Abstract
This study aimed to determine the effect of colchicine use on the risk of stroke among patients with diabetes mellitus (DM). We retrospectively enrolled patients with DM between 2000 and 2013 from the Longitudinal Health Insurance Database and divided them into a colchicine cohort (n = 8761) and noncolchicine cohort (n = 8761) by using propensity score matching (PSM). The event of interest was a stroke, including ischemic stroke and hemorrhagic stroke. The incidence of stroke was analyzed using multivariate Cox proportional hazards models between the colchicine cohort and the comparison cohort after adjustment for several confounding factors. The subdistribution hazard model was also performed for examination of the competing risk. The colchicine cohort had a significantly lower incidence of stroke [adjusted hazard ratios (aHR), 95% confidence intervals (95%CI)] (aHR = 0.61, 95%CI = 0.55–0.67), ischemic stroke (aHR = 0.59, 95%CI = 0.53–0.66), and hemorrhagic stroke (aHR = 0.66, 95%CI = 0.53–0.82) compared with the noncolchicine cohort. Drug analysis indicated that patients in the colchicine cohort who received colchicine of cumulative daily defined dose (cDDD) > 14 and duration > 28 days had a lower risk of stroke and ischemic stroke compared with nonusers. The colchicine cohort (cDDD > 150, duration > 360 days) also had a lower risk of stroke, ischemic stroke, and hemorrhagic stroke. The cumulative incidence of stroke, ischemic stroke, and hemorrhagic stroke in the colchicine cohort was significantly lower than that in the noncolchicine cohort (log-rank P < 0.001). However, the subdistribution hazard model reveal the colchicine was not associated with the hemorrhagic stroke in DM patients without gout (aHR = 0.69, 95%CI = 0.47–1.00). Colchicine use with cDDD > 14 and duration > 28 days was associated with lower risk of stroke and ischemic stroke, and colchicine use with cDDD > 150 and duration > 360 days played an auxiliary role in the prevention of stroke, ischemic stroke, and hemorrhagic stroke in patients with DM. The colchicine for the hemorrhagic stroke in DM patients without gout seem to be null effect.
Similar content being viewed by others
Introduction
The stroke are closely associated with the system inflammation and these diseases having the high level of the C-reactive protein (CRP) and inflammatory cytokines such as interleukin-6 (IL-6)1,2. Meanwhile, previous data provide evidence that nucleotide-binding domain and leucine-rich repeat protein-3 (NLRP3) inflammasome mediated inflammation is implicated in the etiology of DM and promotes DM-induced endothelial inflammation and atherosclerosis1,2. Thus, the NLRP3 may contribute to early neurological deterioration in stroke patients with DM. The colchicine displays its anti-inflammatory effect through inhibition of interleukn-1 (IL-1) and IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and NLRP3 inflammasome, and it is used to prevent the complication of the coronary artery disease (e.g., cardiac arrhythmia). Moreover, the colchicine use are associated with the lower risk of the recurrent ischemic stroke among acute non-cardiogenic ischemic stroke patients3. Altogether, the colchicine may play a role for attenuating the risk of stroke in patients with DM3,4.
A random-effects meta-analysis model was applied by Masson et al.5. They analyzed nine eligible trials of colchicine therapy involving a total of 6630 patients, (colchicine cohort, n = 3359; noncolchicine cohort, n = 3271). The stroke incidence was lower in the colchicine cohort than in the noncolchicine cohort5. However, Khandkar et al. performed a systematic review and meta-analysis of studies and concluded that the results for the effect of colchicine on stroke incidence were inconclusive6. Therefore, the relationship between the colchicine and the stroke is to be debated.
In Taiwan, colchicine is a historic treatment for gout, Familial Mediterranean Fever and its associated complication, amyloidosis. In recent years gout patients who have been taking colchicine for years have demonstrated novel applications within oncology, immunology, cardiology and dermatology7,8. The increasing prevalence of gout is associated with several factors including increasing incidence of metabolic syndromes and, in turn, DM. Meanwhile, the rising pace of aging across the globe is rising joint-related disorders such as joint pain, and gouty arthritis is estimated to enhance the growth of colchicine. Moreover, the micro- and macrovascular complications of DM are predisposing factors of the stroke, leading to the high frequency of the stroke among the those patients with DM. Up to today, no English literature has focused on the association between colchicine use and stroke (including ischemic and hemorrhagic strokes) risk among DM cohort. Thus, we investigated this association in patients with DM from the general population.
Materials and methods
Data source
Our data source was the Longitudinal Health Insurance Database 2000 (LHID2000), which is a representative subset of Taiwanese National Health Insurance Research Database (NHIRD) and contains records of one million people randomly sampled from 23 million beneficiaries of the universal health insurance program in Taiwan. All diagnostic codes for the claims are recorded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Personal identification information was anonymized to protect the privacy of the insured subjects. Because the NHIRD data set comprises anonymized secondary data, informed consent was not required. This study was approved by the Research Ethics Committee of China Medical University and Hospital in Taiwan (CMUH104-REC2-115-AR-4).
Study design and participants
We designed a population-based retrospective cohort study by using the LHID2000 dataset to examine the association between colchicine use and stroke in patients with DM. Patients over 18 years of age with DM (ICD-9-CM code 250) from 2000 to 2013 were included as the target population. The colchicine cohort comprised patients who were prescribed colchicine (Anatomical Therapeutic Chemical (ATC) code M04AC01) for at least 28 days following the DM diagnosis. The date of the first prescription of colchicine was defined as the index date. Patients in the noncolchicine cohort were randomly selected from DM patients who were never prescribed colchicine during the study period. The index date for the noncolchicine cohort was randomly assigned. Patients with stroke history before the index date were excluded from the study.
The classification of stroke
Based on Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, the ischemic stroke included the (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology. In the Goldstein et al. study, 73% of patients with code 434.11 had embolic strokes, and 47% of those with code 436 had an identified stroke cause. Of patients with code 434.91, 39% had stroke of uncertain cause, 25% "lacunar," 17% atherothrombosis, and 15% embolism9,10. Information on the etiology and subtypes of ischemic stroke was not available in the NHIRD. We cannot discriminate the subtypes of ischemic stroke according to the TOAST classification. However, in accordance with Goldstein et study, we designated the ICD-9CM in the appendix Table 4.
In the Taiwan Stroke Registry, ischemic stroke is defined as the “acute onset of neurological deficits with signs or symptoms persisting for longer than 24 h, presenting to the hospital within 10 days of onset, presence or absence of acute ischemic lesion(s) on brain computed tomography, or presence of acute ischemic lesion(s) on diffusion weighted magnetic resonance images that correspond to the clinical manifestations.” The accuracy of recording ischemic stroke diagnoses in the NHIRD is high, and the NHIRD appears to be a valid resource for population-based research on ischemic stroke11.
The pure dissection of carotid artery in collagen disorders may induced the stroke. However, these patients were young, rarely with hypertension, hyperlipidemia and gout. Therein, these patients rarely entry into our study. Diagnosis and treatment of non-atherosclerotic stroke relies heavily upon history and physical exam as well as CT/MR and catheter angiography. Treatment depends upon the etiology of the ischemia but is in general focused on managing the underlying condition and reducing the risk of future stroke either through antithrombotic therapy or revascularization12.
The plausible mechanism for stroke prevention
Primary prevention of ischemic stroke includes lifestyle modification and diet, treatment of risk factors including hypertension, hyperlipidemia, and DM, antiplatelet therapy for high cardiovascular risk patients, and anticoagulation in atrial fibrillation. The primary prevention of hemorrhagic stroke include the aggressive treatment of hypertension, restriction in alcohol intake, and occlusion of the left atrial appendage in patients with atrial fibrillation and permanent contraindications for oral anticoagulation13. The stains seem to play a role for prevention of the ischemic stroke. In contrast, the role of statins for the risk for hemorrhagic stroke is controversial13.
Definition of colchicine cohort
From a mechanistic standpoint, the anti‐inflammatory effects of colchicine are not only mediated by direct interaction with microtubules and by regulation in cytokine secretion but also due to modifications at the transcriptional level14. Thus, longer therapy durations (such as > 28 days) are necessary to establish the full effects of colchicine; thus, we enrolled patients who had tolerated colchicine for > 28 days15,16. In the DM cohort, the colchicine users had higher frequency of pneumonia than nonusers; therefore, we set the 28-day cutoff point to observe tolerance for colchicine17. This policy facilitates decision-making for continuation of colchicine use (Fig. 1).
Propensity score matching
To control for confounding effects, we performed a 1:1 PSM between patients with and without colchicine prescriptions. Logistic regression was used to calculate the propensity score on the basis the following covariates: age, sex, index year, hypertension-related disease (presumed blood pressure, BP ≥ 140/90)18, coronary artery disease, acute pericarditis, endocarditis, myocarditis, other disease of pericardium, other disease of endocardium, cardiomyopathy, conduction disorder, cardiac arrhythmia, heart failure, dyslipidemia, hypoglycemia, obesity, gout, liver cirrhosis, hepatitis B, hepatitis C, pneumonia, adapted Diabetes Complications Severity Index (aDCSI score), and inpatient days. The ATC codes used were other antihypertensive agents, diuretics, beta-adrenoceptor blockers, calcium channel blockers, angiotensin-converting-enzyme inhibitors and angiotensin II antagonists, direct renin inhibitors, oral hypoglycemic agents, insulin injection agents, antithrombotic agents (including aspirin, warfarin, heparin, and ticlopidine), NSAIDs, steroids, antigout benzbromarone, allopurinol, and hypolipidemic drugs such as statins, fibrates, bile acid sequestrantse, nicotinic acid and derivates, and other hypocholesterolaemic and hypotrygliceridaemic drugs.
Diabetes Complications Severity Index score
The aDCSI score was calculated to evaluate the severity of complications among diabetes patients. The severity index included the following complications: nephropathy, retinopathy, peripheral vascular disease, cardiovascular disease, stroke, neuropathy, and metabolic disorders. Renal function was related to colchicine use. Therefore, the components of the aDCSI such as DM nephropathy and renal insufficiency were included in the analysis. Furthermore, aDCSI severity for the variables in the analysis was used to avoid bias due to unequal numbers of male and female enrollees on the risk of stroke19.
Because DM-related complications were the major determinant of hospital mortality rather than diabetes per se, glycated hemoglobin (HbA1c) level, or initial blood glucose level, the progression of aDCSI was an accurate predictor of acute coronary syndrome, ischemic stroke, and hemorrhagic stroke. Therefore, aDCSI severity, concurrent medications, and diseases were included in the analyses instead of laboratory tests to evaluate the effects of these clinical factors on stroke incidence20. Thus, even without using laboratory data, we could monitor the risk of stroke based on the aDCSI following colchicine use21. Wicke et al. reported that higher aDSCI levels are correlated with a higher risk of stroke, supporting our assumption22. Moreover, Lo et al. reported that the three variables in the aDSCI—neuropathy, nephropathy, and retinopathy—indicate a higher risk of hemorrhagic stroke (adjusted hazard ratio [aHR] = 4.12) and ischemic stroke (aHR = 2.64)23. In our study, patients with hypoglycemia were more likely to have a aDSCI score of 2 (27.10% vs 24.58%) and had higher frequencies of ischemic stroke (11.43% vs 6.75%) and hemorrhagic stroke (2.11% vs 1.84%) among the colchicine users (Appendix Tables 1 and 2). These findings support that the aDSCI is a useful index for predicting the effects of colchicine use on stroke among patients with DM.
Study outcome
The analyzed outcome in this study was a new diagnosis of stroke during the follow-up period. We classified stroke into two categories: hemorrhagic stroke and ischemic stroke. All patients were followed up from the index date until stroke diagnosis, death, withdrawal from insurance, or the end of 31 December 2013.
Colchicine prescription
In the colchicine cohort, the history of colchicine use for each patient was measured as the cumulative daily defined dose (cDDD), which was calculated by summing the DDD from the index date to the study endpoint. Dose–response relationships for risk of stroke were evaluated by using the cDDD (none, 14 < cDDD ≤ 20, 21 < cDDD ≤ 50, 51 < cDDD < 150, cDDD > 150), duration of colchicine use (nonusers, 28–60 days, 61–180 days, 181–360 days, > 360 days), and period from last colchicine use to study endpoint (< 30 days, 30–60 days, 61–180 days, > 180 days).
Meanwhile, according to ATC, the cDDD = 1 mg = 0.5MG/tablet × 2. We also calculated cumulative DDD (cDDD) of colchicine per year by summing DDDs prescribed per year per individual, and further classified colchicine users by cDDD per year as follows: (1) > 14 < 20 cDDD (> 28 < 40 tablets) per year, that is, used > 28 < 40 days per year, (2) 21–50 cDDD (41–100 tablets) per year, that is, 41–100 days, (3) 51–150 cDDD (101–300 tablets) per year, that is, 100–300 days, and (4) over 150 cDDD (> 300 tablets) per year, that is, 150 days of using over 1 DDD.
Colchicine users with gout subcohort
Colchicine users who were with DM and gout both used colchicine and antigout drugs such as “allopurinol-colchicine/nonsteroid anti-inflammatory drug (NSAID),” “benzbromarone–colchicine/NSAID,” “for prevention of gout attacks,” and “NSAID–colchicine” for acute gout attacks or for prevention of chronic gout (Appendix Table 3).
Colchicine users without gout subcohort
Colchicine users who were with DM with nongout conditions such as arthritis-crystal arthropathies, systemic inflammatory diseases such as sarcoidosis, Behcet's syndrome, autoimmune disease, chronic idiopathic or spontaneous urticarial skin diseases, allergic purpura, psoriasis, collagen vascular diseases, and hypertension-related diseases (Appendix Table 3 and Appendix Table 4)24,25,26,27,28,29,30.
Statistical analysis
We compared the demographic characteristics and comorbidities between the colchicine and the comparison cohorts by using the standard mean difference (SMD); values less than 0.1 indicated no significant differences. The incidence of stroke, ischemic stroke, and hemorrhagic stroke was calculated for both cohorts as the number of patients with stroke divided by the sum of person-years (per 1000 person-years). Multivariable Cox proportional hazard models were used to estimate the adjusted hazard ratios (aHR) of stroke after adjustment of potential confounding variables. Ignoring competing risks may lead to an overestimation of the cumulative incidence. Depending on the research question, in the presence of competing events, survival data should be analyzed using either a cause-specific hazard model or a subdistribution hazard model. In this study, we performed the subdistribution hazard model for examining this bias31.
We obtained the cumulative incidence curves of stroke, ischemic stroke, and hemorrhagic stroke for the colchicine and the comparison cohorts by using the Kaplan–Meier method and tested the curve differences by using the log-rank test. Further analysis was performed to evaluate the effects of the cDDD, duration of colchicine use, and the last day of colchicine use on the various dose–response categories. All analyses were conducted using SAS statistical software (Version 9.4 for Windows; SAS Institute, Cary, NC, USA). P values of < 0.05 indicated statistical significance.
Results
Table 1 presents the baseline characteristics, comorbidities, and medications of the study patients. After PSM, we identified 8761 colchicine users in the case cohort and 8761 nonusers in the comparison cohort. In the matched cohorts, no significant difference was noted in the distribution of age, sex, comorbidities, aDCSI score, inpatient days, or medications between the colchicine and comparison cohorts. The mean follow-up durations for patients in the colchicine cohort and the comparison cohort were 4.39 ± 3.34 years and 5.24 ± 3.42 years, respectively.
Table 2 presents the incidence rates and hazard ratios of stroke, ischemic stroke, and hemorrhagic stroke for the colchicine cohort relative to the comparison cohort. The incidence rates of stroke, ischemic stroke, and hemorrhagic stroke were 16.05, 12.79, and 3.29 per 1000 person-years, respectively, in the colchicine cohort. The corresponding incidence rates in the comparison group were 26.19, 21.33, and 4.86. According to the multivariable Cox proportional hazards models adjusted for age, sex, comorbidities, aDCSI score, inpatient days, and medication, we observed that the risks of stroke (aHR = 0.61, 95% confidence interval [CI] = 0.55–0.67), ischemic stroke (aHR = 0.59, 95% CI = 0.53–0.66), and hemorrhagic stroke (aHR = 0.66, 95% CI = 0.53–0.82) were significantly lower in the colchicine cohort than in the comparison cohort. The subdistribution hazard model revealed the colchicine was associated with lower risk of ischemic stroke and hemorrhagic stroke.
We found a significant dose–response effect of colchicine prescription on the reduction of the risk of stroke occurrence. Compared with noncolchicine users, patients who were prescribed colchicine at cDDD = 14–20 (aHR = 0.69, 95% CI = 0.55–0.86), cDDD = 21–50 (aHR = 0.61, 95% CI = 0.55–0.67), cDDD = 51–150 (aHR = 0.62, 95% CI = 0.53–0.71), and cDDD > 150 (aHR = 0.51, 95% CI = 0.44–0.58) had lower stroke risk. Similar results were observed for ischemic stroke (cDDD = 14–20: aHR = 0.70, 95% CI = 0.55–0.90; cDDD = 21–50: aHR = 0.71, 95% CI = 0.60–0.85; cDDD = 51–150: aHR = 0.56; 95% CI = 0.48–0.67; cDDD > 150: aHR = 0.52; 95% CI = 0.45–0.61). Additionally, patients who were prescribed colchicine at the highest cDDD of > 150 had lower hemorrhagic stroke risk (aHR = 0.43, 95% CI = 0.30–0.60) compared with nonusers (Table 3).
In the drug duration analysis, the duration of colchicine use was significantly associated with lower risk of stroke (28–60 days: aHR = 0.69, 95% CI = 0.59–0.81; 61–180 days: aHR = 0.66, 95% CI = 0.57–0.76; 181–360 days: aHR = 0.63, 95% CI = 0.52–0.77; > 360 days: aHR = 0.48, 95% CI = 0.41–0.56), ischemic stroke (28–60 days: aHR = 0.67, 95% CI = 0.57–0.80; 61–180 days: aHR = 0.61, 95% CI = 0.52–0.72; 181–360 days: aHR = 0.62, 95% CI = 0.48–0.75; > 360 days: aHR = 0.51, 95%CI = 0.43–0.60), and hemorrhagic stroke (> 360 days: aHR = 0.36, 95%CI = 0.24–0.54) (Table 4).
For the period from the last day of colchicine use until the study endpoint, a significantly lower risk of stroke (61–180 days: aHR = 0.63, 95% CI = 0.47–0.84; > 180 days: aHR = 0.30, 95% CI = 0.24–0.39), ischemic stroke (61–180 days: aHR = 0.61, 95% CI = 0.44–0.85; > 180 days: aHR = 0.30, 95% CI = 0.24–0.39), and hemorrhagic stroke (> 180 days: aHR = 0.31, 95% CI = 0.19–0.50) was observed compared with the < 30 days groups (Table 5).
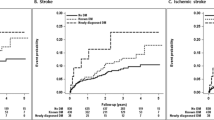
In the Kaplan–Meier analysis, the cumulative incidence of stroke, ischemic stroke, and hemorrhagic stroke was significantly lower in the colchicine cohort than in the comparison cohort (log-rank test, P < 0.001; Fig. 2A–C). Compared with colchicine nonusers, colchicine users in different cDDD groups and different duration groups had a lower cumulative incidence of stroke during the study period (Figs. 3 and 4).
Table 6 reveals the results of the sensitivity analysis for DM patients without gout. Colchicine use was associated with lower risk of stroke (aHR = 0.64, 95%CI = 0.54–0.75). The aHR of ischemic stroke was 0.64 (95% CI = 0.53–0.76) and that of hemorrhagic stroke was 0.62 (95% CI = 0.42–0.91). The p for interaction of colchicine and gout = 0.116. Subgroup analyses of gout and non-gout did not show an interaction. The subdistribution hazard model revealed the colchicine was associated with lower risk of ischemic stroke. However, colchicine was not associated with the hemorrhagic stroke in DM patients without gout (aHR = 0.69, 95%CI = 0.47–1.00).
High frequency of gout in colchicine users with DM
DM and gout had a bidirectional relationship. The frequency of gout in patients with DM before the PSM was 20.88% (7996 + 14,433)/(11,277 + 95,973)32. Colchicine is commonly used for gout management in Taiwan. Therefore, the frequency of gout in the colchicine use cohort was high—up to 66.9% after PSM. Tung et al. suggested that gout is a risk factor for DM, and women with gout have a significantly higher risk of DM than men do33. Consistent with this assertion, a higher proportion of women (68%) than men (32%) had gout in our cohort. Wijnands et al. also noted that DM comorbidities such as hypertension and obesity were also key risk factors for gout34. One explanation is that hypertension, dyslipidemia, low dose aspirin use, and diuretic use may induce hyperuremia, and fluctuations in uric acid may trigger gout attacks. These attacks may be accompanied by sugar fluctuations, leading to stroke29,32,35,36,37. In this study, the high frequency of hypertension-related diseases (78.51%), drug use (86.57%), and dyslipidemia (61.73%) are consistent with the high incidence of gout (66.09%) in the DM cohort38. Moreover, the sensitivity analysis indicated a high incidence of hypertension-related diseases in the DM with gout subcohort (75.13%) and the DM without gout subcohort (74.15%), supporting these assertions (Appendix Table 3 and Appendix Appendix Fig. 1).
Cumulative incidence curves of stroke for the cDDD groups and duration groups and immortal time bias
To account for the immortal time bias, we defined the index date for the case cohort as the first date of colchicine prescription after a diagnosis of DM, and we restricted the case cohort to patients who used colchicine for more than 28 days. Meanwhile, the index date for the control cohort was a randomly assigned date after a diagnosis of DM and was matched to the case patients by propensity score. Moreover, the impact of pay-for-performance (P4P) programs for DM, including the initial enrollment visit, continuing care visit), and annual evaluation visit was analyzed. These strict policies were used to avoid immortal time bias39. To confirm the absence of bias, we determined the cumulative incidence curves of stroke for each cDDD group and each duration group. The results revealed that high-dose colchicine users had a lower risk of stroke (Figs. 3 and 4).
Colchicine use and nephropathy
For the variables, score for chronic renal failure, renal failure not otherwise specified, renal insufficiency, and serum creatinine > 2.0 mg/dL was 2 and that for diabetic nephropathy, acute glomerulonephritis, nephrotic syndrome, hypertension nephrosis, chronic glomerulonephritis, nephritis or nephropathy, urine protein ≥ 30 mg/g of creatinine, or (+) dipstick protein or serum creatinine ≥ 1.5 mg/dL was 1. Most colchicine users (68.93%) and noncolchicine users (68.19%) had a total score of 0 (SMD < 0.1). We thus assumed that most colchicine users had creatinine levels < 1.5 mg/dL (estimated glomerular filtration rate (eGFR) > 50–60 mL/min/1.73 m2) and did not have proteinuria. Renal function, as indicated by impairment (eGFR < 60 mL/min/1.73 m2 or proteinuria) is associated with stroke risk40; because the majority of the cohort did not have these indicators, renal function impairment did not have a significant impact on stroke risk in this study. Long-term use of low doses of colchicine is relatively safe; the dose only requires adjustment if creatinine levels reach 2 mg/dL (eGFR ≈ 30–50 mL/min/1.73 m2)41. Moreover, P4P for the DM multiple disciplinary team is key to monitoring creatinine level fluctuations in DM patients and facilitates recovery of renal function in patients using colchicine42. These strict policies reduce the influence of the confounding factor of renal function impairment on stroke risks in the DM cohort.
Discussion
The two key findings of this study are as follows: first, the colchicine cohort had a lower risk of stroke, ischemic stroke, and hemorrhagic stroke than the noncolchicine cohort did; patients with the longest durations of colchicine use had the lowest risk of ischemic and hemorrhagic stroke. Moreover, during the follow-up period, significant differences were observed in the cumulative incidence of stroke, ischemic stroke, and hemorrhagic stroke. Second, similar results were observed for colchicine users in the gout and nongout subcohorts. Patients in the nongout subcohort also had a lower risk of stroke, ischemic stroke, and hemorrhagic stroke. Studies have suggested that in populations with a high cardiovascular risk (e.g., patients with DM, gout, hypertension, and coronary artery disease), colchicine use results in a significant reduction of stroke risk, in line with our results3,5,15,43 (Appendix Fig. 1).
Pandey et al. reported that higher CRP levels, higher scores on the National Institutes of Health Stroke Scale, high BP, high blood sugar, and higher frequency of hypoglycemia were associated with hemorrhagic stroke2,37,44. These higher levels of inflammation markers in patients with DM contribute to the necessity of higher colchicine doses to attenuate severe inflammation and prevent hemorrhagic stroke37,44. Furthermore, only the group with cDDD > 150 and duration of use > 360 days had a low risks of hemorrhagic stroke. These findings imply a delayed response in the prevention of hemorrhagic stroke in the DM with colchicine use group39. However, the mechanism underlying this effect warrants further research.
Overall, the dose-dependent effect of colchicine use on stroke indicates that colchicine attenuates the risk of stroke, ischemic stroke, and hemorrhagic stroke in patients with complicated DM and high aDCSI scores. Patients with the longest duration of colchicine use had the lowest risk of ischemic stroke and hemorrhagic stroke. A possible explanation for this finding is that these patients have more frequent medical service use and undergo regular follow-up visits in Taiwan39,45.
Notably, compared with last day colchicine use < 30 days, the group with last day colchicine use of 30–60 days did not exhibit a significant reduction in risk of stroke or ischemic stroke. For example, patients using colchicine for the management of acute gout cases did not have a reduced risk of stroke27. By contrast, patients with last day colchicine use > 180 days still had a lower risk of stroke, ischemic stroke, and hemorrhagic stroke. These results indicate colchicine’s role in the prevention of stroke among patients with DM. Therefore, colchicine may be more effective when administered as a prophylactic such as in long-term therapy (e.g., use days > 360 days and cDDD > 150) than as a treatment agent3. Meanwhile, low dose (0.5 mg/d) with low cDDD (> 14 < 20) and low duration use (> 28 < 60 days) with lower risk of ischemic stroke. And as such in this low dose, it is unlikely to increase the risk of intracranial or extracranial bleeding in this vulnerable patient population. If the colchicine is proven to safe and effective, thus this low-cost approach can have a potential to change clinical practice.
Lai et al. that physiological concentrations of serum uric acid displayed anti-inflammatory and chondroprotective effects both in vitro and in vivo46. However, Mohsin et al. that high uric acid level may be considered as a risk factor in patients with acute ischemic stroke47. Our result reveal that the colchicine use was associated with the lower risk of ischemic stroke regardless with gout or not. These findings should be confirmed in further large-scale randomized controlled trials.
Strengths
First, this is the first large-scale study to investigate the effects of colchicine use on ischemic and hemorrhagic stroke risk among patients with DM based on their propensity scores for a long duration: 5.24 ± 3.42 years in the colchicine use cohort and 4.39 ± 3.34 years in the noncolchicine use cohort. Second, PSM ensured robust internal validity48. Third, comorbidities such as hypertension, hyperlipidemia, gout, and obesity were investigated in this study instead of lifestyle factors; due to the intersection of environmental air pollution, pneumonia, and inflammation, pneumonia was used to represent environmental and economic status; and aDCSI replaced drug adherence based on the closely monitoring the drug compliance of the DM cohort49. This approach was applied to avoid interactions between confounding factors. Fourth, aDCSI is a useful tool for predicting the risk of stroke. Variables including the aDCSI score in the NHIRD are similar to those used by studies worldwide, and aDCSI was demonstrated to have external validity50. The index includes biochemical data—such as hbA1c levels, cholesterol levels, triglyceride levels, and body mass index (BMI)—which are related to uric acid levels. Thus, aDCSI is suitable for representing data unavailable in the NHIRD, such as uric acid level, smoking status, and alcoholism49.
Fifth, according to Dhillon et al., fewer diabetes complications, lower diabetes severity, stricter medication adherence, and psychosocial well-being are direct or indirect predictors of better quality of life among patients with DM49. In summary, aDCSI is a tool for the analysis of lifestyle factors—such as smoking, alcohol consumption, exercise, and psychiatric status—for which data are unavailable in the NHIRD. Moreover, aDCSI may be a predictor of hypoglycemia36. Thus, we adopted aDCSI in the present study to improve the results; this observation could be used in future studies on the relationship between stroke risk and colchicine use among patients with DM.
Sixth, in the COVID-19 pandemic era, the colchicine use in the patients having virus with diabetes or hypertension is an important topic. Our study may infer to the investigation of the relationship between the stroke and the colchicine use in the diabetes with gout or without gout in future.
Limitations
This study had several limitations. First, the NHIRD does not provide biochemical data regarding cytokine interleukin or lifestyle data such as smoking status. Second, some patients may not have taken their prescribed medication or may not have taken the prescribed dose, leading to exposure misclassification. This misclassification, if nondifferential, tends to result in hazard ratio underestimation and may explain the lack of associations between stroke risk reduction and colchicine use in some of our findings. The identification of patients with stroke in this study was based on recorded diagnoses or treatment with colchicine rather than a screening of the study population, leading to an underestimation of cases of stroke. Moreover, some patients in the noncolchicine cohort may have had undiagnosed stroke, which would also have resulted in an underestimation of the association with colchicine. Third, although our analysis included a wide range of potential confounding factors, our observational study still had potential residual confounders and indication bias. We aimed to reduce protopathic bias by excluding patients with stroke diagnosis before DM and patients using colchicine. Fourth, the symptoms of hypoglycemia may be similar to those of stroke; however, whether hypoglycemia is a prodromal symptom or a risk factor for stroke remains controversial. Fifth, the HBA1C data and subclassification for chronic kidney disease are unavailable in the NHIRD. Because renal function impairment is a factor for stroke, we replaced the level of renal function impairment with the variables of nephropathy in the aDSCI. A comparison of the frequency of score distributions for scores of 0, 1, and 2 for the variables of nephropathy between the colchicine and noncolchicine groups revealed no significant differences. Altogether, generalization of the results of this study to patients with renal function impairment requires further research.
Sixth, the anti-hypertension drugs have many indications. For example, the beta-blocker is a prescription medicine used to treat migraine, coronary artery disease, cardiac arrhythmia, heart failure and hyperthyroidism. The calcium-blocker was used for the coronary artery disease, pulmonary hypertension. The diuretic was used for edema, heart failure or liver cirrhosis. Thus, the rate of antihypertensive medications (79.3%) was larger than the number of hypertensive-related patients (64.8%). Despite discrepancies in international guidelines, the mantra that every physician should be resumed in “treat the elderly DM patient, not the HbA1c level”. The use of antidiabetic medications (OHA or insulin) in elderly ≥ 75 years may be limited. For example, in elderly DM who have multiple chronic diseases (aDSCI ≥ 1), mild to moderate dementia, and shortened life expectancy, HbA1c should target 7.1–8%, fasting and pre-meal plasma glucose at 90–150 mg/dl, and overnight plasma glucose at 100–180 mg/dl. Thus, if those elderly patients with DM could achieve the target level such as (HBA1c, 6.5–7.5% in healthy older adults, 7.1–7.8% in heart failure, 7.5–8.5% in frail) after lifestyle therapies, the antidiabetic medications may be not necessary in select case such as residential aged care facilities51,52,53,54. Meanwhile, the frail elderly may be censored or dead before starting use of antidiabetic medications. Moreover, Huang et al. reported about 9.8% of DM patients receiving the tradition Chinese drugs were without antidiabetic medications55. Therefore, the high rate of the elderly (44%) and chronic illness (31%) with a higher score of aDSCI (≥ 1, mild to severe frail) in this study may explain the not all DM patients received the antidiabetic medications. In Taiwan, most people take small amounts of colchicine regularly for a long time (> 360 days) to prevent severe attacks or other problems caused by inflammation. For example, people with frequent acute flare-ups or chronic gout tend to use it on a long-term basis. However, the levels of BP, HBA1c, sugar and uric acid were unavailable in NHIRD. These confounding factors were another limitations in this study.
Conclusion
Colchicine use (cDDD > 14, duration > 28 days) was associated with lower risk of stroke and ischemic stroke in patients with DM. Additionally, cDDD > 150, duration > 360 days was an auxiliary protective factor for stroke, ischemic stroke, and hemorrhagic stroke in these patients. However, the subdistribution hazard model reveal the colchicine was not associated with the hemorrhagic stroke in DM patients without gout.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- TIA:
-
Transient ischemic attack
- LHID2000:
-
Longitudinal Health Insurance Database 2000
- NHIRD:
-
National Health Insurance Research Database
- ICD-9-CM:
-
International Classification of Diseases, 9th Revision, Clinical Modification
- aDCSI:
-
Adapted Diabetes Complications Severity Index
References
Hong, P. et al. NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J. Neuroinflamm. 16, 121 (2019).
Pandey, A., Shrivastava, A. & Solanki, A. Study of atherogenic lipid profile, high sensitive C-reactive protein neurological deficit and short-term outcome in stroke subtypes. Iran J. Neurol. 15, 146–152 (2016).
Liu, C.-H. et al. Colchicine use and risks of stroke recurrence in acute non-cardiogenic ischemic stroke patients: A population-based cohort study. J. Pers. Med. 11, 935 (2021).
Kelly, P. J., Lemmens, R. & Tsivgoulis, G. Inflammation and stroke risk: A new target for prevention. Stroke 52, 2697–2706 (2021).
Masson, W., Lobo, M., Molinero, G., Masson, G. & Lavalle-Cobo, A. Role of colchicine in stroke prevention: An updated meta-analysis. J. Stroke Cerebrovasc. Dis. 29, 104756 (2020).
Khandkar, C., Vaidya, K. & Patel, S. Colchicine for stroke prevention: A systematic review and meta-analysis. Clin. Ther. 41, 582-590.e3 (2019).
Lin, Z.-Y. et al. Potential of novel colchicine dosage schedule for the palliative treatment of advanced hepatocellular carcinoma. Kaohsiung J. Med. Sci. 37, 616–623 (2021).
Sun, A., Wang, Y.-P., Chia, J.-S., Liu, B.-Y. & Chiang, C.-P. Treatment with levamisole and colchicine can result in a significant reduction of IL-6, IL-8 or TNF-α level in patients with mucocutaneous type of Behcet’s disease. J. Oral Pathol. Med. 38, 401–405 (2009).
Goldstein, L. B. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: Effect of modifier codes. Stroke 29, 1602–1604 (1998).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24, 35–41 (1993).
Hsieh, C.-Y., Chen, C.-H., Li, C.-Y. & Lai, M.-L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Formos Med. Assoc. 114, 254–259 (2015).
Morris, N. A., Merkler, A. E., Gialdini, G. & Kamel, H. Timing of incident stroke risk after cervical artery dissection presenting without ischemia. Stroke 48, 551–555 (2017).
Diener, H.-C. & Hankey, G. J. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J. Am. Coll. Cardiol. 75, 1804–1818 (2020).
Opstal, T. S. J. et al. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease. Circulation 142, 1996–1998 (2020).
Katsanos, A. H. et al. Colchicine for stroke prevention in patients with coronary artery disease: A systematic review and meta-analysis. Eur. J. Neurol. 27, 1035–1038 (2020).
Kofler, T. et al. Colchicine in patients with coronary artery disease: A systematic review and meta-analysis of randomized trials. J. Am. Heart Assoc. 10, e021198 (2021).
Tsai, T.-L. et al. The association between usage of colchicine and pneumonia: A nationwide, population-based cohort study. Front Pharmacol. 10, 908–908 (2019).
Tseng, C. H., Chong, C. K., Tseng, C. P., Shau, W. Y. & Tai, T. Y. Hypertension is the most important component of metabolic syndrome in the association with ischemic heart disease in Taiwanese type 2 diabetic patients. Circ. J. 72, 1419–1424 (2008).
Huebschmann, A. G. et al. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 62, 1761–1772 (2019).
Young, B. A. et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am. J. Manag. Care. 14, 15–23 (2008).
Hsieh, M.-S. et al. Hospital outcomes and cumulative burden from complications in type 2 diabetic sepsis patients: A cohort study using administrative and hospital-based databases. Ther. Adv. Endocrinol. Metab. 10, 204201881987540 (2019).
Wicke, F. S. et al. Performance of the adapted Diabetes Complications Severity Index translated to ICD-10. Am. J. Manag. Care 25, e45–e49 (2019).
Lo, S.-F. et al. Microvascular parameters help to predict stroke risk in the Asian diabetic population in Taiwan: A population based case-control study. Front. Neurol. 9, 719 (2018).
Rho, Y. H., Zhu, Y., Zhang, Y., Reginato, A. M. & Choi, H. K. Risk factors for pseudogout in the general population. Rheumatology 51, 2070–2074 (2012).
Dasgeb, B. et al. Colchicine: An ancient drug with novel applications. Br. J. Dermatol. 178, 350–356 (2018).
Nicholls, M. Australia and colchicine for coronary heart disease. Eur Heart J. 42, 367–368 (2020).
Raju, N. C. et al. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: A pilot randomized controlled trial. J. Thromb. Thrombolysis. 33, 88–94 (2012).
Solomon, D. H., Liu, C.-C., Kuo, I. H., Zak, A. & Kim, S. C. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: A cohort study using electronic medical records linked with Medicare claims. Ann. Rheum. Dis. 75, 1674–1679 (2016).
Yu, K. H. et al. Management of gout and hyperuricemia: Multidisciplinary consensus in Taiwan. Int. J. Rheum. Dis. 21, 772–787 (2018).
Stewart, S., Yang, K. C. K., Atkins, K., Dalbeth, N. & Robinson, P. C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 22, 28 (2020).
Buzkova, P. Competing risk of mortality in association studies of non-fatal events. PLoS ONE 16, e0255313 (2021).
Suppiah, R., Dissanayake, A. & Dalbeth, N. High prevalence of gout in patients with type 2 diabetes: Male sex, renal impairment, and diuretic use are major risk factors. N. Z. Med. J. 121, 43–50 (2008).
Tung, Y. C. et al. Association between gout and incident type 2 diabetes mellitus: A retrospective cohort study. Am. J. Med. 129, 1219.e17-1219.e25 (2016).
Wijnands, J. M. A. et al. Individuals with type 2 diabetes mellitus are at an increased risk of gout but this is not due to diabetes: A population-based cohort study. Medicine 94, e1358 (2015).
Tseng, W. C. et al. U-shaped association between serum uric acid levels with cardiovascular and all cause mortality in the elderly: The role of malnourishment. J. Am. Heart Assoc. 7, e007523 (2018).
Kornelius, E. et al. Progress of diabetes severity associated with severe hypoglycemia in Taiwan. Am. J. Manag. Care 24, e99–e106 (2018).
Saxena, A. et al. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage. Stroke 47, 682–688 (2016).
Thottam, G. E., Krasnokutsky, S. & Pillinger, M. H. Gout and metabolic syndrome: A tangled web. Curr. Rheumatol. Rep. 19, 60 (2017).
Chen, Y.-C., Lee, C.T.-C., Lin, B. J., Chang, Y.-Y. & Shi, H.-Y. Impact of pay-for-performance on mortality in diabetes patients in Taiwan: A population-based study. Medicine 95, e4197 (2016).
Kelly, D. & Rothwell, P. M. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J. Neurol. Neurosurg. Psychiatry. 91, 88–97 (2020).
Yaxley, J., Yaxley, W. & Scott, T. Safety and adverse effect profile of colchicine in renal impairment: A systematic review of randomised trials. J. Renal Inj Prev. 9, e28 (2020).
Andreis, A., Imazio, M., Casula, M., Avondo, S. & De Ferrari, G. M. Colchicine efficacy and safety for the treatment of cardiovascular diseases. Intern. Emerg. Med. 16, 1691–1700 (2021).
Katsanos, A. H. et al. An updated meta-analysis of RCTs of colchicine for stroke prevention in patients with coronary artery disease. J. Clin. Med. 10, 3110 (2021).
Andersen, K. K., Olsen, T. S., Dehlendorff, C. & Kammersgaard, L. P. Hemorrhagic and ischemic strokes compared. Stroke 40, 2068–2072 (2009).
Chou, C.-W., Kung, P.-T., Chou, W.-Y. & Tsai, W.-C. Pay-for-performance programmes reduce stroke risks in patients with type 2 diabetes: A national cohort study. BMJ Open 9, e026626 (2019).
Lai, J.-H. et al. Physiological concentrations of soluble uric acid are chondroprotective and anti-inflammatory. Sci. Rep. 7, 2359 (2017).
Mohsin, M. et al. Serum uric acid level among acute stroke patients. Mymensingh Med. J. 25, 215–220 (2016).
Lim, S., Marcus, S. M., Singh, T. P., Harris, T. G. & LevanonSeligson, A. Bias due to sample selection in propensity score matching for a supportive housing pro. PLoS ONE 9, e109112 (2014).
Dhillon, H., Nordin, R. & Ramadas, A. Quality of life and associated factors among primary care Asian patients with type 2 diabetes mellitus. Int. J. Environ. Res. Public Health. 16, 3561 (2019).
Noble, D., Mathur, R., Dent, T., Meads, C. & Greenhalgh, T. Risk models and scores for type 2 diabetes: Systematic review. BMJ 343, d7163–d7163 (2011).
McCormick, T. A. et al. Age-dependent hemoglobin A1c therapeutic targets reduce diabetic medication changes in the elderly. EGEMS 7, 46 (2019).
Mangé, A. S., Pagès, A., Sourdet, S., Cestac, P. & McCambridge, C. Diabetes and frail older patients: Glycemic control and prescription profile in real life. Pharmacy 9, 115 (2021).
Yau, C. K. et al. Glycosylated hemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. J. Am. Geriatr. Soc. 60, 1215–1221 (2012).
Aguilar, D., Bozkurt, B., Ramasubbu, K. & Deswal, A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J. Am. Coll. Cardiol. 54, 422–428 (2009).
Huang, C.-Y., Tsai, Y.-T., Lai, J.-N. & Hsu, F.-L. Prescription pattern of Chinese herbal products for diabetes mellitus in Taiwan: A population-based study. Evid. Based Complement. Altern. Med. 2013, 201329 (2013).
Acknowledgements
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), China Medical University Hospital (DMR-109-231, DMR-110-089, DMR-111-090, DMR-111-091); Ministry of Science and Technology (MOST 110-2321-B-039-003). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: conception and design: J-J.Y., I-L.K., C-H.K. Administrative support: C-H.K. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeh, JJ., Kuo, IL., Yip, HT. et al. Effects of colchicine use on ischemic and hemorrhagic stroke risk in diabetic patients with and without gout. Sci Rep 12, 9195 (2022). https://doi.org/10.1038/s41598-022-13133-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13133-0
This article is cited by
-
Angiotensin receptor blockers might be protective against hepatic steatosis after liver transplantation
BMC Gastroenterology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.