the disease.
In this study, we used a single-item unidimensional instrument (fVAS), and two multi-item unidimensional instruments (FSS, SF-36vs). The consistency of the results indicates that the findings derived from the selected fatigue instruments are representative.
Several factors may influence fatigue severity; in our study, female sex and younger age were associated with fatigue. Previous studies on other diseases and conditions have been inconsistent regarding these items; and even though a statistically significant association was found in our study, it is not possible to make a generalized and universal conclusion5,49,50,51,52,53. However, the association between pain and fatigue has been consistent in many studies15,54. Pain elicits signals of bodily damage, and is an inducer of the sickness behavior response that is evolutionarily believed to increase the survival of the individual55; fatigue is a major component of this response15. A genome-wide association study (GWAS) of patients with Sjögren’s syndrome identified an association with genetic variants of the RTP4 gene influencing the severity of fatigue. This gene encodes a protein involved in pain processing and points to a possible molecular explanation for the association15,56.
Depressive mood is a consistent factor associated with fatigue in chronic inflammatory conditions3,5. In addition, this association has been described in both treated and untreated patients37,57. Both depression and fatigue are elements in the sickness behavior response and are possibly signaled by common inflammatory and neurotransmitter pathways58,59. Some studies of people with mental depression show increased proinflammatory cytokine profiles60. The role of IL-1β in depression has been increasingly emphasized, and could be key to elucidating why fatigue and depression are closely associated61. An additional explanation is that the instruments used to assess fatigue and depression often have similar wording, therefore capturing elements of both dimensions. Finally, fatigue can also be a part of depressive disease entities with overlapping symptomatologies62. Although fatigue in celiac disease have been scarcely explored previously, it is likely that the mechanisms that generate fatigue are identical across chronic inflammatory diseases.
Anemia, as well as vitamin D, folic acid, cobalamin, and iron deficiencies, frequently occur in celiac disease63,64,65; however, we could not reveal any association between hemoglobin and fatigue, an observation possibly owing to the low number of patients with anemia (n = 5). The evidence regarding vitamin D deficiency and fatigue is conflicting; no associations were observed. The rates of cobalamin and folic acid deficiencies were too small for adequate analysis, and no associations with fatigue were observed.
This study has some limitations; first, we did not record sleep disturbances. Reduced sleep quality, as recorded by the Pittsburgh Sleep Quality Index, has been previously shown to influence fatigue in patients with celiac disease and IBD38,49. Another limitation is the cross-sectional study design, which cannot point to causality and only describes associations. Even though we selected predefined cut-off values that have been used in other studies to define fatigue prevalence, the validity of these cut-off values is debatable; conceptually it is artificial to dichotomize a continuous trait into “fatigue” or “no fatigue”.
In conclusion, patients with newly diagnosed celiac disease had significantly increased and more prevalent fatigue when compared with matched, healthy control subjects. Fatigue in these patients was associated with female sex, younger age, pain, and depressive mood. This confirms that fatigue is a substantial ailment in untreated celiac disease that deserves increased attention in the management of these patients.
Abbreviations
- BMI:
-
Body mass index
- FSS:
-
Fatigue Severity Scale
- fVAS:
-
Fatigue Visual Analog Scale
- GWAS:
-
Genome-wide association study
- HADS-D:
-
The Hospital Anxiety and Depression Scale, Depression Subscale
- Hb:
-
Hemoglobin
- HCS:
-
Healthy control subjects
- H&E:
-
Hematoxylin and eosin
- IL:
-
Interleukin
- SF-36 Pain:
-
Pain subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey
- SF-36vs:
-
Vitality subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey
- tTG-IgA:
-
Anti-tissue transglutaminase-IgA antibodies
References
Krupp, L. B. & Pollina, D. A. Mechanisms and management of fatigue in progressive neurological disorders. Curr. Opin. Neurol. 9, 456–460 (1996).
Ng, W. F. & Bowman, S. J. Primary Sjogren’s syndrome: Too dry and too tired. Rheumatology (Oxford) 49, 844–853 (2010).
Grimstad, T. et al. Fatigue in newly diagnosed inflammatory Bowel disease. J. Crohns Colitis 9, 725 (2015).
van Hoogmoed, D., Fransen, J., Bleijenberg, G. & van Riel, P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 49, 1294–1302 (2010).
Skoie, I. M. et al. Fatigue in psoriasis: A controlled study. Br. J. Dermatol. 177, 505–512 (2017).
Hewlett, S. et al. Patients’ perceptions of fatigue in rheumatoid arthritis: Overwhelming, uncontrollable, ignored. Arthritis Rheum. 53, 697–702 (2005).
Norheim, K. B., Jonsson, G. & Omdal, R. Biological mechanisms of chronic fatigue. Rheumatology 50, 1009–1018 (2011).
de Rooy, E. C. et al. Concerns of patients with inflammatory bowel disease: Results from a clinical population. Am. J. Gastroenterol. 96, 1816–1821 (2001).
Curt, G. A. Impact of fatigue on quality of life in oncology patients. Semin. Hematol. 37, 14–17 (2000).
Dantzer, R. Cytokine-induced sickness behavior: Where do we stand?. Brain Behav. Immun. 15, 7–24 (2001).
Hart, B. L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (1988).
Swiergiel, A. H., Smagin, G. N., Johnson, L. J. & Dunn, A. J. The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: Effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res. 776, 96–104 (1997).
Kelley, K. W. et al. Cytokine-induced sickness behavior. Brain Behav. Immun. 17, 112–118 (2003).
Larssen, E. et al. Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid. SAGE Open Med. 7, 2050312119850390 (2019).
Bardsen, K. et al. Interleukin-1-related activity and hypocretin-1 in cerebrospinal fluid contribute to fatigue in primary Sjogren’s syndrome. J. Neuroinflamm. 16, 102 (2019).
Vollmer-Conna, U. et al. Cytokine polymorphisms have a synergistic effect on severity of the acute sickness response to infection. Clin. Infect. Dis. 47, 1418–1425 (2008).
Bower, J. E., Ganz, P. A., Irwin, M. R., Arevalo, J. M. & Cole, S. W. Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav. Immun. 25, 147–150 (2011).
Ludvigsson, J. F. et al. The Oslo definitions of coeliac disease and related terms. Gut 62, 43–52 (2013).
Ludvigsson, J. F. et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 63, 1210–1228 (2014).
Jericho, H., Sansotta, N. & Guandalini, S. Extraintestinal manifestations of celiac disease: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 65, 75–79 (2017).
Spijkerman, M. et al. A large variety of clinical features and concomitant disorders in celiac disease—A cohort study in the Netherlands. Dig. Liver Dis. 48, 499–505 (2016).
Leffler, D. A., Green, P. H. & Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 12, 561–571 (2015).
Zipser, R. D., Patel, S., Yahya, K. Z., Baisch, D. W. & Monarch, E. Presentations of adult celiac disease in a nationwide patient support group. Dig. Dis. Sci. 48, 761–764 (2003).
Skjellerudsveen, B. M., Omdal, R. & Grimstad, T. Fatigue in celiac disease: A review of the literature. JGH Open 3, 242–248 (2019).
Jelsness-Jorgensen, L. P., Bernklev, T. & Lundin, K. E. A. Fatigue as an extra-intestinal manifestation of celiac disease: A systematic review. Nutrients 10, 1652 (2018).
Gasche, C. et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm. Bowel Dis. 13, 1545–1553 (2007).
Dickson, B. C., Streutker, C. J. & Chetty, R. Coeliac disease: An update for pathologists. J. Clin. Pathol. 59, 1008–1016 (2006).
Ware, J. E. Jr. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992).
Wolfe, F., Michaud, K. & Pincus, T. Preliminary evaluation of a visual analog function scale for use in rheumatoid arthritis. J. Rheumatol. 32, 1261–1266 (2005).
Krupp, L. B., LaRocca, N. G., Muir-Nash, J. & Steinberg, A. D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 46, 1121–1123 (1989).
Keyser, R. E. et al. Evidence for aerobic insufficiency in women with systemic lupus erythematosus. Arthritis Care Res. 49, 16–22 (2003).
Hewlett, S., Dures, E. & Almeida, C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res. (Hoboken) 63(Suppl 11), S263–S286 (2011).
Dagfinrud, H., Vollestad, N. K., Loge, J. H., Kvien, T. K. & Mengshoel, A. M. Fatigue in patients with ankylosing spondylitis: A comparison with the general population and associations with clinical and self-reported measures. Arthritis Rheum. 53, 5–11 (2005).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Olsson, I., Mykletun, A. & Dahl, A. A. The Hospital Anxiety and Depression Rating Scale: A cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry 5, 46 (2005).
Vissink, A. et al. Health-related quality of life, employment and disability in patients with Sjögren’s syndrome. Rheumatology 48, 1077–1082 (2009).
Siniscalchi, M. et al. Fatigue in adult coeliac disease. Aliment. Pharmacol. Ther. 22, 489–494 (2005).
Zingone, F. et al. The quality of sleep in patients with coeliac disease. Aliment. Pharmacol. Ther. 32, 1031–1036 (2010).
Wolfe, F., Hawley, D. J. & Wilson, K. The prevalence and meaning of fatigue in rheumatic disease. J. Rheumatol. 23, 1407–1417 (1996).
Silvester, J. A., Graff, L. A., Rigaux, L., Walker, J. R. & Duerksen, D. R. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 44, 612–619 (2016).
Ludvigsson, J. F. et al. Symptoms and signs have changed in Swedish children with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 38, 181–186 (2004).
White, L. E. et al. Childhood coeliac disease diagnoses in Scotland 2009–2010: The SPSU project. Arch. Dis. Child. 98, 52 (2012).
Saleem, A., Connor, H. J. & Regan, P. O. Adult coeliac disease in Ireland: A case series. Ir. J. Med. Sci. 181, 225–229 (2012).
Cook, H. B. et al. Adult coeliac disease: Prevalence and clinical significance. J. Gastroenterol. Hepatol. 15, 1032–1036 (2000).
Bodé, S. & Gudmand-høyer, E. Symptoms and haematologic features in consecutive adult coeliac patients. Scand. J. Gastroenterol. 31, 54–60 (1996).
Masjedizadeh, R. et al. Celiac disease in South-West of Iran. World J. Gastroenterol. 12, 4416–4419 (2006).
Pulido, O. et al. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can. J. Gastroenterol. 27, 449–453 (2013).
Leffler, D. A. et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A Randomized controlled trial. Gastroenterology 148, 1311–9.e6 (2015).
Graff, L. A. et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm. Bowel Dis. 17, 1882–1889 (2011).
Keightley, P., Reay, R. E., Pavli, P. & Looi, J. C. L. Inflammatory bowel disease-related fatigue is correlated with depression and gender. Australas. Psychiatry 26, 508–513 (2018).
Villoria, A. et al. Fatigue in out-patients with inflammatory bowel disease: Prevalence and predictive factors. PLoS ONE 12, e0181435 (2017).
Norton, C. et al. Assessing fatigue in inflammatory bowel disease: Comparison of three fatigue scales. Aliment. Pharmacol. Ther. 42, 203–211 (2015).
Loge, J. H., Ekeberg, Ø. & Kaasa, S. Fatigue in the general norwegian population: Normative data and associations. J. Psychosom. Res. 45, 53–65 (1998).
Pollard, L. C., Choy, E. H., Gonzalez, J., Khoshaba, B. & Scott, D. L. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 45, 885–889 (2006).
Omdal, R., Mellgren, S. I. & Norheim, K. B. Pain and fatigue in primary Sjögren’s syndrome. Rheumatology 60, 3099–3106 (2019).
Norheim, K., Alexsson, A., Imgenberg-Kreuz, J. et al. Genetic determinants of fatigue in primary Sjögren’s syndrome—A genome wide association study [abstract]. Arthritis Rheumatol. 69 (2017).
Zingone, F. et al. Psychological morbidity of celiac disease: A review of the literature. United Eur. Gastroenterol. J. 3, 136–145 (2015).
Arnold, L. M. Understanding fatigue in major depressive disorder and other medical disorders. Psychosomatics 49, 185–190 (2008).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008).
Köhler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 135, 373–387 (2017).
Rossi, S. et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology 89, 1338–1347 (2017).
Corfield, E. C., Martin, N. G. & Nyholt, D. R. Co-occurrence and symptomatology of fatigue and depression. Compr. Psychiatry 71, 1–10 (2016).
Sulimani, R. A. Celiac disease and severe vitamin D deficiency: The case for anti-tissue transglutaminase antibody screening. Arch. Osteoporos. 14, 30 (2019).
Freeman, H. J. Iron deficiency anemia in celiac disease. World J. Gastroenterol. 21, 9233–9238 (2015).
Naik, R. D., Seidner, D. L. & Adams, D. W. Nutritional consideration in celiac disease and nonceliac gluten sensitivity. Gastroenterol. Clin. N. Am. 47, 139–154 (2018).
Acknowledgements
We thank the study nurses Merethe Lie Seglem and Anne Brit Meling for their assistance and logistical help during study visits. We thank the pathologist Ole Gunnar Aasprong for his help in grading the biopsies.
Funding
This study was supported by a grant from the Research Department of Stavanger University Hospital. The funding source had no role in the design and conduct of the study, the analysis and interpretation of data, or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
B.S. drafted the manuscript with support from T.G. and R.O. T.G. and R.O. conceived the original idea. A.K.H. and I.M.S. contributed to patient visits and sample preparation. B.S. analysed the data, J.T.K. verified the analytical method. L.A. produced the eCRF-software. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skjellerudsveen, B.M., Omdal, R., Hetta, A.K. et al. Fatigue: a frequent and biologically based phenomenon in newly diagnosed celiac disease. Sci Rep 12, 7281 (2022). https://doi.org/10.1038/s41598-022-11802-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11802-8
This article is cited by
-
High mobility group box 1 and a network of other biomolecules influence fatigue in patients with Crohn’s disease
Molecular Medicine (2023)
-
Conceptualised psycho-medical footprint for health status outcomes and the potential impacts for early detection and prevention of chronic diseases in the context of 3P medicine
EPMA Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.