Abstract
N-acetylaspartate (NAA) is the second most abundant metabolite in the human brain; although it is assumed to be a proxy for a neuronal marker, its function is not fully elucidated. NAA is also detectable in plasma, but its relation to cerebral NAA levels, cognitive performance, or features of cerebral disease has not been investigated. To study whether circulating NAA tracks cerebral NAA levels, and whether circulating NAA correlates with cognitive function and features of cerebral small vessel disease (SVD). Two datasets were analyzed. In dataset 1, structural MRI was acquired in 533 subjects to assess four features of cerebral SVD. Cognitive function was evaluated with standardized test scores (N = 824). In dataset 2, brain 1H-MRS from the occipital region was acquired (N = 49). In all subjects, fasting circulating NAA was measured with mass spectrometry. Dataset 1: in univariate and adjusted for confounders models, we found no correlation between circulating NAA and the examined features of cerebral SVD. In univariate analysis, circulating NAA levels were associated inversely with the speed in information processing and the executive function score, however these associations were lost after accounting for confounders. In line with the negative findings of dataset 1, in dataset 2 there was no correlation between circulating and central NAA or total NAA levels. This study indicates that circulating NAA levels do not reflect central (occipital) NAA levels, cognitive function, or cerebral small vessel disease in man.
Similar content being viewed by others
Introduction
One of the most abundant metabolites in the mammalian brain is N-acetylaspartate (NAA), an aminoacid with concentrations in the central nervous system (CNS) in the millimolar range (~ 10 mM)1. NAA derives from L-aspartic acid, synthesized in neuronal mitochondria from acetyl-coenzyme A. The two-carbon acetate portions of the latter derive predominantly from the oxidation of glucose. Even though NAA is synthesized and stored primarily in neurons, it cannot be catabolized by them2 and is therefore released into the extracellular fluid (ECF). Following diffusion to oligodendrocytes, aspartoacylase (ASPA) cleaves the acetate moiety and aspartate is recycled back to neurons3. N-acetyl aspartate glutamate (NAAG) is also synthesized in neurons and is converted to NAA in astrocytes.
Although the function of NAA in humans remains to be fully elucidated4, it has been proposed that one of its most important functions is acting as a neuronal molecular water pump where each NAA molecule with a minimum of 32 water molecules5 is released by neurons to the ECF down a steep NAA gradient, effectively transporting water to ECF. NAA may also act as a facilitator of energy metabolism and as a source of acetate for fatty acid and steroid synthesis necessary for axonal myelination by oligodendrocytes4.
Thanks to the abundant presence of NAA in neurons and its N-acetyl methyl group resonance, NAA and total NAA (i.e. NAA + NAAG) can be detected with brain 1H magnetic resonance spectroscopy (MRS). NAA is considered to be a proxy marker of neuronal health6; indeed brain spectroscopy studies have shown NAA deficits in normal aging7 and in several neurological disorders such as in Alzheimer’s disease8,9,10, Huntington disease11,12, and multiple sclerosis13,14, and in psychiatric diseases such as bipolar disorders15,16,17, and schizophrenia16,18,19. A recent meta-analysis showed that presence of type 2 diabetes (T2D) is also associated with decreased levels of NAA in the frontal lobe and the lenticular nucleus, while no significant changes in NAA levels were found in the occipital and parietal lobes and in thalamus20. At the other end of the spectrum, excessive NAA accumulation caused by a missense mutation in the ASPA gene results in a fatal neurodegenerative disorder, the Canavan disease, characterized by widespread CNS vacuolization and hypomyelination21.
Neuronal NAA metabolism is dynamic with a turnover rate of ~ 1.4 times/day1. Also, there are now several lines of evidence showing that neuronal stimulation or specific treatments impact on central NAA concentrations. For instance, a study has shown that during periods of visual stimulation, NAA concentrations in the visual cortex are decreased, whereas upon stimulus cessation, central NAA concentrations are restored22. This dynamic modification of central NAA concentrations upon stimulation and stimulus withdrawal has been suggested to indicate an intimate association of NAA metabolism and neurostimulation22. In the same line, treatment with levodopa in patients with Parkinsons’ disease23, antidepressant medication in depressed patients24, or alcohol withdrawal in patients with alcohol abuse25 have all been shown to restore central NAA levels.
Previous studies have linked central NAA concentrations with cognition26, even though the available literature is not unanimous on the topic. For instance, even though reductions in central NAA concentrations have been linked to worse cognitive function, this correlation is rather weak27, and other studies have failed to detect any association between cognition and NAA concentrations28. To some extent the discrepancy in the existing literature could be attributed to varying portions of gray and white matter inside the sampling MRS voxels, thereby affecting the concentrations of the assessed metabolites29 and consequently also their associations to cognitive parameters. Also, it is be reasonable to expect that correlations between neurometabolites and cognitive function might vary according to the cortical region investigated and its functional specialization.
Cerebral small vessel disease (SVD) is an “umbrella term” encompassing a variety of abnormalities related to cerebral microangiopathy30. It is a major cause of cognitive impairment and dementia31. Because of the mild clinical symptoms and the low case fatality rate of cerebral SVD, it is often discovered as incidental findings in neuroimaging studies, and its prevalence increases with advancing age32. Evidence suggests that patients with cerebral SVD have lower brain NAA concentrations compared to healthy controls, and that central NAA concentrations correlate negatively with the lesion volume28.
NAA is also detectable in the plasma, and ASPA is abundantly expressed in peripheral organs in addition to oligodendrocytes33. However, to the best of our knowledge whether circulating NAA tracks central NAA levels has not been investigated. Moreover, whereas previous studies have linked central NAA concentrations to both cognitive function26 and cerebral SVD28, it is not known whether circulating NAA concentrations associate with cognitive function or with cerebral SVD. Thus, the primary aim in the present study was to assess the value of measuring plasma NAA concentrations and their relation to features of cognitive dysfunction and cerebral small vessel disease. Also, in a separate smaller study we assessed the interplay between central (occipital) and circulating NAA concentrations.
Materials and methods
Study subjects
In this study we combined data from two different datasets: dataset 1 is a random subset of The Maastricht Study34; dataset 2 is from a clinical trial (performed at the Turku PET Centre, NCT04343469) whose main aim was to study brain inflammation in human obesity. The Maastricht Study is an observational prospective population-based cohort study. The rationale and methodology have been described previously34. In brief, the study focuses on the etiology, pathophysiology, complications and comorbidities of type 2 diabetes mellitus (T2D) and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2D status, with an oversampling of individuals with T2D, for reasons of efficiency. The examinations of each participant were performed within a time window of three months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088-105234-PG). All participants gave written informed consent. In The Maastricht Study (Dataset 1, n = 824), participants had a thorough evaluation of their cognitive function (three cognitive domains were explored: verbal memory, processing speed and executive function), and 533 of them also underwent a structural brain MRI for the evaluation of cerebral small vessel disease features. Participants in dataset 2 underwent brain 1H-MRS in the fasting state (n = 49). All subjects had fasting circulating NAA measured after an overnight fast. In dataset 1 mean age was 60 ± 8 years, mean BMI was 27.1 ± 4.5 kg/m2, ~ 30% of the population had T2D and ~ 80% of the population had prior cardiovascular disease. In dataset 2, the study participants (45 ± 9 years of age) included morbidly obese individuals (N = 25) and their lean controls (N = 24), none of whom had prior cardiovascular disease. The characteristics of the study participants are given in Tables 1 and 2. The study protocols were approved by the local ethics committee of Maastricht University Medical Centre (NL31329.068.10) and the Ministry of Health, Welfare, and Sports of the Netherlands (Permit 131088-105234-PG), and the local ethics committee of Turku University Hospital, respectively. Written informed consent was obtained from all volunteers before their participation in the studies. In both studies, all procedures performed were in accordance with the Helsinki Declaration.
Brain MRI and 1H-MRS protocols
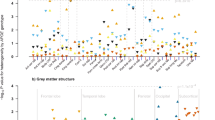
In dataset 1, brain MRI was performed on a 3 T MRI scanner (Siemens Magnetom Prisma-fit Syngo MR D13D, Erlangen, Germany). Data on four cerebral small vessel disease features are available, i.e., total brain parenchyma volume, white matter hyperintensity volume, and presence of lacunar infarcts and cerebral microbleeds35. (Supplementary Information). In dataset 2, subjects were studied with a 3 T MRI scanner (Siemens Magnetom Skyra fit (Siemens Healthcare, Erlangen, Germany). Subjects were positioned supine inside the MRI and the head was immobilized with foam inserts on top of a radiofrequency probe. For signal acquisition, a 20-channel Head/Neck coil was used. After taking brain morphology scans, 1H-MRS spectrum was measured from a 20 × 30 × 30 mm voxel in the occipital lobe using PRESS sequence with 176 water-suppressed signal averages and 4 signal averages without water suppression. The occipital lobe was selected due to its reliability for spectral acquisition36. The voxel was carefully placed in the center of the occipital lobe, making sure that the margins of the voxel would not be close to the skull or the cerebellum (Fig. 1a). The measurement lasted 10 min. The sequence parameters were repetition time = 3000, echo time = 35 ms, bandwidth = 2000 Hz. Water suppression was performed using the CHESS method. Figure 1b shows a representative example of a fitted spectrum.
Quantification of 1H-MRS data
Eddy current corrected 1H-MRS data were analyzed using LCModel (Version 6.3-1N)37. Water scaling was performed and the analysis window was set from 4.0 to 0.2 ppm. A simulated basis set38 was used on the acquired data to obtain the brain total NAA concentrations. Brain tissue fractions (gray matter, white matter, cerebrospinal fluid) were derived for more accurate estimation of the concentration of water which was used as an internal reference39. Subsequently, the metabolite/water ratio was converted to concentrations in mmol/L.
Plasma NAA measurement
Plasma NAA concentrations were measured by HPLC–MS–MS and sample derivatization; the method has been validated in compliance with EMA guidelines and previously described in detail by Campi et al.40. In brief, plasma samples were thawed at room temperature and a 100 μL aliquot was added with 300 μL of a freshly prepared daily precipitation solution containing acetonitrile, formic acid 1% (V%), and internal standard. The resulting suspensions were vortexed, centrifuged, and 300 μL of the supernatants dried under a gentle stream of nitrogen. The dry residues were submitted to Fischer esterification reaction, and drying again under a nitrogen stream. The dry residues were reconstituted with 100 μL of ACN/H2O (20/80; V/V), and put into a 96 wells plate for the HPLC–MS–MS analysis. The injection volume of samples and calibrators was 5 μL. Calibrators were prepared by serial dilution in water at the following concentrations: 0.7813 (L1), 1.563 (L2), 3.125 (L3), 6.250 (L4), 12.50 (L5), 25.00 (L6), 50.00 (L7), 100.0 ng/mL (L8), 200.0 ng/mL (L9) to build calibration curve. The equation of that curve was used to convert the readings (Area analyte/Area Internal Standard) of the unknown samples into concentration.
Cognitive function
Cognitive performance was assessed in dataset 1 using a concise neuropsychological test battery34. Standardized test scores were calculated for the three cognitive domains: verbal memory, processing speed and executive function. We evaluated verbal memory with the Verbal Learning Test; processing speed with the Stroop Color-Word Test Part I and II, Concept Shifting Test Part A and B, and Letter-Digit Substitution Test; and executive function with the Stroop Color-Word Test Part III and Concept Shifting Test Part C41. These tests are presented in more detail in the Supplementary Information.
Statistical analysis
Data are given as mean ± SD. Because plasma NAA concentrations had a skewed distribution, they were summarized as median [interquartile range IQR], and logarithmically transformed for use in parametric statistical tests. Group values were compared by the Mann–Whitney U-test. Linear and logistic regression analysis were used to evaluate the association between log-transformed circulating NAA levels (main exposure) and central NAA levels (main outcome dataset 2), cerebral small vessel disease features and domain-specific cognitive performance (main outcomes dataset 1). Data analyses were performed using JMP® 7.0 (SAS Institute Inc., 2007); a p value < 0.05 was considered statistically significant in two-sided tests.
Results
Circulating NAA, cognitive function and cerebral small vessel disease
In Dataset 1, we evaluated whether circulating NAA levels correlate with total brain parenchyma volume, white matter hyperintensity volume, and presence of lacunes and microbleeds using three statistical models: unadjusted, and adjusted for several potential confounders. The adjusted models showed that circulating NAA did not correlate with any feature of cerebral small vessel disease (Table 3). Similarly, whereas in univariate analysis circulating NAA levels were associated inversely with the speed in information processing and the executive function score, these associations were lost after accounting for confounders (Table 4). We then divided the group into quartiles of circulating NAA (Table 5). Because we noticed that cognitive functions and the features of cerebral small vessel disease varied slightly across quartiles of circulating NAA, we also tested whether there were any significant correlations between circulating NAA and these features when comparing the lowest to the higher quartiles of NAA. Once again, we did not detect any significant correlations between circulating NAA and the features of cognitive function and cerebral small vessel disease (Tables 6, 7).
In the whole dataset, circulating NAA levels correlated positively with age (rho = 0.16, p < 0.0001), and inversely with BMI (rho = − 0.18, p < 0.0001) and HbA1c (rho = − 0.12, p = 0.001).
Central and circulating NAA
Dataset 2 included obese and lean individuals, who were well-matched for sex and age. Along with marked differences in adiposity measures, obese subjects also had higher prevalence of impaired glucose tolerance and T2D and thus higher HbA1c, higher mean arterial blood pressure, C-reactive protein, and triglycerides (Table 2). Central NAA and total NAA concentrations were significantly lower in the patients with obesity compared to the lean controls (Table 2). On the contrary, circulating NAA levels were not different between the two groups.
In univariate analysis, there was no significant correlation between central NAA or central total NAA and circulating NAA concentrations (Fig. 2).
Discussion
Despite recent, major progress in the neurological field and a better understanding of the role of the brain in the orchestration of whole-body metabolism42,43,44,45,46,47,48,49, much remains to be investigated in human brain metabolism. Molecules that originate primarily from the brain but spill over into the blood in quantifiable levels represent good candidate biomarkers for assessing cerebral function. One such molecule is NAA, an aminoacid mainly produced in neurons and secondarily in astrocytes after hydrolysis of the glutamate moiety from NAAG. Several lines of research have shown that cerebral NAA levels are decreased in several cerebral diseases comprising Alzheimer’s, Parkinson and Huntngton’s disease8,11,15. However, thus far it was not known whether circulating NAA levels reflect brain function. Our study shows that, in a large sample of subjects from the general population, circulating NAA does not correlate with any features of cerebral small vessel disease or cognitive function.
First, to test whether circulating NAA reflects brain function, we chose to study functional (cognition) and structural (small vessel disease) characteristics that have been previously linked with central NAA levels. There is a large body of evidence showing that central metabolites associate with cognitive function in both healthy subjects and in patients with neurological damage, even though other studies have shown no correlation between central NAA levels and cognition (as reviewed by Ross and Sachdev26). In healthy subjects, central NAA has been positively related to performance on intelligence tests working memory tasks in children and adults50,51,52, and with executive-attentional cognitive tasks and facial recognition in the elderly53,54,55. As plausible explanations for these findings, it has been proposed that higher central NAA may be associated with greater neuronal density, better metabolic efficiency, or increased synaptic connectivity (i.e. the ensemble of direct chemical and electrical connections between neurons56)26. However, the existing literature on the relationship between central NAA levels and cognition is not unanimous. For instance, Patel and colleagues reviewed 14 studies and reported that the average correlation between NAA and various measures of cognitive ability was 0.39, with a relatively high standard deviation of 0.23, suggesting inhomogeneous effects across studies27.
Cerebral small vessel disease is a major cause of cognitive impairment and dementia31. In a brain 1H-MRS study, Nitkunan et al. showed that, in subjects with cerebral small vessel disease, central NAA concentrations are reduced and negatively correlated to the lesion volume28.
In the current study circulating NAA was associated neither with cognitive function nor with features of small vessel disease. On the contrary, we confirmed the previously described correlations of circulating NAA with age, BMI and HbA1c57. Based on these findings in a large number of subjects, we hypothesized that the lack of association between circulating NAA and functional or structural characteristics of the brain could be explained by assuming that circulating NAA does not reflect central NAA levels.
Indeed, in the small sample of subjects who were studied with brain 1H-MRS, contemporaneous assessment of central and circulating NAA levels showed that the two measurements were unrelated (Fig. 2). Even though our study cannot assess the reason(s) for the discrepancy between central and circulating NAA values, there are several steps where this mismatch could occur, from the spillover from the brain into the bloodstream or from differences in NAA removal from the plasma among different subjects (or a combination thereof)4.
Strengths of the present study are the large number of subjects studied in dataset 1 and the direct comparison of central and peripheral NAA in the same subjects in dataset 2. Our study also has limitations. First, the sample of subjects in whom brain 1H-MRS was performed was rather small; future studies are needed to replicate the present findings. In addition, 1H-MRS was acquired only from the occipital lobe, thus generalization of our 1H-MRS findings to the whole-brain level should be made with caution. Finally, in dataset 1 the acquisition of the fasting samples for the measurement of circulating NAA was not synchronous with the MRI or the cognitive function evaluation (there was a time lag of weeks). Considering the dynamic nature of central NAA, this might have affected the present results; on the other hand, it seems implausible that either cognitive function or cerebral small vessel disease would change in such a short time period in otherwise neurologically healthy subjects.
In conclusion, the current study shows that circulating NAA does not predict presence or severity of small vessel disease or cognitive dysfunction in a large dataset of neurologically healthy individuals. Thus, alterations in circulating NAA levels do not seem of relevant clinical use in predicting—at an early stage—either cognitive decline, or cerebral SVD. However, since the present datasets consisted only of subjects with no previous and current neurologic diagnoses, further research is necessary to assess whether circulating NAA concentrations reflect central NAA levels and/or cognition in patients. Also, whether circulating NAA is merely a “spillover” marker or whether it elicits any cross-talk effects between the brain and the periphery warrants investigation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASPA:
-
Aspartoacylase
- BMI:
-
Body mass index
- CNS:
-
Central nervous system
- HPLC:
-
High-performance liquid chromatography
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- NAA:
-
N-acetylaspartate
- NAAG:
-
N-acetyl aspartate glutamate
- SVD:
-
Small vessel disease
- T2D:
-
Type 2 diabetes
- TNAA:
-
Total NAA
References
Baslow, M. H. N-Acetylaspartate and N-Acetylaspartylglutamate in Handbook of Neurochemistry and Molecular Neurobiology (Springer ScienceþBusiness Media, LLC, 2007).
Baslow, M. H. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: Role in glial cell-specific signaling. J. Neurochem. 75, 453–459 (2000).
Baslow, M. H. N-acetylaspartate in the vertebrate brain: Metabolism and function. Neurochem. Res. 28, 941–953 (2003).
Moffett, J. R., Ross, B., Arun, P., Madhavarao, C. N. & Namboodiri, A. M. A. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 (2007).
Baslow, M. H. Evidence supporting a role for N-acetyl-L-aspartate as a molecular water pump in myelinated neurons in the central nervous system. An analytical review. Neurochem. Int. 40, 295–300 (2002).
Clark, J. B. N-acetyl aspartate: A marker for neuronal loss or mitochondrial dysfunction. Dev. Neurosci. 20, 271–276 (1998).
Kirov, I. I. et al. Global brain volume and N-acetyl-aspartate decline over seven decades of normal aging. Neurobiol. Aging 98, 42–51 (2021).
Zhu, X. et al. Effects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imaging. Alzheimer Dis. Assoc. Disord. 20, 77–85 (2006).
Wong, D. et al. Reduced hippocampal glutamate and posterior cingulate N-acetyl aspartate in mild cognitive impairment and Alzheimer’s disease is associated with episodic memory performance and white matter integrity in the cingulum: A pilot study. J. Alzheimers. Dis. 73, 1385–1405 (2020).
Song, T. et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 72, 101503 (2021).
Tsai, G. & Coyle, J. T. N-acetylaspartate in neuropsychiatric disorders. Prog. Neurobiol. 46, 531–540 (1995).
Sturrock, A. et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology 75, 1702–1710 (2010).
Teunissen, C. E. et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 72, 1322–1329 (2009).
Pardini, M. et al. The association between retinal nerve fibre layer thickness and N-acetyl aspartate levels in multiple sclerosis brain normal-appearing white matter: A longitudinal study using magnetic resonance spectroscopy and optical coherence tomography. Eur. J. Neurol. 23, 1769–1774 (2016).
Winsberg, M. E. et al. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol. Psychiatry 47, 475–481 (2000).
Reynolds, L. M. & Reynolds, G. P. Differential regional N-acetylaspartate deficits in postmortem brain in schizophrenia, bipolar disorder and major depressive disorder. J. Psychiatr. Res. 45, 54–59 (2011).
Croarkin, P. E. et al. N-acetylaspartate normalization in bipolar depression after lamotrigine treatment. Bipolar Disord. 17, 450–457 (2015).
Molina, V. et al. N-acetyl-aspartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease duration. Schizophr. Res. 73, 209–219 (2005).
Whitehurst, T. S. et al. Proton magnetic resonance spectroscopy of N-acetyl aspartate in chronic schizophrenia, first episode of psychosis and high-risk of psychosis: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 119, 255–267 (2020).
Wu, G.-Y. et al. Changes in cerebral metabolites in type 2 diabetes mellitus: A meta-analysis of proton magnetic resonance spectroscopy. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 45, 9–13 (2017).
Namboodiri, A. M. A. et al. Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol. Cell. Endocrinol. 252, 216–223 (2006).
Baslow, M. H., Hrabe, J. & Guilfoyle, D. N. Dynamic relationship between neurostimulation and N-acetylaspartate metabolism in the human visual cortex: Evidence that NAA functions as a molecular water pump during visual stimulation. J. Mol. Neurosci. 32, 235–245 (2007).
Ellis, C. M. et al. Changes in putamen N-acetylaspartate and choline ratios in untreated and levodopa-treated Parkinson’s disease: A proton magnetic resonance spectroscopy study. Neurology 49, 438–444 (1997).
Gonul, A. S. et al. The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 120–125 (2006).
Bartsch, A. J. et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130, 36–47 (2007).
Ross, A. J. & Sachdev, P. S. Magnetic resonance spectroscopy in cognitive research. Brain Res. Brain Res. Rev. 44, 83–102 (2004).
Patel, T., Blyth, J. C., Griffiths, G., Kelly, D. & Talcott, J. B. Moderate relationships between NAA and cognitive ability in healthy adults: Implications for cognitive spectroscopy. Front. Hum. Neurosci. 8, 39 (2014).
Nitkunan, A. et al. Reduced N-acetylaspartate is consistent with axonal dysfunction in cerebral small vessel disease. NMR Biomed. 22, 285–291 (2009).
McLean, M. A., Woermann, F. G., Barker, G. J. & Duncan, J. S. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magn. Reson. Med. 44, 401–411 (2000).
Li, Q. et al. Cerebral small vessel disease. Cell Transplant. 27, 1711–1722 (2018).
Grau-Olivares, M. & Arboix, A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: A forerunner of vascular dementia?. Expert Rev. Neurother. 9, 1201–1217 (2009).
de Leeuw, F. E. et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 70, 9–14 (2001).
Mersmann, N. et al. Aspartoacylase-lacZ knockin mice: An engineered model of Canavan disease. PLoS ONE 6, e20336 (2011).
Schram, M. T. et al. The Maastricht Study: An extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur. J. Epidemiol. 29, 439–451 (2014).
van Agtmaal, M. J. M. et al. Prediabetes is associated with structural brain abnormalities: The Maastricht study. Diabetes Care 41, 2535–2543 (2018).
Hwang, J. J. et al. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2, e95913 (2017).
Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 (1993).
Smith, S. A., Levante, T. O., Meier, B. H. & Ernst, R. R. Computer simulations in magnetic resonance. An object oriented programming approach. J. Magn. Reson. 106a, 75–105 (1994).
Quadrelli, S., Mountford, C. & Ramadan, S. Hitchhiker’s guide to voxel segmentation for partial volume correction of in vivo magnetic resonance spectroscopy. Magn. Reson. Insights 9, 1–8 (2016).
Campi, B. et al. Plasma N-acetylaspartate: Development and validation of a quantitative assay based on HPLC-MS-MS and sample derivatization. Clin. Chim. Acta. 508, 146–153 (2020).
Geijselaers, S. L. C. et al. The role of hyperglycemia, insulin resistance, and blood pressure in diabetes-associated differences in cognitive performance—The Maastricht study. Diabetes Care 40, 1537–1547 (2017).
Rebelos, E., Nummenmaa, L., Dadson, P., Latva-Rasku, A. & Nuutila, P. Brain insulin sensitivity is linked to body fat distribution-the positron emission tomography perspective. Eur. J. Nucl. Med. Mol. Imaging https://doi.org/10.1007/s00259-020-05064-7 (2020).
Rebelos, E., Rinne, J. O., Nuutila, P. & Ekblad, L. L. Brain glucose metabolism in health, obesity, and cognitive decline—Does insulin have anything to do with it? A narrative review. J. Clin. Med. 10, 1532 (2021).
Rebelos, E. et al. Brain glucose uptake is associated with endogenous glucose production in obese patients before and after bariatric surgery and predicts metabolic outcome at follow-up. Diabetes Obes. Metab. 21, 218–226 (2019).
Rebelos, E. et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: A large-scale PET cohort. Diabetes Care 44, 1–7 (2021).
Rebelos, E. et al. Brain free fatty acid uptake is elevated in morbid obesity, and is irreversible 6 months after bariatric surgery: A positron emission tomography study. Diabetes Obes. Metab. 22, 1074–1082 (2020).
Boersma, G. J. et al. Altered glucose uptake in muscle, visceral adipose tissue, and brain predict whole-body insulin resistance and may contribute to the development of type 2 diabetes: A combined PET/MR study. Horm. Metab. Res. 50, 627–639 (2018).
Heni, M. et al. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66, 1797–1806 (2017).
Rebelos, E. et al. Brain substrate metabolism and β-cell function in humans: A positron emission tomography study. Endocrinol. diabetes Metab. 3, e00136 (2020).
Yeo, R. A., Hill, D., Campbell, R., Vigil, J. & Brooks, W. M. Developmental instability and working memory ability in children: A magnetic resonance spectroscopy investigation. Dev. Neuropsychol. 17, 143–159 (2000).
Jung, R. E. et al. Biochemical markers of intelligence: A proton MR spectroscopy study of normal human brain. Proc. Biol. Sci. 266, 1375–1379 (1999).
Jung, R. E., Yeo, R. A., Chiulli, S. J., Sibbitt, W. L. J. & Brooks, W. M. Myths of neuropsychology: Intelligence, neurometabolism, and cognitive ability. Clin. Neuropsychol. 14, 535–545 (2000).
Ferguson, K. J. et al. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain 125, 2743–2749 (2002).
Urenjak, J., Williams, S. R., Gadian, D. G. & Noble, M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J. Neurochem. 59, 55–61 (1992).
Pfefferbaum, A., Adalsteinsson, E., Spielman, D., Sullivan, E. V. & Lim, K. O. In vivo brain concentrations of N-acetyl compounds, creatine, and choline in Alzheimer disease. Arch. Gen. Psychiatry 56, 185–192 (1999).
Jirsa, V. Synaptic connectivity in neural population models. In Encyclopedia of Computational Neuroscience (eds Jaeger, D. & Jung, R.) 1–3 (Springer, 2013). https://doi.org/10.1007/978-1-4614-7320-6_78-1.
Daniele, G. et al. Plasma N-acetylaspartate is related to age, obesity, and glucose metabolism: Effects of antidiabetic treatment and bariatric surgery. Front. Endocrinol. (Lausanne) 11, 216 (2020).
Acknowledgements
We thank Simona Baldi for assisting in the lab work. The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (Grant 31O.041), Stichting De Weijerhorst (Maastricht, The Netherlands), the Pearl String Initiative Diabetes (Amsterdam, The Netherlands), the Cardiovascular Center (CVC, Maastricht, the Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, The Netherlands), CAPHRI Care and Public Health Research Institute (Maastricht, The Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, The Netherlands), Health Foundation Limburg (Maastricht, The Netherlands), and by unrestricted Grants from Janssen-Cilag B.V. (Tilburg, The Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), and Sanofi-Aventis Netherlands B.V. (Gouda, the Netherlands). PN and ER report funding from the European Foundation for the Study of Diabetes (EFSD).
Author information
Authors and Affiliations
Contributions
E.R. and G.D. drafted the manuscript. B.C. and A.S. performed the LC–MS. E.S. and K.K. acquired the 1H-MRS data. K.K. and J.I. analyzed the 1H-MRS data. W.H.B., J.J., P.C.D., S.K., B.E.dG., C.S. acquired the data of Dataset 1. P.N. and T.T.vS. provided clinical samples. E.F. conceived the study design and critically revised the manuscript. E.F. is also the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors provided critical feedback on the manuscript at previous stages and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rebelos, E., Daniele, G., Campi, B. et al. Circulating N-Acetylaspartate does not track brain NAA concentrations, cognitive function or features of small vessel disease in humans. Sci Rep 12, 11530 (2022). https://doi.org/10.1038/s41598-022-15670-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15670-0
This article is cited by
-
Using mass spectrometry imaging to map fluxes quantitatively in the tumor ecosystem
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.