Abstract
Myotonic dystrophy type 1 (DM1) is a severe autosomal dominant neuromuscular disease in which the musculoskeletal system contributes substantially to overall mortality and morbidity. DM1 stems from a noncoding CTG trinucleotide repeat expansion in the DMPK gene. The human skeletal actin long repeat (HSALR) mouse model reproduces several aspects of the disease, but the muscle-wasting phenotype of this model has never been characterized in vivo. Herein, we used quantitative MRI to measure the fat and muscle volumes in the leg compartment (LC) of mice. These acquired data were processed to extract relevant parameters such as fat fraction and fat infiltration (fat LC/LC) in HSALR and control (FBV) muscles. These results showed increased fat volume (fat LC) and fat infiltration within the muscle tissue of the leg compartment (muscle LC), in agreement with necropsies, in which fatty clumps were observed, and consistent with previous findings in DM1 patients. Model mice did not reproduce the characteristic impaired fat fraction, widespread fat replacement through the muscles, or reduced muscle volume reported in patients. Taken together, the observed abnormal replacement of skeletal muscle by fat in the HSALR mice indicates that these mice partially reproduced the muscle phenotype observed in humans.
Similar content being viewed by others
Introduction
Myotonic dystrophy type 1 (DM1) is a debilitating chronic disease caused by an expansion of the unstable CTG triplet repeat in the 3' untranslated region of the DM1 protein kinase (DMPK) gene. DM1 is autosomal dominant and can affect all ages, from newborns to elderly adults. Although DM1 is the most common form of muscular dystrophy in adults and the prevalence of the mutation is 1/2100 births1, there are currently no treatment options for this disease. Adult-onset patients face risks of physical disability and a shortened lifespan. Characteristic neuromuscular symptoms include muscle stiffness and weakness, impaired muscle relaxation capacity (myotonia), and progressive loss of muscle mass (atrophy). However, DM1 is not merely a skeletal muscle disease but a multisystemic disease, as other tissues and organs, such as the smooth muscle, brain, and heart, are also affected2. Premature deaths among DM1 patients are caused by respiratory failure due to muscle weakness and myotonia that affect the peripheral respiratory system. Additionally, failure of the central respiratory drive and the upper airway muscles contributes to an increased risk of pulmonary infections in patients3,4.
Muscle atrophy and wasting are critical signs of DM1, and various factors have been hypothesized to lead to these phenotypes5. The principal factor consists of DMPK transcripts carrying the DM1 mutation. Mutant RNA is retained in the cell nucleus in several tissues, including the heart, brain, and skeletal muscle, where it aggregates into insoluble, stable, membraneless structures known as foci. Ribonuclear foci of expanded CUG repeat hairpins can bind and sequester RNA-binding proteins, thus preventing them from performing their normal functions6.
Magnetic resonance imaging (MRI) is the gold-standard technique in muscle pathology. The benefits include its capacity to discriminate muscular from adipose tissue accurately and to identify fat infiltration into skeletal muscle7,8. Consequently, this technique helps diagnose neuromuscular pathologies, as it can reveal disease-specific patterns of muscle involvement9. Furthermore, as MRI is a noninvasive technique that does not expose patients to ionizing radiation, it can be used to track the evolution of muscular diseases. In musculoskeletal disorders, MRI is being used increasingly often for longitudinal evaluation of candidate drugs10 and validation of clinical trial outcomes11.
Clinical research on DM1 has categorically demonstrated that patients display muscle defects that can be quantitatively analyzed using MRI. Specifically, DM1 patients have almost 4 times more fatty infiltration in the skeletal musculature than unaffected controls. Additionally, fat infiltration correlates with scores on the 6-min walk test and the Muscular Impairment Rating Scale12. Patients also show significantly reduced skeletal muscle volume, especially in the gastrocnemius medialis and soleus, accompanied by increased T2 water relaxation time, indicating muscle atrophy and edema, respectively12,13. Other MRI studies have shown that over 70% of DM1 patients display fatty infiltration in the lower extremities, with especially severe defects in the gastrocnemius and tibialis anterior during the initial stages of the disease14. Interestingly, the authors reported the possibility of detecting affected muscles even before weakness was clinically noted. Recent studies have shown that an increased fat fraction in the muscles of DM1 patients is correlated with muscle weakness, reduced specific strength, and high disease severity15,16. Taken together, these data support the idea that muscle impairment as determined by quantitative MRI is strongly correlated with strength, supporting its feasibility as an approach to predict muscle dysfunction in DM1.
Nearly two dozen experimental treatments against DM1 are in development17, but there are insufficient noninvasive methods for preclinical evaluation of these candidate drugs in DM1 model mice; such methods would allow dynamic evaluation of phenotype progression. Therefore, we decided to optimize quantitative MRI to these circumstances and present an in vivo method to quantitatively measure muscle defects in a murine model expressing 250 CUG repeats in the skeletal muscles, driven by a human skeletal actin transgene18 (HSALR). The HSALR murine model accurately reproduces key DM1 phenotypes such as aberrant alternative splicing, centrally located nuclei in muscular fibers, variable muscle fiber size, and myotonia18,19,20. Some further studies performed in young HSALR mice have reported that chemical inhibition of glycogen synthase kinase 3 beta (GSK3β) corrects impaired Celf1 activity, thus preventing the development of DM1 muscle pathology, including muscle atrophy21. However, independent studies have reported a lack of clear and consistent dystrophic and atrophic features in this model18,19. Conversely, researchers have discovered genetic and drug manipulations that can enhance muscle atrophy phenotypes in HSALR mice22,23. Selecting the disease-related phenotypes already described in DM1 patients, our study aimed to achieve MRI quantitation of muscle and fat volume, fat infiltration, and fat fraction (FF) in HSALR model mice.
Results
MRI acquisition in DM1 model mice
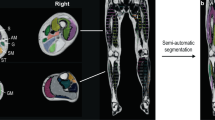
Necropsies of 4.5-month-old HSALR mice showed clear signs of fatty infiltration in the quadriceps and gastrocnemius muscles, as fatty clumps could easily be observed (Fig. 1). To quantitatively analyze these phenotypes in vivo, images from eleven male HSALR mice and eleven age-matched counterpart control (FVB) mice were acquired under anesthesia in an MR scanner. Two individuals from each group had to be removed from the study because their breathing movement impeded the acquisition of images of sufficient quality for further quantitative analyses. Images of both hind limbs from each animal were acquired, and the leg compartment (LC) was segmented manually. The LC consists of all muscles, fat, and bones forming the leg, but the small size of the mice compared to the scanners used in clinical practice precluded us from differentiating small bones from muscle; therefore, the LC was stratified into macroscopic fat (fat LC) and muscle plus bone, defined as muscle LC. Note that, as the animals used in the experiment were of the same sex and age and did not express the transgenic construct in bone or bone precursors, bone structure size and density were assumed to be constant among the different individuals. A schematic workflow of the process is shown in Fig. 2.
Summary of image acquisition and analysis workflow. The image depicts the infrastructure used for mouse sedation and MRI acquisition, showing both the angulation and the main parameters of the sequences used to obtain the cross-sectional images of a DM1 model mouse thigh (A), along with the workflow of muscle compartment segmentation, the automatic segmentation of fat and muscle, and the postprocessing used to obtain the muscle/fat fraction mapping (B). LC leg compartment, FF fat fraction. Figure partially created with BioRender.com.
Different MRI outcome measures between DM1 model mice and controls
Image analysis from manual segmentation provided the whole LC volume of both hind legs, corresponding to the combined muscle LC and fat LC (Table 1, Fig. 3A,B). DM1 model mice had significantly higher LC volume (18%) than the control group (Fig. 4A), consistent with the observed greater weight of DM1 mice. Specifically, the mean weight of control FVB mice (CNT) (n = 14) was 27.9 g vs. 30.71 g for DM1 (n = 20) (p = 0.0423; Mann‒Whitney U test). Individual analysis of both LC components showed that the difference was mainly due to a robust, almost fourfold increase in fat LC volume in the DM1 group relative to controls (Fig. 4B). In contrast, no statistically significant difference in muscle LC volume was detected between the two experimental groups (Fig. 4C).
DM1 model mice show partial pathological phenotypes. Representative axial images from T2W, fat and water mDIXON of two control (A) and two DM1 model mice (B). The position is indicated on the coronal survey, and the red square brackets delimit the coverage of the acquired volume. H head, F foot, A anterior, P posterior, R right, L left. Arrows point to fat in the LC.
HSALR mice display fat infiltration in skeletal muscle. Plots represent the total volume (A), the volume of fat (B), and the volume of muscle (C) in the leg compartments of control (CNT) and DM1 model mice. Values were obtained from quantitative analyses of MRI data (n = 9 mice per group). Muscle-to-fat ratios (D) were also calculated. Disease model mice showed increased fat infiltration levels in skeletal muscle (E). No differences in the fat fraction were detected between control and model mice (F). The boxes extend from the 25th to the 75th percentile, the line in the middle of the box is plotted at the median, and the whiskers reach down to the smallest value and up to the largest. *p < 0.05, ***p < 0.001, ****p < 0.001 according to a 2-tailed Mann‒Whitney U test.
Several fat LC and muscle LC ratios in the LC were generated to determine the relative contributions of each tissue fraction to the disease phenotype. Specifically, the muscle LC/fat LC ratio revealed that although the muscle LC fraction did not differ significantly from controls in volume, its ratio to the amount of fat was significantly reduced in DM1 mice (Fig. 4D). Similarly, fatty infiltration in the LC was calculated as the ratio of fat LC to total LC, and the results confirmed that HSALR mice had more than threefold higher fatty infiltration levels than FVB control mice (Fig. 4E). For quantitative determination of the muscle fat fraction, we employed the fat and water images provided by the mDIXON sequence over the voxels corresponding to the muscle LC. These analyses revealed that the model mice did not reproduce the increased fat fractions observed in humans12,13,15 (Fig. 4F), despite a trend-level increase.
Discussion
Muscle MRI has recently become a prominent diagnostic procedure in neuromuscular disorders24. Recent studies have shown that DM1 patients have several affected muscle parameters that can be quantitatively analyzed by this technique13,14,15,25. These alterations include fat infiltration, fat fraction, reduced muscle mass, muscle atrophy, edema, impaired contractile muscle volume, and altered muscle size. In this work, we sought a more in-depth characterization of a murine model widely used by the scientific community by using MRI to investigate whether it reproduces the muscle defects detected in patients. Some studies have already suggested that HSALR mice are somewhat limited in robustly reproducing muscle atrophy phenotypes18,23. Quantitative analyses of MRI data have confirmed that these mice do not reproduce all the muscle-wasting-related alterations described in recent years in DM1 patients. Specifically, while an increased fat and leg volume ratio were found, analogous to findings in patients, a normal fat fraction was detected. Interestingly, model mice have large lumps of adipose tissue within limb skeletal muscles, whereas in patients, fatty infiltration is more dispersed and heterogeneous12,13,16. In other words, the human phenotype is quantitatively reproduced in mice, but significant qualitative differences exist. However, parameters related to muscle atrophy remain unaltered in model mice, as no difference in muscle LC volume or fat fraction was observed between HSALR mice and the control group. Fat fraction is a key parameter in the context of the disease, given that, in addition to being increased in patients, it strongly correlates with disease severity and progression12,16, but we detected no significant differences in this measure. Interestingly, HSALR mice showed higher body weight and fat volume than controls, in agreement with what is observed in DM1 patients26.
Increased activity and stability of GSK3β have been proposed to explain the atrophic phenotype in DM121,27. In recent years, however, additional molecular alterations have been found to contribute to the phenotype5. Pathogenic upregulation of Musashi 2 (MSI2) in DM1 cells, which inhibits miR-7 biogenesis, leads to increases in autophagy and the activity of the ubiquitin‒proteasome system, two pathways with a strong contribution to the muscle impairment characteristic of DM128,29,30,31,32. Nonetheless, miR-7 levels in the diaphragm and gastrocnemius of HSALR mice were similar to those of control mice29. Therefore, the failure to reduce miR-7 in model mice may explain why no overt muscle atrophy was detected.
Preclinical testing of therapeutic effects in a mammalian animal model is a requirement of any drug development procedure. No studies have yet been published in which MRI determinations have been performed in the widely used HSALR DM1 model mice33,34,35. Therefore, our finding that they reproduce some of the muscle-pathology-related phenotypes observed in patients, such as fat replacement, makes this model a valuable tool during preclinical evaluation. A particularly useful aspect of MRI in this context is that it is a noninvasive technique performed in vivo, enabling scientists to evaluate the effect of a candidate treatment on the same individual at different time points during the experiment. This brings two advantages: first, it allows researchers to study the phenotype's evolution over time; second, it allows them to identify the optimal treatment duration or point of maximum effect. Moreover, it allows the study of all muscles, rather than only a few, as with biopsy or electromyography.
In conclusion, we observed evidence of fatty infiltration in the leg muscles of DM1 mice in necropsies, and quantitative MRI confirmed this phenotype, which has been reported to affect more than 70% of DM1 patients14,36. Our results are valuable for therapy research due to the vast difference between control and DM1 mice, allowing researchers to identify molecules with strong therapeutic potential.
Methods
Animal experimentation
Mouse handling and experimental procedures were performed according to the European law regarding laboratory animal care and experimentation (2003/65/CE) and the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines. All procedures were approved by the Consejeria de Agricultura, Generalidad Valenciana (Reference Number 2019/VSC/PEA/0198).
Eleven male homozygous transgenic HSALR mice (line 20b) were used as DM1 disease models; they were compared to 11 controls, which were mice with the same genetic background (FVB). Mice were provided by C. Thornton (University of Rochester Medical Center, Rochester, NY)18. All animals were 4.5 months old at the time of experimentation and were randomly selected for the experiment. The animals were bred in the animal facilities of the University of Valencia and then transferred to the facilities of the La Fe University Hospital. Once the acclimatization period had elapsed, images were acquired by MR while the animals were anesthetized with isoflurane and immobilized in the supine position; after acquisition was completed, the animals were sacrificed by cervical dislocation.
For the necropsy study, six HSALR and six FVB mice were euthanized by cervical dislocation. All animals were 4.5-month-old males. The procedure was approved by the Consejería de Agricultura, Generalidad Valenciana (Reference Number 2018/VSC/PEA/0182).
MR acquisition
MR images were obtained using a Philips Achieva 3.0 TX (Amsterdam, The Netherlands) using an 8-Channel Sense Wrist Coil. Animals were placed in a prone position for transverse acquisition; the protocol was created with 22 slices to cover the full thigh. This plan was made on the basis of sagittal and coronal scout images. The acquisition protocol consisted of a T2-weighted multi-slice turbo spin‒echo (T2W MS TSE) sequence with a field of view (FOV) of 50 × 50 × 22 mm in the transverse plane (22 slices), voxel size = 0.14 × 0.14 × 1 mm; TE = 97 ms and TR = 2800 ms and an mDIXON sequence with the same FOV and voxel size; TE = 1.18/2.3 ms, TR = 6.4 ms and a flip angle of 10°. The mDIXON (modified Dixon) sequence employed in this study was developed by Philips researchers; this sequence employs two echoes with unrestricted TE values, a 7-peak fat model, and a B0 correction algorithm to improve fat-free imaging.
Image processing
The purpose of the image processing was to identify fat LC and muscle LC, from which fat infiltration, defined by the ratio between fat LC to the total LC volume and by the fat fraction over muscle LC, was determined.
For this purpose, acquired images were converted from DICOM to NIFTI, and the LC was manually segmented in both muscles using open-access ITK-SNAP software37; two individuals from each group were excluded during this process due to excessive movement.
The Otsu method38 (an image processing method to separate voxels into two classes) was implemented in MATLAB (R2016b, MathWorks, Natick, MA) and applied to the fat image provided by the mDIXON sequence to separate muscle LC from fat LC in the regions delineated as LC. The fat fraction mapping was obtained employing the water and fat images provided by mDIXON over muscle LC voxels according to the following formula: \(FF=\frac{fat\, mDIXON}{fat\, mDIXON+water\, mDIXON}\). This procedure is schematically represented in Fig. 2. LC, muscle LC, and fat LC volumes were obtained from the sum of voxels belonging to each region and voxel size, and these and other ratios between these regions are presented in the results section (Table 1 and Fig. 4); among these ratios, fat infiltration is highlighted as the ratio of fat LC to LC.
Statistical analyses
The Pingouin 0.5.1 library developed in Python for image processing results was used for statistical analysis. First, the Shapiro–Wilk test and Levene’s test, respectively, were applied to determine the normality and homoscedasticity of the distributions. ANOVA tests were performed using ANOVA for comparisons if both groups showed homoscedasticity and Welch’s ANOVA otherwise. In cases where the samples of either group did not follow a normal distribution, differences between control and DM1 model mice were assessed with a 2-tailed Mann‒Whitney U test.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Johnson, N. E. et al. Population based prevalence of myotonic dystrophy type 1 using genetic analysis of state-wide blood screening program. Neurology https://doi.org/10.1212/WNL.0000000000011425 (2021).
Smith, C. A. & Gutmann, L. Myotonic dystrophy type 1 management and therapeutics. Curr. Treat. Options Neurol. 18, 52 (2016).
Rossi, S. et al. Prevalence and predictor factors of respiratory impairment in a large cohort of patients with myotonic dystrophy type 1 (DM1): A retrospective, cross sectional study. J. Neurol. Sci. 399, 118–124 (2019).
Hawkins, A. M. et al. Respiratory dysfunction in myotonic dystrophy type 1: A systematic review. Neuromuscul. Disord. 29, 198–212 (2019).
Ozimski, L. L., Sabater-Arcis, M., Bargiela, A. & Artero, R. The hallmarks of myotonic dystrophy type 1 muscle dysfunction. Biol. Rev. https://doi.org/10.1111/brv.12674 (2020).
Pettersson, O. J., Aagaard, L., Jensen, T. G. & Damgaard, C. K. Molecular mechanisms in DM1—A focus on foci. Nucleic Acids Res. https://doi.org/10.1093/nar/gkv029 (2015).
Sergi, G., Trevisan, C., Veronese, N., Lucato, P. & Manzato, E. Imaging of sarcopenia. Eur. J. Radiol. 85, 1519–1524 (2016).
Sanz-Requena, R. et al. The role of imaging biomarkers in the assessment of sarcopenia. Diagnostics 10, 534 (2020).
Díaz-Manera, J., Llauger, J., Gallardo, E. & Illa, I. Muscle MRI in muscular dystrophies. Acta Myol. 34, 95–108 (2015).
Carlier, P. G. et al. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J. Neuromuscul. Dis. 3, 1–28 (2016).
Mitchell, W. K. et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. https://doi.org/10.3389/fphys.2012.00260 (2012).
Heskamp, L. et al. Lower extremity muscle pathology in myotonic dystrophy type 1 assessed by quantitative MRI. Neurology 92, e2803–e2814 (2019).
van der Plas, E. et al. Quantitative muscle MRI as a sensitive marker of early muscle pathology in myotonic dystrophy type 1. Muscle Nerve 63, 553–562 (2021).
Peric, S. et al. Magnetic resonance imaging of leg muscles in patients with myotonic dystrophies. J. Neurol. 264, 1899–1908 (2017).
Steenkjaer, C. H., Mencagli, R. A., Vaeggemose, M. & Andersen, H. Isokinetic strength and degeneration of lower extremity muscles in patients with myotonic dystrophy; an MRI study. Neuromuscul. Disord. 31, 198–211 (2021).
Garibaldi, M. et al. Muscle magnetic resonance imaging in myotonic dystrophy type 1 (DM1): Refining muscle involvement and implications for clinical trials. Eur. J. Neurol. https://doi.org/10.1111/ene.15174 (2021).
Pascual-Gilabert, M., López-Castel, A. & Artero, R. Myotonic dystrophy type 1 drug development: A pipeline toward the market. Drug Discov. Today 26, 1765–1772 (2021).
Mankodi, A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773 (2000).
Mankodi, A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44 (2002).
Vihola, A. et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology 60, 1854–1857 (2003).
Wei, C. et al. Correction of GSK3beta at young age prevents muscle pathology in mice with myotonic dystrophy type 1. FASEB J 32, 2073–2085 (2018).
Li, M. et al. HNRNPA1-induced spliceopathy in a transgenic mouse model of myotonic dystrophy. Proc. Natl. Acad. Sci. 117, 5472–5477 (2020).
Crawford Parks, T. E., Marcellus, K. A., Péladeau, C., Jasmin, B. J. & Ravel-Chapuis, A. Overexpression of Staufen1 in DM1 mouse skeletal muscle exacerbates dystrophic and atrophic features. Hum. Mol. Genet. https://doi.org/10.1093/hmg/ddaa111 (2020).
Carlier, P. G. & Reyngoudt, H. The expanding role of MRI in neuromuscular disorders. Nat. Rev. Neurol. 16, 301–302 (2020).
Park, D., Lee, S.-H., Shin, J.-H. & Park, J.-S. Lower limb muscle magnetic resonance imaging in myotonic dystrophy type 1 correlates with the six-minute walk test and CTG repeats. Neuromuscul. Disord. 28, 29–37 (2018).
Perseghin, G. et al. Postabsorptive and insulin-stimulated energy and protein metabolism in patients with myotonic dystrophy type 1. Am. J. Clin. Nutr. 80, 357–364 (2004).
Jones, K. et al. GSK3β mediates muscle pathology in myotonic dystrophy. J. Clin. Invest. https://doi.org/10.1172/JCI64081 (2012).
Sabater-Arcis, M. et al. Musashi-2 contributes to myotonic dystrophy muscle dysfunction by promoting excessive autophagy through miR-7 biogenesis repression. Mol. Ther. Nucleic Acids 25, 652–667 (2021).
Sabater-Arcis, M., Bargiela, A., Furling, D. & Artero, R. miR-7 restores phenotypes in myotonic dystrophy muscle cells by repressing hyperactivated autophagy. Mol. Ther. Nucleic Acid 19, 278–292 (2020).
Loro, E. et al. Normal myogenesis and increased apoptosis in myotonic dystrophy type-1 muscle cells. Cell Death Differ. https://doi.org/10.1038/cdd.2010.33 (2010).
Huguet, A. et al. Molecular, physiological, and motor performance defects in DMSXL mice carrying >1,000 CTG repeats from the human DM1 locus. PLoS Genet. 8, e1003043 (2012).
Morriss, G. R., Rajapakshe, K., Huang, S., Coarfa, C. & Cooper, T. A. Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1. Hum. Mol. Genet. 27, 2789–2804 (2018).
Brockhoff, M. et al. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J. Clin. Invest. 127, 549–563 (2017).
Jenquin, J. R. et al. Furamidine rescues myotonic dystrophy type I associated mis-splicing through multiple mechanisms. ACS Chem. Biol. 13, 2708–2718 (2018).
Bisset, D. R. et al. Therapeutic impact of systemic AAV-mediated RNA interference in a mouse model of myotonic dystrophy. Hum. Mol. Genet. 24, 4971–4983 (2015).
Greve, T. et al. Regional variation of thigh muscle fat infiltration in patients with neuromuscular diseases compared to healthy controls. Quant. Imaging Med. Surg. 11, 2610–2621 (2021).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979).
Acknowledgements
This work was made possible by research grant RTI2018-094599-B-100 from the Ministerio de Ciencia e Innovación, including funds from the European Regional Development Fund, to R.A.; GV/2021/014 from the Consejería de Innovación, Universidades, Ciencia y Sociedad Digital, Generalidad Valenciana to A.B; and PI21/00311 from the Instituto de Salud Carlos III, including funds from the European Regional Development Fund, to M.P.A. A.B. was supported by a Sara Borrell fellowship from the Spanish Instituto de Salud Carlos III (CD21/00031). A.T.E. is a recipient of a PFIS grant (FI20/00239) from the Instituto de Salud Carlos III. We acknowledge Juan Carbonell from the Bioinformatics and Biostatistics Unit of INCLIVA Biomedical Research Institute for his advice on the statistical analysis of our data.
Author information
Authors and Affiliations
Contributions
Conception and design, R.A., T.S., and A.B.; financial support, R.A., M.P.A. and A.B.; investigation A.B., A.T. and L.M.B.; collection and/or assembly of data A.T. and L.M.B. data analysis and interpretation A.T., L.M.B. and A.B.; manuscript writing, A.B.; final approval of the manuscript R.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bargiela, A., Ten-Esteve, A., Martí-Bonmatí, L. et al. Quantitative magnetic resonance imaging assessment of muscle composition in myotonic dystrophy mice. Sci Rep 13, 503 (2023). https://doi.org/10.1038/s41598-023-27661-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27661-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.