Abstract
Serum glypican-4 (GPC4) has been identified as an insulin-sensitizing adipokine serving as a marker for body mass index and insulin resistance in humans. The association of circulating GPC4 with kidney function is to date largely unexplored. Therefore, we aimed to evaluate the association between serum GPC4 and prevalent as well future kidney function in a prospective cohort study. The study included 456 Caucasian coronary angiography patients. After a median follow up period of 3.4 years, data on kidney function was reassessed in all patients. Chronic kidney disease (CKD) was defined by decreased estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or albuminuria. At baseline, serum GPC4 was significantly associated with decreased eGFR (adjusted odds ratio (OR) per standard deviation = 4.75 [2.66–8.48]; P < 0.001), albuminuria (OR = 1.49 [1.15–1.92]; P = 0.002), and, accordingly, with CKD (OR = 1.75 [1.35–2.26]; P < 0.001). GPC4 levels also significantly and independently predicted the incidence of newly diagnosed decreased eGFR (OR = 2.74 [1.82–4.14]; P < 0.001, albuminuria (OR = 1.58 [1.01–2.46]; P = 0.043, and CKD (OR = 2.16 [1.45–3.23]; P < 0.001). ROC analysis indicated an additional predictive value of GPC4 to a basic prediction model for newly diagnosed CKD and eGFR < 60 mL/min/1.73 m2. Our study, therefore, indicates that high serum GPC4 is associated with decreased prevalent and future kidney function.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is strongly associated with an increased risk of cardiovascular and all-cause mortality1,2. Main risk factors for CKD are hypertension and diabetes3. Criteria for the definition of CKD are based on decreased glomerular filtration rate (GFR) or markers of kidney damage such as albuminuria4. Much interest has focused on further kidney markers to improve early disease management and risk prediction, particularly in high risk subjects such as coronary patients. In this regard, uromodulin5,6,7 and fibroblast growth factor 23 (FGF23)8,9,10 have been proposed as new promising biomarkers of kidney disease.
Adipose tissue has been recognised as a highly active endocrine organ secreting various biologically active adipokines, whose altered expression and secretion have been associated with a number of pathologies, including CKD11,12. In 2012, Ussar et al. showed that the cell surface protein glypican-4 (GPC4) also plays the role of an adipokine13. GPC4 belongs to the family of heparan sulfate proteoglycans (HSPGs), which are linked to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor and mediate interactions with a variety of extracellular ligands such as growth factors and adhesion molecules14. GPC4 as well as other HSPGs can be released from the cell surface into extracellular space by phospholipase‐mediated cleavage of the GPI anchor or by proteolytic shedding15,16. Ussar et al. demonstrated that both membrane-bound and released GPC4 can interact with the insulin receptor and enhance insulin signalling in pre-adipocytes promoting adipocyte differentiation13. The authors further showed that serum GPC4 is a marker for body mass index (BMI) and insulin resistance in mice and humans13, which has been confirmed by subsequent studies17,18,19,20. Recently, Cha et al. revealed that circulating GPC4 is linked with estimated GFR (eGFR) and urinary albumin excretion in individuals with diabetes21. Another study showed a significant correlation of GPC4 with creatinine in patients with metabolic syndrome22. However, to our best knowledge, no other reports on the association of serum GPC4 with kidney function exist so far. Moreover, it is unclear, whether GPC4 can predict the development of future decreased kidney function.
In order to get deeper insights into the association of GPC4 with kidney function, we investigated the association of serum GPC4 with CKD as well as with decreased estimated GFR and albuminuria at baseline and after a 3.4-year follow up period in a well characterized cohort of coronary angiography patients. In addition, we evaluated its diagnostic and prognostic value as a new biomarker for decreased kidney function.
Results
Baseline
GPC4 levels ranged from 1.6 ng/mL to 12.8 ng/mL; the median GPC4 level was 5.5 [interquartile range: 4.6–6.9] ng/mL. Patients’ baseline characteristics of the total study cohort as well as stratified according to GPC4 quartiles are given in Table 1. Overall, the characteristics of our patients were representative for patients undergoing coronary angiography for the evaluation of coronary artery disease (CAD), with a high prevalence of male gender, type 2 diabetes mellitus (T2DM), and hypertension. Age, BMI, and parameters of kidney function were significantly associated with increasing GPC4 quartiles. Results from correlation analyses confirmed these observations (Table 2). Results from correlation analyses further showed that GPC4 serum levels were significantly linked with systolic blood pressure, urea, uromodulin, and FGF23 (Table 2). Analysis of covariance revealed that serum GPC4 was significantly associated with eGFR and the albumin/creatinine concentration ratio (ACR) independently from classic risk factors for decreased kidney function (each P-value < 0.001; Supplemental Table S1). Importantly, serum GPC4 was associated with ACR independently from eGFR (F = 9.3; P = 0.002) and with eGFR independently from ACR (F = 113.0; P < 0.001).
Figure 1a–c shows GPC4 levels stratified by categories of eGFR, albuminuria, and risk of CKD progression, respectively. Increased GPC4 levels were significantly associated with categories of decreased eGFR (Fig. 1a; Ptrend < 0.001). Particularly, serum GPC4 differed highly significantly between patients with normal filtration rates (G1) and mildly decreased filtration rates (G2) and between individuals with mildly decreased filtration rates and moderately decreased filtration rates (G3a). GPC4 differed significantly between categories of severity of albuminuria (Fig. 1b; Ptrend < 0.001) and between risk categories of CKD progression (Fig. 1c; Ptrend < 0.001). Consequently, serum GPC4 was significantly elevated in patients with CKD compared to patients without CKD (median [interquartile range] = 6.4 [5.3–8.0] ng/mL versus 5.3 [4.5–6.4] ng/mL; P < 0.001).
Association between glypican-4 levels and kidney function. Glypican-4 levels are expressed as median with interquartile range. (a) Estimated glomerular filtration rate categories are assigned as follows: G1: eGFR ≥ 90 mL/min/1.73 m2 (n = 196); G2: eGFR 60–89 mL/min/1.73 m2 (n = 227); G3a: eGFR 45–59 mL/min/1.73 m2 (n = 23); G3b: eGFR 30–44 mL/min/1.73 m2 (n = 9); G4: eGFR 15–29 mL/min/1.73 m2 (n = 1); due to the low number of G4 subjects, G4 was combined with G3b; (b) Albuminuria categories are based on the urinary albumin-to-creatinine ratio and are assigned as follows: A1: ACR < 30 mg/g (n = 357); A2: 30–300 mg/g (n = 83); A3: ACR > 300 mg/g (n = 16). (c) Chronic kidney disease (CKD) risk categories of the prognosis of CKD are based on eGFR and albuminuria categories according to the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD4 revealing that 341 subject were at low risk, 84 patients were at moderately increased risk, 20 patients were at high risk, and 11 patients were at very high risk. CKD, chronic kidney disease; GPC4, glypican-4. ***P < 0.001; **P < 0.005; *P < 0.05.
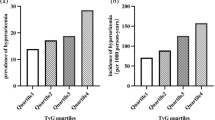
Association of serum GPC4 with eGFR < 60 mL/min/1.73 m2, albuminuria, and CKD remained significant after correction for age, sex, BMI, T2DM, CAD, smoking, blood pressure, and C-reactive protein (CRP) in multivariable logistic regression analysis. Respective odds ratios with 95% confidence intervals for one standard deviation change of log-transformed GPC4 are illustrated in Fig. 2a.
Associations between glypican-4 levels and (a) prevalence of eGFR < 60 mL/min/1.73 m2, albuminuria, and chronic kidney disease at baseline or (b) incidence of newly diagnosed eGFR < 60 mL/min/1.73 m2, albuminuria, and chronic kidney disease at follow up. Chronic kidney disease was attested in case of eGFR < 60 mL/min/1.73 m2 or albuminuria. Odds ratios with 95% confidence intervals [95%CI] per standard deviation of log-transformed GPC4 were obtained by univariable (crude) and multivariable logistic regression analyses adjusted for age, sex, body mass index, systolic and diastolic blood pressure, type 2 diabetes mellitus, history of smoking, C-reactive protein, and significant coronary artery disease.
Area under the receiver operating characteristic curve (ROC-AUC) analyses were used to compare the performance of GPC4 as a biomarker for kidney function with the new circulating biomarkers uromodulin and FGF23. GPC4 showed higher area under the curve (AUC) values for the diagnosis of eGFR < 60 mL/min/1.73 m2, albuminuria, and CKD at baseline as well as at follow up compared to uromodulin and FGF23 and, therefore, outperformed these markers for the diagnosis of baseline and future kidney function (Supplemental Table S2).
Follow up
After a median follow up period of 3.4 [3.1–3.5] years, kidney function was reassessed in all patients. Serum GPC4 determined at baseline was significantly correlated with eGFR and ACR assessed at follow up (r = −0.406; P < 0.001 and r = 0.245; P < 0.001, respectively; adjusted results from analysis of covariance are shown in Supplemental Table S1).
eGFR < 60 mL/min/1.73 m2, albuminuria, and CKD were newly diagnosed in 50, 31, and 50, respectively, patients at follow up. GPC4 levels were significantly increased in patients with newly diagnosed eGFR < 60 mL/min/1.73 m2 at follow up compared to subjects with eGFR ≥ 60 mL/min/1.73 m2 at baseline as well as at follow up (6.97 [5.49–7.93] ng/mL versus 5.23 [4.42–6.28] ng/mL; P < 0.001). Similarly, patients with newly diagnosed albuminuria at follow up showed significant higher baseline GPC4 levels compared to patients with no albuminuria at baseline and at follow up (5.69 [4.86–7.09] ng/mL versus 5.27 [4.49–6.48] ng/mL; P = 0.042). Consequently, GPC4 levels were also significantly increased in patients with newly diagnosed CKD at follow up compared to patients with no CKD at baseline and at follow up (6.17 [5.12–7.70] ng/mL versus 5.13 [4.36–6.24] ng/mL; P < 0.001).
Logistic regression analyses showed that baseline GPC4 levels significantly and independently predicted the incidence of newly diagnosed eGFR < 60 mL/min/1.73 m2, albuminuria, and CKD during a 3.4-year period (Fig. 2b). GPC4 outperformed uromodulin, and FGF23 for the diagnosis of incident eGFR < 60 mL/min/1.73 m2, incident albuminuria, and incident CKD (Supplemental Table S3). ROC analysis further indicated an additional predictive value of GPC4 to a basic prediction model including baseline ACR, age, sex, BMI, blood pressure, T2DM, CRP, smoking, and the presence of CAD for incident CKD and incident eGFR < 60 mL/min/1.73 m2 (Table 3).
Discussion
In the present study, we showed significant associations between GPC4 and decreased eGFR, albuminuria, and as a consequence CKD in a cohort of coronary angiography patients. Previously, GPC4 has been associated with eGFR and urinary albumin excretion in a single, relatively small-sized study including 161 Korean patients with diabetes21. Our study does not only confirm these findings in a much larger cohort of patients but additionally shows that serum GPC4 predicts the future risk of decreased kidney function. In this regard, circulating GPC4 even outperforms other serum markers recently proposed for the detection of kidney disease such as uromodulin5,7 or FGF239,10.
Our study is in line with previous findings demonstrating significant associations of serum GPC4 with BMI13,17,18,19,23 and systolic blood pressure18. The linkage between GPC4 and obesity-related traits may have contributed to the significant association between GPC4 and kidney function but does not fully explain it. Multivariable logistic regression analyses revealed that the association between GPC4 and kidney function remained robust when metabolic parameters and other conventional risk factors such as smoking or significant CAD were included in the model. Therefore, it can be assumed that GPC4 is linked with kidney function decline either as factor of kidney perfusion or as a product of unsatisfactory glycaemic or metabolic control beyond its association with a traditional kidney risk profile predisposing to CKD.
Increased GPC4 levels in patients with decreased kidney function may be a result of impaired glomerular filtration, responsible for elevated plasma levels of creatinine and urea as well. In fact, among the investigated parameters the strongest correlation was found between serum GPC4 und eGFR. However, unlike circulating creatinine and urea, serum GPC4 was strongly correlated with urinary albuminuria too. Albuminuria associated with progression of kidney disease is likely caused by the degradation of the glycocalyx, a hydrogel comprised of glycosoaminoglycans, glycoproteins and associated serum proteins that covers the luminal surface of the glomerular endothelium and that normally acts as a barrier against albumin filtration24,25. Degradation of the endothelial glycocalyx layer (“shedding”) occurs in inflammatory states and during ischemia and results in the release of measurable glycocalyx components into the circulation26,27. As GPC4 contributes to the formation of the glycocalyx, circulating GPC4, derived from endothelial glycocalyx shedding may, therefore, represent a surrogate marker of kidney dysfunction caused by damage to the glycocalyx. In fact, the glycocalyx shed markers syndecan-1 and hyaluronan have been previously associated with different CKD stages supporting this hypothesis28,29.
Since GPC4 is highly expressed in tubular epithelial cells in the adult kidney, it may also have a physiological function30. GPC4 can interact with the insulin receptor enhancing insulin signalling in preadipocytes and plays a role in adipocyte differentiation13. Insulin also performs distinct actions in kidney tissue that regulate metabolic and growth pathways as well as the kidney microcirculation. Consequently, resistance to the metabolic actions of insulin affects kidney structure and function31. It has been speculated that an increase of circulating GPC4 could represent a regulatory mechanism by which fat acts to counteract insulin resistance13,32. This hypothesis could be applicable for kidney tissue as well, assuming that increased GPC4 levels mirror elevated demand of insulin needed for maintenance of kidney function and kidney repair.
GPC4 is also involved in the control of the wingless/int-1 (Wnt) signalling pathway33, which triggers adaptive responses involved in kidney repair and regeneration, but may also promote disease progression, depending on the magnitude and duration of its activation34,35,36. The picture is even more complex, as membrane-bound GPC4 enhances Wnt signalling, but GPC4 secreted to the extracellular environment acts as competitive inhibitor of the Wnt signalling pathway33. Therefore, functional studies are needed to understand the biological role of circulating GPC4 in kidney disease.
Our study has several strengths and limitations. By design, our study population was composed of angiography coronary patients of European ancestry; our results therefore are not necessarily applicable to other ethnicities or the general population. However, the high-risk patient population of coronary angiography patients we chose to investigate is of particular clinical interest. The prospective part of our study represents the first observation linking GPC4 to the future decline of kidney function. However, GPC4 levels have been associated with various obesity-related traits13,17,18,19,20,21,23 and, therefore, may not be specific to the malfunction of a particular organ. In addition, despite the statement of the manufacturer, that the used ELISA shows high specificity to human GPC4, any assay cross- reactivity to GPC4 analogues cannot be fully excluded. Therefore, the specificity of the GPC4 ELISA needs to be confirmed before it could be used as a predictive biomarker in the future. Importantly, due to the observational design of the present study, the pathophysiological mechanisms of the association between circulating GPC4 and impaired kidney function remain speculative and need to be investigated in further studies.
In summary, our study shows that serum GPC4 is strongly associated with kidney function-related traits and for the first time predicts the development of CKD. The value of GPC4 as a new biomarker for kidney malfunction and kidney disease progression has to be further evaluated in subsequent studies.
Methods
Study population
From September 2005 through April 2008, we enrolled 1048 consecutive Caucasian patients who were referred to elective coronary angiography for the evaluation of established or suspected stable CAD at the Academic Teaching Hospital Feldkirch, Austria. Out of these, 456 subjects had available serum samples for GPC4 analysis and data on kidney function at baseline as well as at follow up. Patients with acute kidney injury were not included. Coronary angiography was performed with the Judkin's technique and coronary artery stenoses with stenotic narrowing ≥ 50% were defined as significant CAD37. Hypertension was defined according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure38. T2DM was diagnosed according to American Diabetes Association criteria39. The study has been approved by the Ethics Committee of the University of Innsbruck, Austria, and written informed consent was given by all participants before taking part in the study. All methods were performed in accordance with the relevant guidelines and regulations.
Laboratory measurements
Venous blood samples were collected after an overnight fast of 12 h before angiography was performed. Routine blood variables were determined in fresh serum samples by standard laboratory methods at the Medical Central Laboratories, Feldkirch, Austria. Serum samples were stored at −80 °C until used for analysis of FGF23, uromodulin, and GPC4. FGF23 measurements were performed at Immundiagnostik AG (Bensheim, Germany) and serum uromodulin was detected as previously described7. Serum GPC4 levels were determined using the same batch of a commercial enzyme-linked immunosorbent assay (ELISA) kit (Cloude-Clone, Houston, Texas; article number: SEA998Hu) following the manual of the manufacturer. The kit had a detection range of 0.031–2.000 ng/mL; the intra-assay coefficient of variation (CV) was below 10% and the inter-assay CV was below 12%. Serum samples were diluted 1:4 using phosphate buffered saline as a dilution buffer prior to analysis.
Parameters of kidney function
GFR was estimated (eGFR) using the ‘Chronic Kidney Disease Epidemiology Collaboration’ (CKD-EPI) serum creatinine equation. GFR categories were defined as follows: G1: eGFR ≥ 90 mL/min/1.73 m2; G2: eGFR = 60–89 mL/min/1.73 m2; G3a: eGFR = 45–59 mL/min/1.73 m2; G3b: eGFR = 30–44 mL/min/1.73 m2; G4: eGFR = 15–29 mL/min/1.73 m2. Urinary albumin excretion was expressed as ACR in a random morning urine specimen.
Urinary albumin concentration was determined by immunoturbidometry (Tina-quant Albumin Gen.2 Assay, Roche Diagnostics, Vienna, Austria). Both serum and urinary creatinine concentrations were measured with a modified Jaffé method (Creatinine Jaffé Gen.2 Assay, Roche Diagnostics). ACR levels lower than 30 mg/g (category A1) were defined as normal and albuminuria was diagnosed as an ACR of 30 mg/g or greater. Moderately increased albuminuria (category A2; formerly microalbuminuria) was defined as an ACR of 30 to 300 mg/g and severely increased albuminuria (category A3; formerly macroalbuminuria) as an ACR of 300 mg/g or greater. CKD was diagnosed in case of eGFR ˂ 60 mL/min/1.73 m2 or albuminuria. Due to the observational design of the study, our definition of CKD was based on single measurements of kidney function. Prognosis of CKD was classified into four risk-categories according to the classification system proposed by the 2012 KDIGO recommendations4, which were based on GFR categories (G1-G5) and ACR categories (A1-A3) and were as follows: Low risk: G1 and A1 or G2 and A1; moderately increased risk: G1 and A2, G2 and A1, G2 and A2 or G3a and A1; high risk: G1 and A3, G2 and A3, G3a and A2 or G3b and A1; very high risk: G3a and A3, G3b and A2, G3b and A3, G4 or G5.
Prospective study
After a median period of 3.4 years, patients were re-invited to undergo a follow-up investigation and kidney function was reassessed.
Statistical analyses
Differences in baseline characteristics according to GPC4 quartiles were tested for statistical significance with Chi-squared tests for trend for categorical and Jonckheere Terpstra tests for continuous variables, respectively. Continuous variables are given as median and interquartile range (defined as the range from the 25th percentile to the 75th percentile. Normal distribution was assessed using Kolmogorov–Smirnov and Shapiro–Wilk test, respectively, concluding that GPC4 values were not normally distributed. Correlation analyses were performed calculating non-parametric Spearman rank correlation coefficients. In addition, analysis of covariance models were built using a general linear model approach. Non-normally distributed variables were log-transformed by the base of 10 before they were entered into parametric models. Statistically significant differences between GPC4 levels and categorical variables were determined by the Mann–Whitney U test and the Jonckheere Terpstra test, respectively. Odds ratios and 95% confidence intervals [95%CI] were obtained from univariable and multivariable logistic regression analyses adjusting for age, sex, BMI, blood pressure, T2DM, smoking, CRP, and the presence of CAD. Z-transformation was applied before logistic regression analysis. All data were analysed according to complete-case analysis due to neglible percentage of missing data. Additionally, ROC-AUC analyses were applied to compare the performance of GPC4 as a diagnostic biomarker with other kidney markers. To examine the potential utility of GPC4 as a predictive biomarker, binary logistic regression models were fitted with eGFR < 60 mL/min per 1.73 m2, albuminuria, and CKD, respectively, as the dependent variable. A basic model comprising ACR, age, sex, blood pressure, BMI, T2DM, CRP, smoking history, and the presence of significant CAD as independent variables was compared with a second model including GPC4 as an additional marker. Receiver operating characteristic and the respective AUC were calculated. The statistical significance of the difference between AUCs was tested with the method of DeLong40. All statistical analyses were performed with SPSS 27.0 (SPSS, Inc., Chicago, IL) software.
Data availability
The datasets generated and/or analysed during the current study are available in the Mendeley Data repository, ‘Data on the association of serum glypican-4 with prevalent and future kidney function’ (https://doi.org/10.17632/nx94z23388.1; https://data.mendeley.com/datasets/nx94z23388/1).
References
Weiner, D. E. et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am. J. Kidney Dis. 44, 198–206 (2004).
Tonelli, M. et al. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 (2006).
Carmena, R., Ascaso, J. F. & Redon, J. Chronic kidney disease as a cardiovascular risk factor. J. Hypertens. 38, 2110–2121 (2020).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3, 1–150 (2013).
Steubl, D. et al. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Med. (United States) 95, 1 (2016).
Steubl, D. et al. Association of serum uromodulin With death, cardiovascular events, and kidney failure in CKD. Clin. J. Am. Soc. Nephrol. 15, 616–624 (2020).
Leiherer, A. et al. The value of uromodulin as a new serum marker to predict decline in renal function. J. Hypertens. 36, 110–118 (2018).
Portale, A. A. et al. Fibroblast growth factor 23 and risk of CKD progression in children. Clin. J. Am. Soc. Nephrol. 11, 1989–1998 (2016).
Heine, G. H., Seiler, S. & Fliser, D. FGF-23: The rise of a novel cardiovascular risk marker in CKD. Nephrol. Dial. Transplant. 27, 3072–3081 (2012).
Damasiewicz, M. J., Toussaint, N. D. & Polkinghorne, K. R. Fibroblast growth factor 23 in chronic kidney disease: New insights and clinical implications. Nephrology 16, 261–268 (2011).
Rüster, C. & Wolf, G. Adipokines promote chronic kidney disease. Nephrol. Dialys. Transpl. 28, 1 (2013).
Briffa, J. F., Mcainch, A. J., Poronnik, P. & Hryciw, D. H. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. - Renal Physiol. 305, 1 (2013).
Ussar, S., Bezy, O., Blüher, M. & Kahn, C. R. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes 61, 2289–2298 (2012).
Tumova, S., Woods, A. & Couchman, J. R. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32, 269–288 (2000).
Huang, K. & Park, S. Heparan Sulfated Glypican-4 is Released from Astrocytes Predominantly by Proteolytic Shedding. bioRxiv 2021.02.17.431702 (2021). https://doi.org/10.1101/2021.02.17.431702
Traister, A., Shi, W. & Filmus, J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 410, 503–511 (2008).
Yoo, H. J. et al. Association of glypican-4 with body fat distribution, insulin resistance, and nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 98, 2897–2901 (2013).
Zhu, H. J. et al. The changes of serum glypican4 in obese patients with different glucose metabolism status. J. Clin. Endocrinol. Metab. 99, E2697–E2701 (2014).
Li, K. et al. Glypican-4 is increased in human subjects with impaired glucose tolerance and decreased in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 51, 981–990 (2014).
Zhang, K. et al. Serum glypican4 and glycosylphosphatidylinositol-specific phospholipase D levels are associated with adipose tissue insulin resistance in obese subjects with different glucose metabolism status. J. Endocrinol. Invest. 44, 781–790 (2021).
Cha, J. J. et al. Long-term study of the association of adipokines and glucose variability with diabetic complications. Kor. J. Intern. Med. 33, 367–382 (2018).
Ning, D. P. et al. Serum glypican 4 levels are associated with metabolic syndrome in a han population from Guizhou Province, China. Biomed. Environ. Sci. 32, 383–388 (2019).
Leelalertlauw, C. et al. Serum glypican 4 level in obese children and its relation to degree of obesity. Clin. Endocrinol. (Oxf) 87, 689–695 (2017).
Korakas, E. et al. The endothelial glycocalyx as a key mediator of albumin handling and the development of diabetic nephropathy. Curr. Vasc. Pharmacol. 18, 619–631 (2019).
Rabelink, T. J. & De Zeeuw, D. The glycocalyx - Linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 11, 667–676 (2015).
Lepedda, A. J. et al. Circulating heparan sulfate proteoglycans as biomarkers in health and disease. Semin. Thromb. Hemost. 47, 295–307 (2021).
Hahn, R. G., Patel, V. & Dull, R. O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 65, 590–606 (2021).
Liew, H., Roberts, M. A., Pope, A. & McMahon, L. P. Endothelial glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol. 22, 1 (2021).
Padberg, J. S. et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 234, 335–343 (2014).
Watanabe, K., Yamada, H. & Yamaguchi, Y. K-glypican: A novel GPI-anchored heparan sulfate proteoglycan that is highly expressed in developing brain and kidney. J. Cell Biol. 130, 1207–1218 (1995).
Whaley-Connell, A. & Sowers, J. R. Insulin resistance in kidney disease: Is there a distinct role separate from that of diabetes or obesity. CardioRenal Med. 8, 41–49 (2017).
Tamori, Y. & Kasuga, M. Glypican-4 is a new comer of adipokines working as insulin sensitizer. J. Diabet. Investig. 4, 250–251 (2013).
Sakane, H., Yamamoto, H., Matsumoto, S., Sato, A. & Kikuchi, A. Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. J. Cell Sci. 125, 449–460 (2012).
Kawakami, T., Ren, S. & Duffield, J. S. Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J. Pathol. 229, 221–231 (2013).
Zhou, D., Tan, R. J., Fu, H. & Liu, Y. Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab. Investig. 96, 156–167 (2016).
Wang, Y., Zhou, C. J. & Liu, Y. Wnt Signaling in Kidney Development and Disease. in Progress in Molecular Biology and Translational Science 153, 181–207 (Elsevier B.V., 2018).
Drexel, H. et al. Plasma triglycerides and three lipoprotein cholesterol fractions are independent predictors of the extent of coronary atherosclerosis. Circulation 90, 2230–2235 (1994).
Chobanian, A. V. et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. J. Am. Med. Assoc. 289, 2560–2572 (2003).
American Diabetes Association. Standards of medical care in diabetes-2015. Diabetes Care 38, S1–S93 (2015).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 44, 837 (1988).
Acknowledgements
The authors sincerely thank Dr. Thomas Bernd Dschietzig (Immundiagnostik AG, Bensheim, Germany) and Dr. Franz Paul Armbruster (Immundiagnostik AG, Bensheim, Germany) for performing FGF23 measurements. We are grateful to the Vorarlberger Landesregierung (Bregenz, Austria) for continuously supporting our research institute.
Author information
Authors and Affiliations
Contributions
Conception and design: A.M., H.D. Patients’ recruitment, sample and data collection: C.H.S., H.H., D.H., P.F., C.H. Sample analysis: E.M.B., K.G., S.G., C.H. Statistical analysis: A.M., A.L., C.H.S. Data analysis and interpretation: A.M., H.D., E.M.B., C.H.S., P.F., D.H. Drafting of the manuscript: A.M., E.M.B, H.D. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muendlein, A., Brandtner, E.M., Leiherer, A. et al. Evaluation of the association of serum glypican-4 with prevalent and future kidney function. Sci Rep 12, 10168 (2022). https://doi.org/10.1038/s41598-022-14306-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14306-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.