Abstract
One of the tomato’s acutely devastating diseases is Alternaria leaf spot, lowering worldwide tomato production. In this study, one fungal isolate was isolated from tomatoes and was assigned to Alternaria alternata TAA-05 upon morphological and molecular analysis of the ITS region and 18SrRNA, endoPG, Alt a1, and gapdh genes. Also, Urtica dioica and Dodonaea viscosa methanol leaf extracts (MLEs) were utilized as antifungal agents in vitro and compared to Ridomil, a reference chemical fungicide. The in vitro antifungal activity results revealed that Ridomil (2000 µg/mL) showed the highest fungal growth inhibition (FGI) against A. alternata (96.29%). Moderate activity was found against A. alternata by D. viscosa and U. dioica MLEs (2000 µg/mL), with an FGI value of 56.67 and 54.81%, respectively. The abundance of flavonoid and phenolic components were identified by HPLC analysis in the two plant extracts. The flavonoid compounds, including hesperidin, quercetin, and rutin were identified using HPLC in D. viscosa MLE with concentrations of 11.56, 10.04, and 5.14 µg/mL of extract and in U. dioica MLE with concentrations of 12.45, 9.21, and 5.23 µg/mL, respectively. α-Tocopherol and syringic acid, were also identified in D. viscosa MLE with concentrations of 26.13 and 13.69 µg/mL, and in U. dioica MLE, with values of 21.12 and 18.33 µg/mL, respectively. Finally, the bioactivity of plant extracts suggests that they play a crucial role as antifungal agents against A. alternata. Some phenolic chemicals, including coumaric acid, caffeic acid, ferulic acid, and α-tocopherol, have shown that they may be utilized as environmentally friendly fungicidal compounds.
Similar content being viewed by others
Introduction
Tomato (Solanum lycopersicum L.) is one of the world’s key important vegetable crops1,2. Fresh tomatoes suit the human body's fundamental nutritional needs as a functional food since they include a consistent amount of minerals and antioxidant chemicals like polyphenols3. Globally, tomato is the world’s largest produced vegetable crop with an annual creation of 182.26 million metric tons4. Tomato is sensitive to various diseases caused by bacteria, viruses, nematodes, fungi, and other pathogens5. Diseases induced by phytopathogenic fungi cause significant crop losses in tomato farming, accounting for up to 100% of the crop6.
Alternaria species continue to be an increasing hazard to a wide variety of crops around the world, producing a variety of illnesses. Alternaria disease produces significant yield losses and decreases the economic value of agricultural plants in present production methods7. Alternaria infections cause necrotic areas in circular rings with a yellow chlorotic halo, influencing plants by reducing the photosynthetic surface8. A. solani produces early blight disease in various solanaceous agricultural plant hosts9,10. Indeed, the presence of A. alternata tomato is often linked to the creation of mycotoxins including alternariol, altertoxin-I, II, alternariol methyl ether, and tenuazonic acid, which are all harmful to animal and human health11.
In Egypt, the symptoms of A. alternata leaf spot and blight have been commonly observed on tomatoes and potatoes12. Alternaria black spots are brown or black patches on leaves, stems, or pods that expand in warm, humid environments, reduce the photosynthetic area, defoliation, and accelerate the senescence13. Infection caused severe defoliation and significant production losses before flowering under ideal conditions14.
PCR was previously used to detect Alternaria spp. in tomatoes based on ribosomal internal transcribed spacer (ITS) DNA sequence analysis15. Meanwhile, ITS region sequencing revealed that A. solani and A. alternata species were the two most harmful isolated pathogens from tomato16. The two approaches addressed the inside transcribed spacer sections ITS1 and ITS2 of the rRNA gene, as well as a positive magnification control based on the 18S rRNA gene, using Alternaria-specific primers and probes17. Several investigators used other genes like endoPG, Alt a1, and gapdh for more accurate identification of Alternaria species18,19.
Chemical management of Alternaria infections improves crop output, but is ineffective and non-discriminatory in human and animal health and damages the environment20,21. As a result, numerous research has recommended employing ecologically friendly substances to counteract the spread of Alternaria diseases, such as biocontrol agents or extracts. Urtica dioica L. (popular nettle or sting nettle) plants are deemed medicinal herbs because of their pharmacological and natural properties22,23. The antifungal activity against A. alternata by all parts of the plant can be medicinally used; the leaves offer the most potent antioxidant, antibacterial, and anti-inflammatory properties24,25.
Caffeic and chlorogenic acids, β-sitosterol, and stigmasterol were detected in the extracts of U. urens and U. dioica26. Some other chemical compounds, such as volatile chemicals, lectins, terpenes, sterols, fatty acids, proteins, polysaccharides, vitamins, phenolics, and flavonoids were also detected in U. dioica extracts23,27. The ethyl acetate fraction extract of U. dioica had the most potent antimicrobial activity against Aeromonas hydrophila, Salmonella typhi, Staphylococcus aureus, Bacillus cereus, and Escherichia coli, with its highest content of polyphenols (48.3 mg GAE/gdw)28. Staphylococcus aureus, B. subtilis, and Salmonella spp. exhibited the greatest vulnerability to the antibacterial activity of U. dioica extracts29. Many herbal plants such as U. dioica are natural causes of mixtures with antibacterial, antifungal, and antioxidant effects27.
Dodonaea viscosa extracts showed antifungal activity against the three diseases that target commercial crops (A. solani, Rhizoctonia solani, and Macrophomina phaseolina)30. According to phytochemical screening, alkaloids, flavonoids, fixed oil and fat, steroids, phenolics, saponins, tannins, gums, mucillages, carbohydrates, reducing sugar, and glycosides were detected in D. viscosa extracts31,32. D. viscosa has antidiabetic, antimicrobial, insecticidal, antioxidant, cytotoxic, antifertility, wound, anti-inflammatory, analgesic, anti-ulcer, antispasmodic, anti-diarrheal, and detoxifying effects, according to pharmacological studies31. The HPLC investigation revealed the polyphenolic components i.e., rutin, gallic acid, catechin, caffeic acid, myricetin, and apigenin in various solvent extracts from D. viscosa33.
The study aimed to assess the ability of D. viscosa, and U. Dioica methanolic leaf extracts as an eco-friendly antifungal to suppress the A. alternata fungus.
Materials and methods
Isolation and purification of the fungal pathogen
This study has complied with relevant institutional, national, and international guidelines and legislation. This study does not contain any studies with human participants or animals performed by any of the authors, where one fungal isolate was retrieved from leaf spot symptoms on tomato plants at Rashid, El Behira governorate, Egypt. The isolation process was started with slicing the symptomatic leaves into small pieces (approx. 5 × 5 mm) and then surface-sterilized for 2 min with sodium hypochlorite solution (2%), ensued by excessive rinsing (2–3 times) with sterile dH2O. The small parts were put onto potato dextrose agar (PDA) media plates and then incubated at 25 ± 2 °C for a week. After that, the single spore method was used to obtain pure fungal culture, which was maintained in a fridge at 4 °C on PDA slants for additional studies.
Pathogen’s recognition
Cultural and morphological features
A pathogen slide of the fungal isolate from pure cultures was produced and examined under a light microscope for identification. Isolated fungus morphological and cultural features were documented and compared to the standard keys to determine its identification34.
Molecular characterization of Alternaria isolate
Total genomic DNA was isolated from the mycelia of Alternaria isolate using CTAB (hexacetyl trimethyl ammonium bromide; Sigma-Aldrich, Germany) method35. The molecular identification of A. alternata using specific primers based on the internal transcripted spacer region (ITS) and small subunit ribosomal RNA gene (18SrRNA) gene as well as endoPG, Alt a1 and gapdh genes.
The molecular identification was based on the internal transcripted spacer region (ITS) and small subunit ribosomal RNA gene (18SrRNA) gene. The ITS region was amplified with primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′), ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), and the 18SrRNA gene was amplified by the two primers NS1(5′-GTAGTCATATGCTTGTCTC-3′) and NS8 (5′-TCCGCAGGTTCACCTACGGA-3′)36. While the endoPG gene region was amplified using the primers PG3 (5′-TACCATGGTTCTTTCCGA-3′) and PG2b (5′-GAGAATTCRCARTCRTCYTGRTT-3′)37, Aalt For (5′-GTGCCTTCCCCCAAGGTCTCCG-3′) and Aalt Rev (5′-CGGAAACGAGGTGGTTCAGGTC-3′) was used for amplification of Alt a1 gene region38, the last specific gene (gapdh) was amplified using two primers gab1(5′-CAACGGCTTCGGTCGCATTG-3′) and gpd2 (5′-GCCAAGCAGTTGGTTGTGC-3′) as described by Berbee et al.39. PCR reactions were done using 0.5 µL of each primer pair, 12.5 µL of 2 × Taq Ready Mix (Enzynomics Inc., Daejeon, Korea), and 1 µL of template DNA; then, the molecular grade water was up to a capacity of 25 µL. In a thermal cycler (Techne Prime, Cole-Parmer, Staffordshire, UK), a PCR program was performed as follows: pre-denaturation of 95 °C for 4 min, 35 cycles of three stages, 94 °C/45 s, annealing according to gene primer as described in references provided above, and 72 °C/1 min, and a final elongation step at 72 °C/5 min.
Sequencing and phylogenetic analysis
The obtained ITS (~ 600 bp), 18SrRNA (~ 1500 bp), endoPG (~ 500 bp), Alt a1 (~ 500 bp), and gapdh (~ 600 bp) amplicons of the selected isolate were delivered for sequencing (Macrogen Co, Seoul, Korea). The obtained sequences were equated to those in GenBank using the BLASTn tool. The alignment of ITS and 18SrRNA of Alternaria sequences were used to establish the phylogenetic trees compared with GenBank accessioned Alternaria isolates. The alignment of endoPG, Alt a1, and gapdh genes of Alternaria sequences was used to confirm the identification of Alternaria isolate and deposited in NCBI GenBank. The evolutionary history was implied using the highest parsimony method40.
In vitro-evaluation of plant extracts versus the leaf spot pathogen equated to the chemical fungicide
Plant leaves from U. dioica and D. viscosa were gathered in Alexandria, Egypt. Under laboratory conditions, the samples were air-dried and pulverized in a tiny laboratory mill. Approximately 50 g of U. dioica and D. viscosa leaf samples were extracted for 3 days at room temperature with 200 mL of MeOH, then filtered using Whatman No. 1 filter paper41. The solvent was then evaporated, and the extracts were concentrated using a rotary evaporator at 45 °C under a vacuum. Furthermore, the crude extracts were kept in sealed vials at 4 °C until they were used for in vitro antimicrobial activity screening41. By dissolving the extract in dimethyl sulfoxide (DMSO 99.99%), the extracts and the chemical fungicide Ridomil (Metalaxyl-M (Mefenoxam)-Mancozeb, Germany) were prepared at concentrations of 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, 2000 µg/mL and tested against the growth of the isolated fungus using the food poison technique42. A 5 mm plug of the pure isolate was obtained from the borders of the pathogen's actively developing culture and put into each Petri plate. The Petri plates were then incubated at 25 ± 2 °C for 2 h. When the hyphal development in the control treatment was complete, mycelial growth was measured. Each treatment was given three times in total. The radial mycelial growth of the fungus was measured against both extracts, and the results were translated into the FGI % using a formula created by Naz et al.43 and were subjected to statistical analysis.
HPLC analysis of phenolic and flavonoid components
The phenolic and flavonoid components from the methanol extracts of U. dioica and D. viscosa leaves were categorized by HPLC (Agilent 1100, USA). A binary LC pump, a UV/Vis detector, and a C18 column (125 mm, 4.60 mm, 5 m) make up this apparatus. The Agilent ChemStation was used to acquire and analyze chromatograms. A gradient mobile phase of two solvents—Solvent A (MeOH) and Solvent B [Acetic acid in H2O (1:25)]—was used to separate phenolic acids. The gradient program began with 100% B and remained there for 3 min. Thin was followed by 5 min of 50% eluent A, followed by 2 min of 80% eluent A, followed by 5 min of 50% eluent A, followed by 2 min of 80% eluent A, followed by 5 min of 50% eluent A, followed by 5 min of 50% eluent A, followed by 5 min of detection wavelength at 250 nm. As a result, the phenolic components were arranged in order to authenticate standard components by this mobile phase44.
Antifungal assay of phenolic acids and mixtures
Based on the HPLC analysis of the studied extracts, the highest and more available phenolic acid compounds identified in both extracts were used for the bioassay against the growth of the isolated fungus by poisoned technique in the lab, as described previously in Sect. 2.4. The individual compounds and their combination/mixture with their exact percentage from the HPLC analysis were used against the growth of identified fungus.
Statistical analysis
The experiment was statically analyzed by CoStat program ver., 6.303 (CoHort software, Monterey, CA, USA). A completely randomized design45 was performed and the means were equated by Duncan’s multiple range test46. The data were expressed as means ± SD values and were deemed statistically significant when p ≤ 0.05.
Results
Isolation and identification of the fungal pathogen
The symptoms of tomato leaf spot on field-infected plants yielded the same fungus strain from 20 leaves that showed the same signs. To identify the fungal isolate, morphological and molecular characterization were carried out.
Cultural and morphological traits
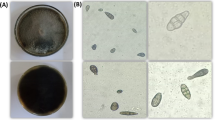
The PDA media plate fungal growth was smooth and deep brown or olivaceous brown (Fig. 1). According to microscopic features of the pathogenic fungus, the conidia were brown to olivaceous brown, solitary, straight, or ellipsoidal tapering, and possessed transverse and longitudinal septate. All these phenotypic traits aided in the initial identification of Alternaria sp.
Molecular genes analyses
The amplified sequences of ITS, 18SrRNA, endoPG, Alt a1, and gapdh genes of TAA-05 isolate were deposited in GenBank under accession numbers OL673807, OL674053, OP311598, OP311599, and OP311600, respectively. According to sequences data retrieved from aligning endoPG, Alt a1, and gapdh genes, the blasted results confirmed the identity of the fungal isolate as A. alternata isolate TAA_05 (Supplementary material).
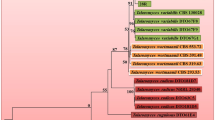
Our isolate TAA-05 showed the maximum homogeneity of 100% with A. alternata fungus, aligning different gene sequences with matched sequences in GenBank. The ITS phylogenetic tree showed a maximum nucleotide sequence similarity (100%) with A. alternata isolates from India (MH084265), China (MT093259), and Iraq (MF099865), a minimum nucleotide sequence similarity (99%) with A. alternata from China (JX406501), Egypt (MW850355) and A. tenuissima from China (JX406499) as shown in Fig. 2.
The 18SrRNA gene phylogenetic tree showed a maximum nucleotide sequence similarity (100%) with A. alternata isolates from India (KX494864), China (HM165489), and Portugal (MF072541). The minimum nucleotide sequence similarity (99%) with A. alternata from Saudi Arabia (MZ314132) and (MZ314135) is portrayed in Fig. 3.
In vitro-evaluation of plant extracts versus A. alternata
The data tabulated in Table 1 show the extremely substantial impacts of U. dioica and D. viscosa methanolic leaf extracts (MLEs) against the growth of A. alternata isolate compared to the fungicide Ridomil as positive chemical control. At the level of concentrations from 100 to 2000 µg/mL, the inhibition percentages of A. alternata growth as affected by U. dioica MLE, D. viscosa MLE, and Ridomil fungicide were ranged between 38.89–54.81, 34.44–56.67, and 66.67–96.29%, respectively (Fig. 4).
It is evident that the positive chemical control Ridomil fungicide was the most suppressive agent against A. alternata (TAA-05) isolate, with the highest mean growth reduction of 79.51%. Furthermore, the IFG value of the U. dioica MLE was 41.64%. With mean growth reduction values of 36.57%, the D. viscosa MLE was shown to have moderate activity (Table 1).
HPLC testing of flavonoids in U. dioica and D. viscosa extracts
The HPLC chromatograms of the flavonoids detected in U. dioica and D. viscosa MLEs are shown in Fig. 5. The abundant identified flavonoid compounds by µg/mL in U. dioica MLE were chrysoeriol (22.08), hesperidin (12.45), quercetin (9.21), and catechin (7.14). While in D. viscosa MLE were hesperidin (11.56), quercetin (10.04), and catechin (6.52) as indicated in Table 2.
HPLC analysis of phenolic components in U. dioica and D. viscosa leaf extracts
The abundant phenolic compounds identified in U. dioica MLE by µg/mL were ferulic acid (21.12), caffeic acid (19.63), ellagic acid (18.33) and pyrogallol (14.51). While in D. viscosa MLE were α-tocopherol (26.13), syringic acid (13.69), and p-coumaric acid (10.11) as presented in Table 3 and Fig. 6.
Antifungal assay of phenolic acids and mixtures
According to the HPLC analysis of phenolic compounds, percentages results; the following phenolic compounds (coumaric acid, caffeic acid, ferulic acid, and α-tocopherol) were chosen to assess their antifungal activity against A. alternata alone or in mixes (the phenolic compounds concentrations of each extract shown in Table 3 were used). All the treatments are listed in Table 4.
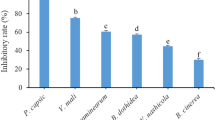
According to the previous results from HPLC analysis, Table 4 shows the significant effects of U. dioica and D. viscosa MLEs against the growth of A. alternata isolate compared to the fungicide Ridomil as a positive chemical control in vitro. It is evident that the positive chemical control Ridomil fungicide was the most suppressive agent against A. alternata (TAA-05) isolate, with the highest mean growth reduction of 83.33%. In addition, the total combinations of U. dioica MLE were found to have a high FGI value of 69.25%, followed by ferulic and caffeic acids combination of 60.74%. The highest significance was shown with ferulic and coumaric acids of D. viscosa MLE was applied with mean growth reduction values of 67.03%, followed by total combinations of 62.96%. On the other hand, the lowest significance showed with caffeic acid and α-tocopherol combinations of U. dioica MLE, and D. viscosa MLE was found to have low FGI values of 39.25 and 42.59%, respectively (Fig. 7).
Antifungal activity of Urtica dioica and Dodonaea viscosa most abundant compounds and mixtures. A = Control, B = Positive control (Ridomil), C = Caffeic acid, D = Ferulic acid, E = α-Tocopherol, F = Ferulic acid + caffeic acid, G = Ferulic acid + coumaric acid, H = Ferulic acid + α-tocopherol, I = Caffeic acid + coumaric acid, J = Caffeic acid + α-tocopherol, K = Coumaric acid + α-tocopherol, L = Coumaric acid, M = Total mixture.
Discussion
Leaf spot disease caused by A. alternata is a significant disease in tomato and potato47,48. Our study investigated one fungal isolate of the leaf spot disease pathogen of A. alternata (TAA-05). The fungal strain was identified morphologically using Simmons' morphological parameters, including outpost morphology, size, conidia shape, and conidial septation pattern49.
The attributes of this pathogen were reliable with the features depicted by the Commonwealth Mycological Institute, Kew, Surrey, England50. Therefore, the pathogen was recognized as A. Alternata51, based on the morphological characteristics, ITS region, 18SrRNA, endoPG, Alt a1, and gapdh genes amplification and sequencing. Based on earlier findings, A. alternata is the crucial reason for black spot blight symptoms in the family Solanaceae52. Additionally, molecular methods are appropriate assays for analysis, especially for scientists unfamiliar with the traditional features of fungi53. Sequencing the ITS region of ribosomal DNA, which identifies Alternaria spp. from other infections quite well49,50, is one of these approaches. The morphological characterization of the examined fungal isolates acquired in this study was corroborated by data collected from genetic studies54,55.
The antifungal effect of dual plant extracts U. dioica and D. viscosa were assayed against A. alternata growth. Both plants extract with a range of 100 to 2000 µg/mL were exhibited an antifungal possibility versus the pathogenic fungus. Previously, U. dioica extract inhibited A. alternata growth by 59.78 and 77.81%, at 1000 and 1500 µg/mL, respectively24. These findings revealed that U. dioica extract was highly effective against A. alternata and R. solani, suggesting that it might be used as a chemical additive to manage fungal infections in plants56.
D. viscosa was revealed to be highly efficient, suppressing the radial mycelial growth of A. solani (56.96%), Macrophomina phaseolina (52.06%), and Rhizoctonia solani (51.54%)57. D. viscosa extract significantly suppressed the circular mycelial growing of A. solani with a value of 56.96%30. While other study showed inhibition values of 68.48%, in in vitro and in vivo conditions58.
D. viscosa extract at 300 μL showed the maximum scavenging activity with an inhibition rate of 82.09%, observed by 200 μL with values of 81.02%, and 100 μL with a rate of 79.91%59. The most excellent extracts with > 50% reticence against A. solani were Elaeagnus angustifolia, D. viscosa, Haplophyllum perforatum, and inflorescence of Allium hirtifolium, respectively60.
The abundant identified flavonoid compounds from U. dioica MLE were chrysoeriol, hesperidin, quercetin and catechin. While in D. viscosa MLE were hesperidin, quercetin, and catechin. The abundant phenolic components identified in the U. dioica MLE were ferulic acid, caffeic acid, ellagic acid, and pyrogallol, while in D. viscosa MLE were ferulic, ellagic, syringic, and p-coumaric acids. The flavonoid compounds identified in the U. dioica MLE were rutin, naringin, quercetin, kaempferol, luteolin, hesperidin, catechin, and chrysoeriol, while all of them were identified except chrysoeriol in D. viscosa MLE. Phenolic compounds of acids of syringic, p-coumaric, caffeic, ferulic, and ellagic, as well as pyrogallol, α-tocopherol, and catechol were identified in both extracts. Flavonoids and diterpenoids are the strongest secondary metabolites formerly recognized and separated from D. viscosa61.
Several chemical groups were identified in D. viscosa extracts like alkaloids, flavonoids, phenolics, steroids, saponins, tannins, and gums31. In the several plant sections of D. viscosa, HPLC–DAD examination found considerable amounts of rutin, vanillic acid, coumaric acid, ferulic acid, gallic acid, syringic acid, cinnamic acid, gentisic acid, catechin, caffeic acid, apigenin, and myricetin33. Furthermore, cafeoil-malic acid, chlorogenic acid, ferulic acid, rutin, isoquercitrin ,and astragalin were identified in U. dioica and their antioxidant activity was reported62.
U. dioica LE collected in March had the greatest levels of polyphenolcarboxilic acids (expressed as a percentage of chlorogenic acid) and flavonoids (expressed as a percentage of rutin) (4.2295% chlorogenic acid and 0.6237% rutin)63. The p-hydroxybenzoic acid, gentisic acid, quinic acid, protocatechuic vanillic acid, caffeic acid, ferulic acid, 5-O-caffeoylquinic, esculetin, scopoletin, chrysoeriol, α-sitosterol, scopoletin are polyphenols found in extracts from U. dioica64. Polyphenols also are included isorhamnetin, kaempferol, kaempferol plastocyanins, quercitrin, glycoproteins, rutin, amentofavon, 3-O-glucoside, quercetin 3-O-glucoside, plastocyanins, quercitrin, glycoproteins, rutin, and amentofavon65. Caffeic acid, chlorogenic acid, -sitosterol, stigmasterol, rutin, and ergosterol were found in U. dioica, according to the chromatographic data26. The methanolic extracts of U. dioica showed a synergistic effect in mixture with an inhibition effect on bacteria and fungi antibiotics. Furthermore, HPTLC showed amounts of phenolics, tannin, flavonoids, carbohydrates, glycosides, and saponins were detected in U. dioica MeOH-extracts66.
Extracts containing flavonoids and phenolic compounds have been intended to suppress fungal diseases by inhibiting the germination of fungal spores44,67,68,69,70,71,72. Flavonoids inhibit many types of eukaryotic enzymes. This suppression may be due may be owing to the enzyme interactions with various portions of the flavonoid molecule, i.e., carbohydrates, phenyl rings, phenols, and benzopyrone rings73.
Using the higher abundant compounds of U. dioica and D. viscosa, MLEs showed the most elevated significance from the mixture combinations of U. dioica and the mixture of ferulic and coumaric acids of U. dioica. In contrast, mixture compounds from D. viscosa MLE followed by ferulic and coumaric compounds showed the highest significance.
Phenolic acids (ferulic and p-coumaric) were also estimated. In a synthetic medium, ferulic and p-coumaric acids had considerably increased inhibitory capability; ferulic acid was continued active against M. fructicola and A. alternata and was more effective than p-coumaric acid in controlling B. cinerea47. Phenolic compounds, like caffeic, 2,3,4-trihydroxybenzoic, p-coumaric, and protocatechuic acids achieved the highest antifungal effects and almost wholly inhibited A. alternata on early and late-ripening sweet cherries74. Furthermore, 4 phenolic components i.e., salicylic acid, catechol, trans-cinnamic acid, and p-coumaric acid, prevented the expansion of A. solani more efficiently than others75. p-Coumaric acid also totally inhibited the Fusarium growth. In contrast, ferulic acid prevented 64% of Fusarium mycelial growing at the same dose of 1000 μg/g and was particularly efficient against B. cinerea growth76,77. Vanillic and caffeic acids (0.2 mg/mL) suppressed the development and formation of aflatoxin by A. flavus and parasiticus. In comparison, 0.3 mg/mL of p-hydroxy benzoic, protocatechuic, syringic, and p-coumaric acids, and quercetin completely inhibited the molds (Supplementary information)78.
Conclusion
Herein, the methanolic leaf extracts of U. dioica and D. viscosa demonstrated a strong antifungal efficacy against the A. alternata pathogen, the causal agent of tomato leaf spot disease. Among many polyphenolic compounds that were detected in the HPLC of the two extracts, coumaric acid, caffeic acid, ferulic acid, and α-tocopherol showed potent in vitro fungicidal activity against A. alternata, either applied alone or in combination at low concentrations. Consequently, the application of polyphenolic compounds could offer an alternative way, environmentally safe and economically acceptable, for the management of plant fungal diseases. However, additional research is required to corroborate these results in the field.
Data availability
All data generated or analyzed during this study are included in this published article.
References
El-Nagar, A., Elzaawely, A. A., Taha, N. A. & Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 10, 1402 (2020).
Attia, M. S., El-Sayyad, G. S., Abd Elkodous, M. & El-Batal, A. I. The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Sci. Hortic. 266, 109289 (2020).
Htun, A., Aung, S., He, L., Liu, H. & Deng, J. First report of Alternaria blumeae causing leaf blight on tomato in Myanmar. Plant Dis. (2020).
Nakai, J. Food and agriculture organization of the united nations and the sustainable development goals. Sustain. Dev. 22 (2018).
Sahu, D., Khare, C., Singh, H. & Thakur, M. Evaluation of newer fungicide for management of early blight of tomato in Chhattisgarh. Bioscan 8, 1255–1259 (2013).
Chowdappa, P., Kumar, S. M., Lakshmi, M. J. & Upreti, K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 65, 109–117 (2013).
Gaya Karunasinghe, T., Hashil Al-Mahmooli, I., Al-Sadi, A. M. & Velazhahan, R. The effect of salt-tolerant antagonistic bacteria from tomato rhizosphere on plant growth promotion and damping-off disease suppression under salt-stress conditions. Acta Agricu. Scand. Sect. B: Soil Plant Sci. 70, 69–75 (2020).
Kokaeva, L. Y., Belosokhov, A. F., Doeva, L. Y., Skolotneva, E. S. & Elansky, S. N. Distribution of Alternaria species on blighted potato and tomato leaves in Russia. J. Plant Dis. Prot. 125, 205–212 (2018).
Song, W., Ma, X., Tan, H. & Zhou, J. Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol. Biochem. 49, 693–700 (2011).
Mohamed, A. A. et al. Ecofriendly bioagents, Parthenocissus quinquefolia, and Plectranthus neochilus extracts to control the early blight pathogen (Alternaria solani) in tomato. Agronomy 11, 911. https://doi.org/10.3390/agronomy11050911 (2021).
Habib, W. et al. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 13, 513 (2021).
El-Gazzar, N. & Ismail, A. M. The potential use of titanium, silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 27, 101708 (2020).
Conn, K., Tewari, J. & Dahiya, J. Resistance to Alternaria brassicae and phytoalexin-elicitation in rapeseed and other crucifers. Plant Sci. 56, 21–25 (1988).
Akhtar, K., Saleem, M., Asghar, M. & Haq, M. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant. Pathol. 53, 816–816 (2004).
Pavón, M. Á. et al. PCR-based assay for the detection of Alternaria species and correlation with HPLC determination of altenuene, alternariol and alternariol monomethyl ether production in tomato products. Food Control 25, 45–52 (2012).
AbdElfatah, H.-A.S., Sallam, N. M., Mohamed, M. S. & Bagy, H. M. K. Curvularia lunata as new causal pathogen of tomato early blight disease in Egypt. Mol. Biol. Rep. 48, 3001–3006 (2021).
Pavón, M. Á., González, I., Martín, R. & Lacarra, T. G. ITS-based detection and quantification of Alternaria spp. in raw and processed vegetables by real-time quantitative PCR. Food Microbiol. 32, 165–171 (2012).
Woudenberg, J. H. et al. Alternaria section Alternaria: Species, formae speciales or pathotypes?. Stud. Mycol. 82, 1–21. https://doi.org/10.1016/j.simyco.2015.07.001 (2015).
Praveen, B. et al. First report of Alternaria alternata causing Leaf blight on little millet (Panicum sumatrense) in India. Plant Dis. 105, 1202. https://doi.org/10.1094/PDIS-06-20-1373-PDN (2020).
Al-Rahmah, A., Mostafa, A., Abdel-Megeed, A., Yakout, S. & Hussein, S. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr. J. Microbiol. Res. 7, 517–524 (2013).
Xie, Y., Wang, Z., Huang, Q. & Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 108, 278–285 (2017).
Motamedi, H., Seyyednejad, M., Dehghani, F. & Hasannejad, Z. Investigation of antibacterial activity of ethanolic and methanolic extracts of Mentha pulegium L.. Zahedan J. Res. Med. Sci. 16, 55–59 (2014).
Joshi, B. C., Mukhija, M. & Kalia, A. N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 8, 201–209 (2014).
Nematollahi, S. & Sayidi, M. Antifungal activity of nettle (Urtica dioica L.) and European pennyroyal (Mentha pulegium L.) extracts on Alternaria alternata. Int. J. Mol. Clin. Microbiol. 7, 869–874 (2017).
Zarafshan, K. S. S. Stinging nettle (Urtica dioica L.): A reservoir of nutrition and bioactive components with great functional potential. Food Meas. 11, 423–433 (2017).
Nencu, I., Vlase, L., Istudor, V. & Mircea, T. Preliminary research regarding Urtica urens L. and Urtica dioica L.. Amino Acids 63, 710–715 (2015).
Asgarpanah, J. & Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L.. J. Med. Plants Res. 6, 5714–5719 (2012).
Ghaima, K. K., Hashim, N. M. & Ali, S. A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 3, 96 (2013).
Salih, N. Antibacterial effect of nettle (Urtica dioica). Al-Qadisiyah J. Vet. Med. Sci. 13, 1–6 (2014).
Aslam, A., Naz, F., Arshad, M., Qureshi, R. & Rauf, C. In vitro antifungal activity of selected medicinal plant diffusates against Alternaria solani, Rhizoctonia solani and Macrophomina phaseolina. Pak. J. Bot. 42, 2911–2919 (2010).
Al-Snafi, A. E. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J. Pharm. 7, 10–21 (2017).
Kumar, V., Chopra, A. & Srivastava, S. Available online at www. Pelagiaresearchlibrary. com. Asian J. Plant Sci. Res. 3, 120–128 (2013).
Malik, M. N. et al. Bioprospecting Dodonaea viscosa Jacq.; a traditional medicinal plant for antioxidant, cytotoxic, antidiabetic and antimicrobial potential. Arab. J. Chem. 15(3), 103688 (2022).
Booth, C. & Sutton, B. Fusarium pallidoroseum, the correct name for F. semitectum Auct. Trans. Br. Mycol. Soc. 83, 702–704 (1984).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4326. https://doi.org/10.1093/nar/8.19.4321 (1980).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc.: Guide Methods Appl. 18, 315–322 (1990).
Andrew, M., Peever, T. L. & Pryor, B. M. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia 101, 95–109. https://doi.org/10.3852/08-135 (2009).
Kordalewska, M., Brillowska-Dąbrowska, A., Jagielski, T. & Dworecka-Kaszak, B. PCR and real-time PCR assays to detect fungi of Alternaria alternata species. Acta Biochim. Polonica 62, 707–712. https://doi.org/10.18388/abp.2015_1112 (2015).
Berbee, M. L., Pirseyedi, M. & Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91, 964–977. https://doi.org/10.1080/00275514.1999.12061106 (1999).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Salem, M. Z., Mansour, M. M. & Elansary, H. O. Evaluation of the effect of inner and outer bark extracts of sugar maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 39, 136–147 (2019).
Schmitz, H. Poisoned food technique. Ind. Eng. Chem.-Anal. Ed. 2, 361–363 (1930).
Naz, F., Rauf, C., Haque, I. & Ahmad, I. Management of Rhizoctonia solani with plant diffusates and chemicals. Pakistan J. Phytopathol. (Pakistan) (2006).
Hassan, H. S. et al. Natural plant extracts and microbial antagonists to control fungal pathogens and improve the productivity of Zucchini (Cucurbita pepo L.) in vitro and in greenhouse. Horticulturae https://doi.org/10.3390/horticulturae7110470 (2021).
Gomez, K. A. & Gomez, A. A. Statistical Procedures for Agricultural Research (Wiley, 1984).
McDonald, J. H. Handbook of Biological Statistics Vol. 2 (Sparky House Publishing, 2009).
Hernández, A. et al. Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol. 125, 143–152 (2021).
Adhikari, T. B., Muzhinji, N., Halterman, D. & Louws, F. J. Genetic diversity and population structure of Alternaria species from tomato and potato in North Carolina and Wisconsin. Sci. Rep. 11, 1–19 (2021).
Simmons, E. G. Alternaria: An indentification manual. (2007).
Ellis, M. B. Dematiaceous hyphomycetes. Dematiaceous hyphomycetes. (1971).
Ramjegathesh, R. & Ebenezar, E. Morphological and physiological characters of Alternaria alternata causing leaf blight disease of onion. Int. J. Plant Pathol. 3, 34–44 (2012).
Dang, H. X., Pryor, B., Peever, T. & Lawrence, C. B. The Alternaria genomes database: A comprehensive resource for a fungal genus comprised of saprophytes, plant pathogens, and allergenic species. BMC Genom. 16, 1–9 (2015).
Pryor, B. M. & Michailides, T. J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 92, 406–416 (2002).
Peralta, I. E., Knapp, S. & Spooner, D. M. New species of wild tomatoes (Solanum section Lycopersicon: Solanaceae) from Northern Peru. Syst. Bot. 30, 424–434 (2005).
Chaerani, R. & Voorrips, R. E. Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 72, 335–347 (2006).
Hadizadeh, I., Peivastegan, B. & Kolahi, M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pakistan J. Biol. Sci. 12, 58–63 (2009).
Aqsa, A., Farah, N., Muhammad, A., Rahmatullah, Q. & Rauf, C. In vitro antifungal activity of selected medicinal plant diffusates against Alternaria solani, Rhizoctonia solani and Macrophomina phaseolina. Pak. J. Bot. 42, 2911–2919 (2010).
Kumari, N., Sharma, J. & Singh, D. Efficacy of bio-products against post harvest rots of apple. J. Hill Agric. 9, 442–446 (2018).
Shafek, R. E., Shafik, N. H., Michael, H. N., El-Hagrassi, A. M. & Osman, A. F. Phytochemical studies and biological activity of Dodonaea viscosa flowers extract. J. Chem. Pharm. Res. 7, 109–116 (2015).
Bahraminejad, S., Amiri, R. & Abbasi, S. Anti-fungal properties of 43 plant species against Alternaria solani and Botrytis cinerea. Arch. Phytopathol. Plant Prot. 48, 336–344 (2015).
Simpson, B. S. et al. Flavonoids from the leaves and stems of Dodonaea polyandra: A Northern Kaanju medicinal plant. Phytochemistry 72, 1883–1888 (2011).
Ioana, N., Viorica, I., Diana-Carolina, I. & Valeria, R. Preliminary research regarding the therapeutic uses of Urtica dioica l note ii.The dynamics of accumulation of total phenolic compounds and ascorbic acid. Farmacia 61, 276–283 (2013).
Nencu, I., Vlase, L., Istudor, V., Duţu, L. E. & Gird, C. E. Preliminary research regarding the therapeutic uses of Urtica dioica L. Note I. The polyphenols evaluation. Farmacia 60, 493–500 (2012).
Orčić, D. et al. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 143, 48–53 (2014).
Sajfrtová, M., Sovová, H., Opletal, L. & Bártlová, M. Near-critical extraction of β-sitosterol and scopoletin from stinging nettle roots. J. Supercrit. Fuids 35, 111–118 (2005).
Rolta, R., Kumar, V., Sourirajan, A., Upadhyay, N. K. & Dev, K. Phytocompounds of three medicinal plants (Juniperus communis, Urtica dioica and Coleus forskohlii) of North West Himalayas increases the potency of antibacterial and antifungal antibiotics. Plant Archiv 20, 481–489 (2020).
Peralta, M. A., da Silva, M. A., Ortega, M. G., Cabrera, J. L. & Paraje, M. G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 22, 975–980 (2015).
Cushnie, T. T. & Lamb, A. J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26, 343–356 (2005).
Mosa, W. F. A. et al. Pomegranate trees quality under drought conditions using potassium silicate, nanosilver, and selenium spray with valorization of peels as fungicide extracts. Sci. Rep. 12, 6363. https://doi.org/10.1038/s41598-022-10354-1 (2022).
Salem, M. Z. M., Ali, H. M. & Akrami, M. Moringa oleifera seeds-removed ripened pods as alternative for papersheet production: Antimicrobial activity and their phytoconstituents profile using HPLC. Sci. Rep. 11, 19027. https://doi.org/10.1038/s41598-021-98415-9 (2021).
Salem, M. Z. M. et al. Plants-derived bioactives: Novel utilization as antimicrobial, antioxidant and phytoreducing agents for the biosynthesis of metallic nanoparticles. Microb. Pathog. 158, 105107. https://doi.org/10.1016/j.micpath.2021.105107 (2021).
Salem, M. Z. M., Mohamed, A. A., Ali, H. M. & Al Farraj, D. A. Characterization of phytoconstituents from alcoholic extracts of four woody species and their potential uses for management of six Fusarium oxysporum isolates identified from some plant hosts. Plants 10, 1325. https://doi.org/10.3390/plants10071325 (2021).
Havsteen, B. H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 96, 67–202 (2002).
Wang, M., Jiang, N., Wang, Y., Jiang, D. & Feng, X. Characterization of phenolic compounds from early and late ripening sweet cherries and their antioxidant and antifungal activities. J. Agric. Food Chem. 65, 5413–5420 (2017).
Zafar, H. Studies on Biological Control of Alternaria Solani (Federal Urdu University of Arts Sciences & Tech, 2018).
Barral, B., Chillet, M., Minier, J., Léchaudel, M. & Schorr-Galindo, S. Evaluating the response to Fusarium ananatum inoculation and antifungal activity of phenolic acids in pineapple. Fungal Biol. 121, 1045–1053. https://doi.org/10.1016/j.funbio.2017.09.002 (2017).
Patzke, H. & Schieber, A. Growth-inhibitory activity of phenolic compounds applied in an emulsifiable concentrate: Ferulic acid as a natural pesticide against Botrytis cinerea. Food Res. Int. 113, 18–23. https://doi.org/10.1016/j.foodres.2018.06.062 (2018).
Aziz, N. H., Farag, S. E., Mousa, L. A. & Abo-Zaid, M. A. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 93, 43–54 (1998).
Acknowledgements
This paper was a part of a Ph.D. thesis based upon work supported by Faculty of Agriculture Saba Basha, Alexandria University, Alexandria, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
B.P., M.Z.M.S, A.H., M.A.A., I.A.E., A.A., and S.I.B. wrote the main manuscript text, and B.P., M.Z.M.S, and S.I.B. prepared figures, B.P., M.Z.M.S, and S.I.B. carried out the methodology, B.P., M.Z.M.S, A.H., M.A.A., I.A.E., A.A., and S.I.B. investigated the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behiry, S.I., Philip, B., Salem, M.Z.M. et al. Urtica dioica and Dodonaea viscosa leaf extracts as eco-friendly bioagents against Alternaria alternata isolate TAA-05 from tomato plant. Sci Rep 12, 16468 (2022). https://doi.org/10.1038/s41598-022-20708-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20708-4
This article is cited by
-
Trichoderma afroharzianum TRI07 metabolites inhibit Alternaria alternata growth and induce tomato defense-related enzymes
Scientific Reports (2024)
-
Growth, productivity and phytochemicals of Coriander in responses to foliar application of Acacia saligna fruit extract as a biostimulant under field conditions
Scientific Reports (2024)
-
The characteristics, occurrence, and toxicological effects of alternariol: a mycotoxin
Archives of Toxicology (2024)
-
Phytochemical analysis and insight into insecticidal and antifungal activities of Indian hawthorn leaf extract
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.