Abstract
The adverse effects of maternal prenatal stress (PS) on child’s neurodevelopment warrant the establishment of biomarkers that enable early interventional therapeutic strategies. We performed a prospective matched double cohort study screening 2000 pregnant women in third trimester with Cohen Perceived Stress Scale-10 (PSS-10) questionnaire; 164 participants were recruited and classified as stressed and control group (SG, CG). Fetal cord blood iron parameters of 107 patients were measured at birth. Transabdominal electrocardiograms-based Fetal Stress Index (FSI) was derived. We investigated sex contribution to group differences and conducted causal inference analyses to assess the total effect of PS exposure on iron homeostasis using a directed acyclic graph (DAG) approach. Differences are reported for p < 0.05 unless noted otherwise. Transferrin saturation was lower in male stressed neonates. The minimum adjustment set of the DAG to estimate the total effect of PS exposure on fetal ferritin iron biomarkers consisted of maternal age and socioeconomic status: SG revealed a 15% decrease in fetal ferritin compared with CG. Mean FSI was higher among SG than among CG. FSI-based timely detection of fetuses affected by PS can support early individualized iron supplementation and neurodevelopmental follow-up to prevent long-term sequelae due to PS-exacerbated impairment of the iron homeostasis.
Similar content being viewed by others
Introduction
In the second and third trimester of pregnancy maternal iron requirements can increase up to eightfold or a total of 1 g of additional iron, due to expanding maternal and fetal erythropoiesis1,2. Iron homeostasis dysregulation of pregnant mothers and/or children is known to induce lasting neurological damage in the offspring3.
Prenatal maternal stress (PS), including both pregnancy-specific and general psychosocial stress and anxiety, can jeopardize the balance of the maternal iron homeostasis4,5,6,7. Pregnant women are especially vulnerable to chronic stress as they face new and potentially challenging situations such as body image issues, lifestyle changes, and fluctuating hormones8. PS induces lasting changes to fetal stress response, in a process known as “fetal programming”9 which might be partly transmitted by the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) system. In an interim analysis of pregnant women with PS and controls, we showed that PS results in entrainment of fetal heart rate (fHR) by maternal heart rate (mHR), thus yielding a non-invasively obtainable PS biomarker in mother–fetus dyads that we refer to as Fetal Stress Index (FSI)10. HPA dysregulation increases the risk of newborn impairment and higher vulnerability toward certain chronic diseases and neurobehavioral disorders11,12.
Sex-specific PS effects are well described and recommended for the general consideration as part of human PS studies13. However, the contribution of child’s sex to the PS-induced alterations in iron homeostasis of the neonate is unclear and suffers from contradictions4.
Consequently, we tested the hypothesis that PS influences the fetal ANS which results in sex-specific changes to FSI during third trimester and the iron homeostasis in human neonates.
To gauge the fetal iron homeostasis, we assessed the following iron biomarkers in umbilical cord blood serum: ferritin, transferrin, hepcidin14 and iron1. The putative relationships between hepcidin, PS and ANS are summarized in Fig. 1.
Putative link of PS with iron homeostasis and maternal–fetal heart rate coupling. A proposed simplified model of the hepcidin–placental–ferroportin axis based on the articles of Cortes et al.15 and Lobmaier et al.10: Hepcidin is the key regulator within this system. By binding to ferroportin, it prevents the release of iron into the bloodstream and therefore the synthesis of the transport protein transferrin and the storage protein ferritin. Hepcidin levels are influenced strongly by inflammatory processes, especially the cytokine IL-6. Another possible pathway influencing the child's stress phenotype is assumed to be realized by interference in the coupling between maternal and fetal heart rate (mHR, fHR) as seen during maternal expiration. PSS perceived stress scale, FSI fetal stress index.
Results
Sociodemographic parameters and perinatal outcomes
Women enrolled in the study had a mean age of 33.0 (± 4.4) years and a median gestational age of 36.4 (35.3–37.4) weeks of gestation at study entry. Demographics of the excluded participants did not differ from those with all measures. Statistical comparison of SG and CG showed no differences in the used matching criteria (Table S1).
Maternal and fetal iron homeostasis
10.4% of the included women were anemic prior to delivery (Hb < 11 mg/dL). However, we found no differences in fetal iron parameters, maternal intake of iron supplements, fetal and maternal hemoglobin, RBC indices and anemia status between SG and CG (Table S2).
FSI
MHR and fHR coupling analysis revealed a higher FSI among SG than among CG (0.38 (− 0.22 to 0.75) versus − 0.01 (− 0.36 to 0.34); p = 0.024) (Table S1). FSI showed no correlation to any measured fetal iron biomarker for either sex (Table S3).
However, within the automatically binned ranges of cord blood serum iron biomarker values, we observed FSI differences. FSI was higher in SG than in CG for ferritin levels between 153 and 279 μg/L ((n = 42; 24 CG; 18 SG); 0.40 (± 0.57) versus 0.01 (± 0.47); p = 0.03, transferrin saturation of 32–47% ((n = 24; 12 CG; 12 SG); 0.30 (± 0.66) versus − 0.24 (± 0.27); p = 0.045), and hepcidin values between 0 and 57 ng/mL ((n = 92; 47 CG; 45 SG) (0.34 (± 0.68) versus − 0.01 (± 0.56); p = 0.01). Using current newborn guidelines and validated ranges the above-mentioned values of iron markers would be normal16,17,18. Overall, FSI at ~ 36 weeks of gestation was higher in SG fetuses averaging 0.34 compared with − 0.10 in CG fetuses within these cord blood iron biomarker ranges.
Sex-specific differences
We identified sex-specific differences in iron homeostasis among male infants and showed that the PS effect on iron homeostasis depends on the neonates’ sex.
Cord blood transferrin saturation was lower in SG male neonates compared with those in male CG, regardless of iron supplementation. For ferritin levels, we observed a trend towards lower values in male SG (Table 1).
The GEE model revealed that sex is a significant effect modifier that exhibited differences for ferritin (p = 0.038, Fig. 2), and a trend for transferrin saturation (p = 0.070, Fig. S1). For hepcidin, we found no significant sex-driven differences.
Interestingly, maternal hair cortisol tended to increase in SG mothers of female neonates (Table 1). FSI group differences were explained by male neonates only.
Estimated causal effect
We conducted causal inference to investigate the effects of the aforementioned relationships more explicitly. We identified certain variables as adjustment sets in blocking all non-causal paths between the treatment and outcome variables while leaving all causal paths unblocked (Fig. 3). Examination of the causal model on the PS → Cord Blood Ferritin and PS → Bayley Score pathway demonstrated two minimum adjustment sets: “Maternal Age” and “SES” or “Maternal Age” and “Education.” Either set could be used to obtain an estimate for the causal effect. Our SES data are represented by “Household income > 5000 €/month,” and maternal education by “University Degree.'' Controlling for the minimum adjustment set “University Degree” and “Maternal Age” revealed an estimated average exposure effect of lowered cord blood ferritin at the alpha = 0.10 level at − 38.06 μg/L (95% CI − 79.91 to 3.78) in SG compared with that in CG (Table S4). This average exposure effect became obscured when fetal sex was included (p-value increased from 0.07 to 0.19) demonstrating that sex is a strong effect modifier on the causal pathway between PS → Fetal Iron Biomarker.
Maternal age, socioeconomic status, and education as confounding factors within the prenatal stress trajectory. Directed acyclic graph analysis of the relationships between maternal and fetal antenatal, perinatal, and postnatal exposures, covariates, and outcomes. PSS perceived stress scale, FSI fetal stress index, HR heart rate, BMI body-mass index, IVF in-vitro-fertilization.
ML for group classification
First, we considered iron biomarkers and FSI, which predicted the groups at an AUROC = 0.706 (± 0.194). Next, we added the salient clinical and demographic data (gestational and maternal age, BMI at study entry, pre-pregnancy BMI, planned/non-planned pregnancy, higher education yes/no, income over 5000€ yes/no). These are the features that we also used in the DAG approach and that were available at the time of the taECG measurement at study entry. Doing so, we achieved an AUROC = 0.759 (± 0.082). The corresponding feature importance ranking is shown in the supplement (Fig. S2). Use of clinical and demographic data reduced the classification performance to AUROC = 0.688 (± 0.142), similar to using FSI (AUROC = 0.665 ± 0.126) or iron parameters (AUROC = 0.587 ± 0.183) alone. In general, sex ranked in the lower 5% of variable importance, yielding only a slight improvement.
Discussion
PS disrupts fetal iron homeostasis in a sex-specific manner
This study indicates a sex-dependent difference in fetal iron homeostasis and FSI due to PS in an otherwise healthy cohort, mainly driven by the male sex. Causal inference approach allowed us to independently verify fetal sex as an important effect modifier on the causal pathway between PS and cord blood ferritin. The findings strengthen previous published FSI results10.
The PS effect on the fetal iron biomarkers has been poorly understood. Rhesus monkey infants born to stressed mothers were more likely to develop iron deficiency7. Likewise, several studies in humans have shown a correlation between PS and cord blood zinc protoporphyrin/heme index as well as PS and ferritin levels4,5,6.
During pregnancy, maternal stress hormones such as cortisol influence the growing fetus and its neurodevelopment, presumably via epigenetic mechanisms9,19. Cortes and colleagues proposed an influence of chronic stress through a stress-induced altered expression of a variant of the enzyme acetylcholinesterase on the iron-regulating system in fetal sheep brain-derived primary microglia cultures15. They assumed the afferent cholinergic anti-inflammatory pathway signaling on microglial α7 nicotinic acetylcholine receptors to down-regulate metal ion transporter and ferroportin, which acts as a hepcidin receptor (Fig. 1).
Animal studies observed stress-dependent cognitive deficits mainly seen in males20,21. In humans, sex-specific PS effects are reflected by lower scores in conduct assessments and higher test scores for emotional disturbance in males compared to females13,22,23. Campbell et al. applied six specific PS questionnaires, each twice in the second and third trimester, to 428 ~ 28-years-old mothers and found newborns of pregnant women exposed to violence to be stronger associated with cord blood ferritin levels lower in boys than in girls4.
The relation of iron homeostasis biomarkers to PS
Our results show no relationship between the presence of maternal anemia, fetal iron deficiency and PS. These findings are in agreement with literature suggesting that the fetus is robust against moderate changes of the maternal iron homeostasis24,25.
Within the DAG framework, we estimated that PS reduced the cord blood serum ferritin levels by approximately 15%. These findings are exceeding the adaption factor for inflammatory processes in infants the WHO uses in a current guideline26. We assume that during pregnancy even relatively small additional shifts in fetal iron homeostasis, especially in ferritin levels, may induce sex-specific neurodevelopmental effects27. Our observations regarding the link between PS, fetal iron homeostasis and the postnatal neurodevelopmental trajectories warrant further investigations, because this condition may be corrected therapeutically via targeted prenatal and/or postnatal iron supplementation2.
The PS effect transmitted by maternal cortisol on the fetal neurodevelopment may depend on the time course of exposure28,29. Hypothetically, taking our explanation further antepartum, i.e., to ~ 3.5 weeks earlier at the time of taECG recording, we speculate that PS-induced differences in hepcidin at that time may lead to the reported changes in iron parameters that could still be detected in the cord blood1. Our exploratory findings of higher FSI within certain ranges of at-birth iron biomarkers support this notion. The absence of group differences of cord blood iron parameters including the whole cohort may reflect adaptions (more pronounced in females) that occur as pregnancy progresses20.
The role of the immune system
Our data in leukocytes showed no evidence of increased inflammatory processes in SG neonates (Table S1). Nevertheless, acute inflammatory processes, a common phenomenon during delivery, may have had an effect on our cord blood findings transmitted by other cellular messengers such as the cytokine IL-6 (Fig. 1). In general, inflammation upregulates the acute phase protein ferritin influencing its role as a biomarker of the iron storage30. Inflammation also upregulates hepcidin levels leading to an intestinal sequestration of iron14. Cord blood interleukin levels were increased in chronically stressed mother’s infants31. Taken together, the effects of PS can be mediated by inflammatory processes and this link should be investigated further in future studies including a broader characterization of the maternal and neonatal inflammatory profiles32.
FSI as a potential biomarker of PS in late gestation
The present findings confirm that FSI is increased in PS during the third trimester of pregnancy10. Because the FSI showed poor association with the measured iron biomarkers, we assume that PS influences fHR and mHR coupling by different pathways. Moreover, in our DAG framework it is conceivable that FSI may serve as an indicator of subsequent altered neurodevelopmental trajectories, even in the absence of biochemical PS correlates such as alterations in iron homeostasis33,34.
ML-based predictions of PS
With our ML approach, we mimicked a real-life scenario to identify mother–fetus dyads affected by PS. Our results are consistent with findings in other clinical settings where electronic medical record mining identified patients at risk even without additional biophysical assessments, such as ECG35. Notably, adding biophysical characteristics improves ML model performance, thus emphasizing the potential of antepartum mother–child monitoring using taECG to improve the early detection of health abnormalities such as PS.
Strengths and limitations
Strengths of the FELICITy study are the prospective design preventing recall bias and the definition of criteria for a matching system to exclude possible confounders. Additionally, eventual confounding factors such as the intake of iron supplement and ethnic group showed no group differences (Fig. S1). This is the first prospective longitudinal study starting in utero aiming to assess PS and fetal biomarkers. Additionally, it is the first study to use causal inference and machine learning approaches to investigate sex-dependent influence of PS on the fetal iron homeostasis. There are certain limitations. Our inclusion criteria prevented us from enrolling non-German-speaking patients. This may have biased how the PS effects are represented in the multicultural Munich population. Also, we used a matching system that could not include every screened CG patient. Due to the uncertainties of a human study, several subject numbers for different sub-analyses were lower. Furthermore, we focused on measuring PS in the third trimester which necessarily neglected earlier stages of pregnancy and a possible temporal dynamic of PS over the entire course of pregnancy.
We chose not to include other potential effect modifiers on the causal pathway of the DAG such as inflammatory processes as they are difficult to define quantitatively and were not the focus of this study. However, future studies could further refine estimates of PS → Iron Biomarker average exposure effect by adjusting for these covariates.
This study did not differentiate between arterial or venous origin of the analyzed cord blood samples. To our knowledge this issue has not been addressed in literature so far. In general, the placental iron transfer and the assessment of the fetal iron status using cord blood parameters are poorly understood36,37. As of the date of the manuscript’s submission no commonly used normal ranges of cord blood iron parameters exist. The established ranges start with the child's birth26 but are not applicable to cord blood ranges since in cord blood usually, iron parameters are higher38. These issues warrant further research to identify potential biasing effects on cord blood analysis.
Conclusions
We show that during third trimester PS exerts a sex-dependent effect on fetal iron homeostasis and on the fetal ANS measured by FSI, an ECG-derived measure of chronic stress transfer from mother to fetus. The reported biomarkers open novel avenues of research into the association between PS and adverse neurodevelopmental outcomes. They can contribute to development of novel therapeutic intervention strategies39,40.
We propose the following aspects of future research: First, do the changes we report represent a healthy or maladaptive response to PS? Second, will applying FSI monitoring during pregnancy permit to track a “deviating neurodevelopmental trajectory” and when exactly during gestation do these changes occur? The non-invasive fetal monitoring with FSI tracking may help answer these questions. Third, what is the significance and therapeutic opportunity of the discovery that PS can impact fetal iron homeostasis? Do female fetuses possess more successful compensatory mechanisms in response to PS than males do? The sex-specific effects of this impact warrant further investigations. Fourth, which role may iron supplementation play in this context as a corrective therapeutic option2,41? Our findings indicate that we will need to consider the sex when devising therapeutic strategies to compensate for the intrauterine adversity due to PS42.
Methods
Ethical considerations
The study protocol is in strict accordance with the Committee of Ethical Principles for Medical Research from Technical University of Munich (TUM) and has the approval of the “Ethikkommission der Fakultät für Medizin der TUM” (registration number 151/16S). ClinicalTrials.gov registration number is NCT03389178. Written informed consent was obtained from each subject for participation in this study after having read an informative brochure and after PS screening via questionnaires and before data collection in the third trimester.
Procedures
Study design and study population
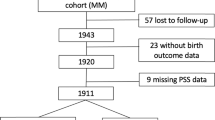
A prospective matched double cohort study was performed between June 2016 and July 2019 at the Department of Obstetrics and Gynecology at “Klinikum rechts der Isar” of the TUM, Germany (Fig. 4). We screened 2000 women using the validated German version of “Cohen Perceived Stress Scale-10” (PSS-10) questionnaire43. This test quantifies the overall chronic stress based on 10 items including anxiety, depression, abnormal fatigue, and general dissatisfaction as symptoms of a generally perceived stress44. By means of PSS-10 we classified the patients into either stressed group (SG) or control group (CG) using a cutoff PSS-10 score of ≥ 1910.
Singleton pregnant women between 18 and 45 years of age in their third trimester (at least 28 weeks gestation) were included. Exclusion criteria were serious placental alterations (e.g., IUGR), fetal malformations, maternal severe illness during pregnancy45, preterm birth, cord blood pH < 7.10, and maternal drug or alcohol abuse. The CG (n = 85; PSS-10 < 19) was additionally matched with SG patients (n = 79; PSS-10 ≥ 19) for parity and gestational and maternal age at study entry (Fig. 4). 728 participants returned the PSS-10 questionnaire and a total of 164 pregnant women were recruited.
Data collection
Participants received a sociodemographic and medical history questionnaire including a bivariate question regarding iron supplementation of usually 100 mg per day. Additionally, we recorded a transabdominal electrocardiogram (taECG) at 900 Hz sampling rate of at least 40 min duration using AN24 (GE HC/Monica Health Care, Nottingham, UK). Maternal and fetal ECGs were extracted from taECG signal as described before10. FHR and mHR coupling was estimated using the bivariate phase‑rectified signal averaging method yielding the FSI46 which provided a measure for the fetal response to mHR decreases10. FSI data was evaluable from n = 139 study subjects (SG: n = 74; CG: n = 65).
During delivery, cord blood samples from a total of n = 107 patients (SG: n = 53; CG: n = 54) were extracted (Fig. 4). EDTA tubes were directly analyzed to receive an adequate hemogram and serum samples were stored at − 80 °C until analysis47,48. Serum iron, transferrin, and ferritin were measured at the internal clinical laboratory of “Klinikum rechts der Isar,” and hepcidin was determined using the commercial competitive “Hepcidin 25 (bioactive) HS ELISA” (DRG Instruments GmbH, Marburg, Germany).
Maternal hair samples were taken during postnatal hospitalization at the posterior vertex region of the scalp49 for cortisol measurement using auto-analyzers50. Cortisol levels in 3-cm hair samples reflect chronic stress exposure of approximately 3 months prior to delivery.
After childbirth, the CG and SG perinatal outcomes were assessed. Covariates reviewed were gestational age, maternal age, gravidity, body-mass index (BMI), ethnicity, nicotine use, socioeconomic status (SES), birth weight, sex, Apgar score, and cord blood gas analysis. Additionally, clinical routine laboratory parameters were recorded including maternal hemoglobin and anemia status at the moment of hospital admission for delivery51.
Statistics for between-group comparison
Continuous data were tested via Shapiro–Wilk test for normal distribution. We used t-tests for independent samples to compare SG and CG when data followed a Gaussian distribution. For non-normally distributed data, the Mann–Whitney U test was performed. Pearson’s Chi-squared test compared binary coded data, and Spearman’s rank-order correlation examined the relations between two variables. To assess exploratively differences in FSI in relation to iron biomarkers due to PS, we binned the value distribution of each iron biomarker automatically into five categories of equal width and compared the corresponding FSI values within each small subset. We used generalized estimating equations (GEE) to model the main effects of sex and study group and their interaction (sex*group) on iron biomarkers. All statistical tests executed were two-sided, and we assumed a significance level (α) of 0.05. IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA) and Exploratory version 6.2.2 were used for modeling, statistical analysis, and visualization.
Causal graph analysis
We used DAGitty (www.dagitty.net)52 to construct a directed acyclic graph (DAG) which defined the causal relationships between exposure (PS) and the two outcome measures: Iron biomarkers and a composite measure of neurodevelopment using the German adaptation of “Bayley Scales of Infant and Toddler Development—Third Edition” (Bayley Score) (Fig. 3). The Bayley score has not yet been calculated for the present cohort, however we included it as an outcome measure in the causal inference framework to allow for adjustment of unobserved variables and to prevent conflict with the iron biomarker outcome. The purpose of the DAG was to visualize the structural relationships between variables and minimize bias in our statistical analysis53 (additional Explanation in Supplement N1). The estimation of the total PS effect on both outcomes was performed in SAS version 3.8 using the “CAUSALGRAPH” function with robust error estimation.
Machine learning (ML)
To test the clinical utility and relative contribution of the measured parameters we classified the participants as SG or CG using ML approaches on the collected demographic, clinical, biophysical (i.e., FSI) and biochemical features (scikit-learn on Dataiku DSS 8.0.2)54. Binary features were expressed as categorical variables and dummy-encoded. No imputation was undertaken; rather, the missing rows were dropped. Using the conventional 80 : 20 data split for training : validation, we tested the classification performance of the following algorithms: random forest, gradient tree boosting, logistic regression, decision tree, K nearest neighbors (grid), extra trees, artificial neural network, LASSO-LARS, SVM and SGD. Threefold cross-validation with randomized grid search was used for hyperparameter optimization and fivefold cross-validation to rank the models created by each algorithm on AUROC (area under receiver operating curve). The highest-ranking model was selected and reported for each test.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to possible links to patients and especially newborns identities but are available from the corresponding author on reasonable request.
References
Fisher, A. L. & Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 106, 1567S-1574S. https://doi.org/10.3945/ajcn.117.155812 (2017).
Georgieff, M. K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 223, 516–524. https://doi.org/10.1016/j.ajog.2020.03.006 (2020).
Iglesias, L., Canals, J. & Arija, V. Effects of prenatal iron status on child neurodevelopment and behavior: A systematic review. Crit. Rev. Food Sci. Nutr. 58, 1604–1614. https://doi.org/10.1080/10408398.2016.1274285 (2018).
Campbell, R. K. et al. Maternal prenatal psychosocial stress and prepregnancy BMI associations with fetal iron status. Curr Dev Nutr. 4, nzaa018. https://doi.org/10.1093/cdn/nzaa018 (2020).
Rendina, D. N., Blohowiak, S. E., Coe, C. L. & Kling, P. J. Maternal perceived stress during pregnancy increases risk for low neonatal iron at delivery and depletion of storage iron at one year. J. Pediatr. 200, 166-173.e162. https://doi.org/10.1016/j.jpeds.2018.04.040 (2018).
Armony-Sivan, R. et al. Prenatal maternal stress predicts cord-blood ferritin concentration. J. Perinat. Med. 41, 259–265. https://doi.org/10.1515/jpm-2012-0125 (2013).
Coe, C. L., Lubach, G. R. & Shirtcliff, E. A. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr. Res. 61, 520–524. https://doi.org/10.1203/pdr.0b013e318045be53 (2007).
Bjelica, A., Cetkovic, N., Trninic-Pjevic, A. & Mladenovic-Segedi, L. The phenomenon of pregnancy—a psychological view. Ginekol. Pol. 89, 102–106. https://doi.org/10.5603/GP.a2018.0017 (2018).
Alyamani, R. A. S. & Murgatroyd, C. Epigenetic programming by early-life stress. Prog. Mol. Biol. Transl. Sci. 157, 133–150. https://doi.org/10.1016/bs.pmbts.2018.01.004 (2018).
Lobmaier, S. M. et al. Fetal heart rate variability responsiveness to maternal stress, non-invasively detected from maternal transabdominal ECG. Arch. Gynecol. Obstet. 301, 405–414. https://doi.org/10.1007/s00404-019-05390-8 (2020).
Van den Bergh, B. R. H. et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 117, 26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003 (2020).
Frasch, M. G. et al. Non-invasive biomarkers of fetal brain development reflecting prenatal stress: an integrative multi-scale multi-species perspective on data collection and analysis. Neurosci. Biobehav. Rev. 117, 165–183. https://doi.org/10.1016/j.neubiorev.2018.05.026 (2020).
Sutherland, S. & Brunwasser, S. M. Sex differences in vulnerability to prenatal stress: A review of the recent literature. Curr. Psychiatry Rep. 20, 102. https://doi.org/10.1007/s11920-018-0961-4 (2018).
Hare, D. J. Hepcidin: A real-time biomarker of iron need. Metallomics 9, 606–618. https://doi.org/10.1039/c7mt00047b (2017).
Cortes, M. et al. alpha7 nicotinic acetylcholine receptor signaling modulates the inflammatory phenotype of fetal brain microglia: first evidence of interference by iron homeostasis. Sci. Rep. 7, 10645. https://doi.org/10.1038/s41598-017-09439-z (2017).
Donker, A. E. et al. Standardized serum hepcidin values in Dutch children: Set point relative to body iron changes during childhood. Pediatr. Blood Cancer 67, e28038. https://doi.org/10.1002/pbc.28038 (2020).
Siddappa, A. M., Rao, R., Long, J. D., Widness, J. A. & Georgieff, M. K. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology 92, 73–82. https://doi.org/10.1159/000100805 (2007).
Higgins, V., Chan, M. K. & Adeli, K. Pediatric reference intervals for transferrin saturation in the CALIPER cohort of healthy children and adolescents. EJIFCC 28, 77–84 (2017).
Rakers, F. et al. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 117, 185–197. https://doi.org/10.1016/j.neubiorev.2017.02.019 (2017).
Hodes, G. E. & Epperson, C. N. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatry. 86, 421–432. https://doi.org/10.1016/j.biopsych.2019.04.028 (2019).
Bronson, S. L. & Bale, T. L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. https://doi.org/10.1210/en.2014-1040 (2014).
Loomans, E. M. et al. Antenatal maternal anxiety is associated with problem behaviour at age five. Early Hum. Dev. 87, 565–570. https://doi.org/10.1016/j.earlhumdev.2011.04.014 (2011).
Glasheen, C. et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev. Psychopathol. 25, 1045–1063. https://doi.org/10.1017/S0954579413000369 (2013).
Sifakis, S. & Pharmakides, G. Anemia in pregnancy. Ann. N Y Acad. Sci. 900, 125–136. https://doi.org/10.1111/j.1749-6632.2000.tb06223.x (2000).
Bencaiova, G. & Breymann, C. Mild anemia and pregnancy outcome in a Swiss collective. J. Pregnancy. 2014, 307535. https://doi.org/10.1155/2014/307535 (2014).
WHO. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. https://www.who.int/docs/default-source/micronutrients/ferritin-guideline/ferritin-guidelines-executivesummary.pdf?sfvrsn=8c98babb_2 (2020).
Tamura, T. et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr. 140, 165–170. https://doi.org/10.1067/mpd.2002.120688 (2002).
Davis, E. P. & Sandman, C. A. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child. Dev. 81, 131–148. https://doi.org/10.1111/j.1467-8624.2009.01385.x (2010).
Howland, M. A., Sandman, C. A. & Glynn, L. M. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev. Endocrinol. Metab. 12, 321–339. https://doi.org/10.1080/17446651.2017.1356222 (2017).
Comes, A. C., Modreira, A. C., Mesquita, G. & Gomes, M. S. Modulation of iron metabolism in response to infection: Twists for all tastes. Pharmaceuticals 11, 84. https://doi.org/10.3390/ph11030084 (2018).
Andersson, N. W. et al. Influence of prenatal maternal stress on umbilical cord blood cytokine levels. Arch. Womens Ment. Health 19, 761–767. https://doi.org/10.1007/s00737-016-0607-7 (2016).
Farajdokht, F., Soleimani, M., Mehrpouya, S., Barati, M. & Nahavandi, A. The role of hepcidin in chronic mild stress-induced depression. Neurosci. Lett. 588, 120–124. https://doi.org/10.1016/j.neulet.2015.01.008 (2015).
Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086. https://doi.org/10.1016/j.neubiorev.2008.03.002 (2008).
Mastorci, F. et al. Long-term effects of prenatal stress: Changes in adult cardiovascular regulation and sensitivity to stress. Neurosci. Biobehav. Rev. 33, 191–203. https://doi.org/10.1016/j.neubiorev.2008.08.001 (2009).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56. https://doi.org/10.1038/s41591-018-0300-7 (2019).
Sangkhae, V. & Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 133, 254–261. https://doi.org/10.1016/j.freeradbiomed.2018.07.001 (2019).
Delaney, K. M. et al. Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples. J. Nutr. 149, 406–415. https://doi.org/10.1093/jn/nxy286 (2019).
Lorenz, L., Peter, A., Poets, C. F. & Franz, A. R. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology 104, 194–202. https://doi.org/10.1159/000353161 (2013).
Babbar, S. & Shyken, J. Yoga in pregnancy. Clin. Obstet. Gynecol. 59, 600–612. https://doi.org/10.1097/GRF.0000000000000210 (2016).
Hutchon, B. et al. Early intervention programmes for infants at high risk of atypical neurodevelopmental outcome. Dev. Med. Child. Neurol. 61, 1362–1367. https://doi.org/10.1111/dmcn.14187 (2019).
Dumrongwongsiri, O. et al. Effect of maternal nutritional status and mode of delivery on zinc and iron stores at birth. Nutrients 13, 860. https://doi.org/10.3390/nu13030860 (2021).
Sharma, R. et al. Association between prenatal stress and infant DNA methylation. Eur. J. Hum. Genet. 28, 754–762 (2020).
Klein, E. M. et al. The German version of the Perceived Stress Scale - psychometric characteristics in a representative German community sample. BMC Psychiatry 16, 159. https://doi.org/10.1186/s12888-016-0875-9 (2016).
Cohen, S., Kamarck, T. & Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396 (1983).
screening and review. American College of, O., Gynecologists, the Society for Maternal-Fetal, Medicine & Kilpatrick, S. K., Ecker, J. L. Severe maternal morbidity. Am. J. Obstet. Gynecol. 215, B17-22. https://doi.org/10.1016/j.ajog.2016.07.050 (2016).
Bauer, A., Barthel, P., Müller, A., Kantelhardt, J. & Schmidt, G. Bivariate phase-rectified signal averaging—a novel technique for cross-correlation analysis in noisy nonstationary signals. J. Electrocardiol. 42, 602–606. https://doi.org/10.1016/j.jelectrocard.2009.06.023 (2009).
Jansen, E. H., Beekhof, P. K. & Schenk, E. Long-term stability of biomarkers of the iron status in human serum and plasma. Biomarkers 18, 365–368. https://doi.org/10.3109/1354750X.2013.781223 (2013).
Pfeiffer, C. M. & Looker, A. C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 106, 1606S-1614S. https://doi.org/10.3945/ajcn.117.155887 (2017).
SoHT. Consensus on hair analysis: recommendations for hair testing in forensic cases. https://soht.org/images/pdf/Consensus_on_Hair_Analysis.pdf (2003).
Gonzalez, D. et al. Hair cortisol measurement by an automated method. Sci. Rep. 9, 8213. https://doi.org/10.1038/s41598-019-44693-3 (2019).
Centers for Disease, C. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb. Mortal Wkly Rep. 38, 400–404 (1989).
Textor, J., van der Zander, B., Gilthorpe, M. S., Liskiewicz, M. & Ellison, G. T. Robust causal inference using directed acyclic graphs: The R package “dagitty”. Int. J. Epidemiol. 45, 1887–1894. https://doi.org/10.1093/ije/dyw341 (2016).
Greenland, S., Pearl, J. & Robins, J. M. Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999).
Frasch, M. G. et al. Brief report: can a composite heart rate variability biomarker shed new insights about autism spectrum disorder in school-aged children?. J. Autism Dev. Disord. 51, 346–356. https://doi.org/10.1007/s10803-020-04467-7 (2021).
Acknowledgements
We are grateful to all female patients who participated in the FELICITy study while having to deal with the challenging last weeks of pregnancy and first years of childcare. MCA was awarded with the August Wilhelm Scheer Professorship Program (TUM) twice for a 6-month period stay at the Klinik und Poliklinik für Frauenheilkunde, TUM, Klinikum rechts der Isar, Munich for the start-up of the FELICITy project and a Hans Fischer Senior Fellowship from IAS-TUM (Institute for Advanced Study-TUM).
Funding
Open Access funding enabled and organized by Projekt DEAL. This article was funded by Institute for Advanced Study, Technische Universität München, August Wilhelm Scheer Professorship.
Author information
Authors and Affiliations
Contributions
P.Z.: patient recruitment, data collection and management, data analysis, and manuscript writing. M.F.: data collection and management, data analysis, machine learning analysis and manuscript writing and editing. S.M.L. and M.C.A.: protocol and project development, data collection and management, data analysis, and manuscript writing and editing. A.M. and H.T.W.: machine learning analysis and manuscript writing and editing. N.W.: DAG analysis, manuscript reviewing and editing. R.S.: manuscript reviewing and editing, CZ: patient recruitment and data collection. B.F.: cortisol analysis in hair samples and manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
MGF holds patents on fetal ECG technologies. No other disclosures were reported.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zimmermann, P., Antonelli, M.C., Sharma, R. et al. Prenatal stress perturbs fetal iron homeostasis in a sex specific manner. Sci Rep 12, 9341 (2022). https://doi.org/10.1038/s41598-022-13633-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13633-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.