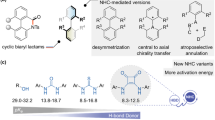
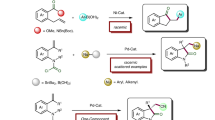
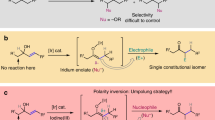
Abstract
Carbocation intermediacy is postulated in numerous organic transformations and provides the foundation for retrosynthetic logics in chemical synthesis. Although a number of catalytic approaches are designed to generate transient carbocations under mild conditions, there is room for improvement in the context of selectivity control and synthetic utility. Here we present an approach that enables catalytic access to carbocation intermediates via metal-nitrenoid transfer into alkenes, which subsequently allows a regiocontrolled elimination reaction. Customized catalysts are capable of bypassing competing pathways of the reactive intermediates to furnish valuable allylic lactams with excellent regioselectivity. Mechanistic investigations suggest that the ligand plays a critical role as an internal base in the selectivity-determining proton transfer process. This protocol is broadly applicable for preparing both five- and the more challenging four-membered allylamides. The virtue of this platform is further demonstrated by achieving the enantioselective construction of γ-lactams.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The X-ray crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition nos. 1999590–1999595. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the authors upon reasonable request.
References
Norris, J. F. On the nonexistence of trivalent carbon. Am. Chem. J. 25, 117–122 (1901).
Kehrmann, F. W. & Ueber, F. Die basischen eigenschaften des kohlenstoffs und die constitution des sogennanten triphenylmeths. Ber. Dtsch. Chem. Ges. 34, 3815–3819 (1901).
Olah, G. A. 100 years of carbocations and their significance in chemistry. J. Org. Chem. 66, 5943–5957 (2001).
Naredla, R. R. & Klumpp, D. A. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev. 113, 6905–6948 (2013).
Olah, G. A. & Lukas, J. Stable carbonium ions. XXXIX. Formation of alkylcarbonium ions via hydride ion abstraction from alkanes in fluorosulfonic acid–antimony pentafluoride solution. Isolation of some crystalline alkylcarbonium ion salts. J. Am. Chem. Soc. 89, 2227–2228 (1967).
Zhang, F. et al. Cu-catalyzed cascades to carbocycles: union of diaryliodonium salts with alkenes or alkynes exploiting remote carbocations. J. Am. Chem. Soc. 136, 8851–8854 (2014).
Nitsch, D. et al. Chiral propargylic cations as intermediates in SN1-type reactions: substitution pattern, nuclear magnetic resonance studies, and origin of the diastereoselectivity. J. Am. Chem. Soc. 136, 2851–2857 (2014).
Zhu, Q., Gentry, E. C. & Knowles, R. R. Catalytic carbocation generation enabled by the mesolytic cleavage of alkoxyamine radical cations. Angew. Chem. Int. Ed. 55, 9969–9973 (2016).
Shao, B., Bagdasarian, A. L., Popov, S. & Nelson, H. M. Arylation of hydrocarbons enabled by organosilicon reagents and weakly coordinating anions. Science 355, 1403–1407 (2017).
Phipps, R. J., McMurray, L., Ritter, S., Duong, H. A. & Gaunt, M. J. Copper-catalyzed alkene arylation with diaryliodonium salts. J. Am. Chem. Soc. 134, 10773–10776 (2012).
Wigman, B. et al. Vinyl carbocations generated under basic conditions and their intramolecular C–H insertion reactions. J. Am. Chem. Soc. 141, 9140–9144 (2019).
Kutateladze, D. A., Strassfeld, D. A. & Jacobsen, E. N. Enantioselective tail-to-head cyclizations catalyzed by dual-hydrogen-bond donors. J. Am. Chem. Soc. 142, 6951–6956 (2020).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Isomura, M., Petrone, D. A. & Carreira, E. M. Coordination-induced stereocontrol over carbocations: asymmetric reductive deoxygenation of racemic tertiary alcohols. J. Am. Chem. Soc. 141, 4738–4748 (2019).
Degennaro, L., Trinchera, P. & Luisi, R. Recent advances in the stereoselective synthesis of aziridines. Chem. Rev. 114, 7881–7929 (2014).
Lee, S., Lei, H. & Rovis, T. A Rh(iii)-catalyzed formal [4+1] approach to pyrrolidines from unactivated terminal alkenes and nitrene sources. J. Am. Chem. Soc. 141, 12536–12540 (2019).
Rigoli, J. W., Weatherly, C. D., Alderson, J. M., Vo, B. T. & Schomaker, J. M. Tunable, chemoselective amination via silver catalysis. J. Am. Chem. Soc. 135, 17238–17241 (2013).
Stokes, B. J., Liu, S. & Driver, T. G. Rh2(ii)-catalyzed nitro-group migration reactions: selective synthesis of 3-nitroindoles from β-nitro styryl azides. J. Am. Chem. Soc. 133, 4702–4705 (2011).
Kong, C., Jana, N. & Driver, T. G. Rh2(ii)-catalyzed selective aminomethylene migration from styryl azides. Org. Lett. 15, 824–827 (2013).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Nay, B., Riache, N. & Evanno, L. Chemistry and biology of non-tetramic γ-hydroxy-γ-lactams and γ-alkylidene-γ-lactams from natural sources. Nat. Prod. Rep. 26, 1044–1062 (2009).
Kambe, T. et al. Discovery of novel prostaglandin analogs as potent and selective EP2/EP4 dual agonists. Bioorg. Med. Chem. 20, 2235–2251 (2012).
Meiries, S. & Marquez, R. Convergent synthesis of (2R,3R,8R,9R)-N-Boc-ADDA. J. Org. Chem. 73, 5015–5021 (2008).
Griesbeck, A. G., Bondock, S. & Lex, J. Synthesis of erythro-α-amino β-hydroxy carboxylic acid esters by diastereoselective photocycloaddition of 5-methoxyoxazoles with aldehydes. J. Org. Chem. 68, 9899–9906 (2003).
Ferreira, P. M. T., Monteiro, L. S. & Pereira, G. Synthesis of substituted oxazoles from N-acyl-β-hydroxyamino acid derivatives. Eur. J. Org. Chem. 2008, 4676–4683 (2008).
Shigehisa, H., Aoki, T., Yamaguchi, S., Shimizu, N. & Hiroya, K. Hydroalkoxylation of unactivated olefins with carbon radicals and carbocation species as key intermediates. J. Am. Chem. Soc. 135, 10306–10309 (2013).
Whitmore, F. C. The common basis of intramolecular rearrangements. J. Am. Chem. Soc. 54, 3274–3283 (1932).
Fujita, K.-i, Tanino, N. & Yamaguchi, R. Ligand-promoted dehydrogenation of alcohols catalyzed by Cp*Ir complexes. A new catalytic system for oxidant-free oxidation of alcohols. Org. Lett. 9, 109–111 (2007).
Royer, A. M., Rauchfuss, T. B. & Gray, D. L. Organoiridium pyridonates and their role in the dehydrogenation of alcohols. Organometallics 29, 6763–6768 (2010).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Hong, S. Y., Son, J., Kim, D. & Chang, S. Ir(iii)-catalyzed stereoselective haloamidation of alkynes enabled by ligand participation. J. Am. Chem. Soc. 140, 12359–12363 (2018).
Hong, S. Y. & Chang, S. Stereodefined access to lactams via olefin difunctionalization: iridium nitrenoids as a motif of LUMO-controlled dipoles. J. Am. Chem. Soc. 141, 10399–10408 (2019).
Hwang, Y., Park, Y., Kim, Y. B., Kim, D. & Chang, S. Revisiting arene C(sp2)−H amidation by intramolecular transfer of iridium nitrenoids: evidence for a spirocyclization pathway. Angew. Chem. Int. Ed. 57, 13565–13569 (2018).
Filler, R. & Schure, R. M. Highly acidic perhalogenated alcohols. A new synthesis of perfluoro-tert-butyl alcohol. J. Org. Chem. 32, 1217–1219 (1967).
Park, Y., Park, K. T., Kim, J. G. & Chang, S. Mechanistic studies on the Rh(iii)-mediated amido transfer process leading to robust C–H amination with a new type of amidating reagent. J. Am. Chem. Soc. 137, 4534–4542 (2015).
Fisher, J. F., Meroueh, S. O. & Mobashery, S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 105, 395–424 (2005).
Fernandes, R., Amador, P. & Prudêncio, C. β-Lactams: chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 24, 7–17 (2013).
Minatti, A. & Muñiz, K. Intramolecular aminopalladation of alkenes as a key step to pyrrolidines and related heterocycles. Chem. Soc. Rev. 36, 1142–1152 (2007).
Xiong, P., Xu, H.-H. & Xu, H.-C. Metal- and reagent-free intramolecular oxidative amination of tri- and tetrasubstituted alkenes. J. Am. Chem. Soc. 139, 2956–2959 (2017).
Race, N. J., Hazelden, I. R., Faulkner, A. & Bower, J. F. Recent developments in the use of aza-Heck cyclizations for the synthesis of chiral N-heterocycles. Chem. Sci. 8, 5248–5260 (2017).
Shuler, S. A., Yin, G., Krause, S. B., Vesper, C. M. & Watson, D. A. Synthesis of secondary unsaturated lactams via an Aza–Heck reaction. J. Am. Chem. Soc. 138, 13830–13833 (2016).
Wang, H. et al. Iridium-Catalysed enantioselective C(sp3)–H amidation controlled by attractive noncovalent interactions. J. Am. Chem. Soc. 141, 7194–7201 (2019).
Dolan, N. S., Scamp, R. J., Yang, T., Berry, J. F. & Schomaker, J. M. Catalyst-controlled and tunable, chemoselective silver-catalyzed intermolecular nitrene transfer: experimental and computational studies. J. Am. Chem. Soc. 138, 14658–14667 (2016).
Lebel, H., Huard, K. & Lectard, S. N-Tosyloxycarbamates as a source of metal nitrenes: rhodium-catalyzed C−H insertion and aziridination reactions. J. Am. Chem. Soc. 127, 14198–14199 (2005).
Jiang, H., Lang, K., Lu, H., Wojtas, L. & Zhang, X. P. Asymmetric radical bicyclization of allyl azidoformates via cobalt(ii)-based metalloradical catalysis. J. Am. Chem. Soc. 139, 9164–9167 (2017).
Bickelhaupt, F. M. & Houk, K. N. Analyzing reaction rates with the distortion/interaction–activation strain model. Angew. Chem. Int. Ed. 56, 10070–10086 (2017).
Olah, G. A. Stable carbocations, 189. The σ-bridged 2-norbornyl cation and its significance to chemistry. Acc. Chem. Res. 9, 41–52 (1976).
Hammett, L. P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 59, 96–103 (1937).
Lee, J. et al. Versatile Cp*Co(iii)(LX) catalyst system for selective intramolecular C–H amidation reactions. J. Am. Chem. Soc. 142, 12324–12332 (2020).
Thai, T.-T., Therrien, B. & Süss-Fink, G. Pentamethylcyclopentadienyl rhodium and iridium complexes containing oxinato ligands. Inorg. Chem. Commun. 12, 806–807 (2009).
Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R010-D1) in Korea. The authors thank S. Seo (Institute for Basic Science) for helpful discussions and critical reading.
Author information
Authors and Affiliations
Contributions
S.Y.H. and S.C. conceived and designed the project and wrote the manuscript. S.Y.H. carried out the experiments and DFT calculations. D.K. performed the X-ray crystallographic analysis. S.C. organized the research. All the authors analysed the data, discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Forrest Michael and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21 and Tables 1–9.
Supplementary Data 1
Cartesian coordinates of DFT-optimized structures.
Supplementary Data 2
Crystallographic Data for compound 40 (CCDC 1999590).
Supplementary Data 3
Crystallographic Data of compound 36 (CCDC 1999591).
Supplementary Data 4
Crystallographic Data of compound 19 (CCDC 1999592).
Supplementary Data 5
Crystallographic Data of compound Ir11 (CCDC 1999593).
Supplementary Data 6
Crystallographic Data of compound 6-OAc (CCDC 1999594).
Supplementary Data 7
Crystallographic Data of compound 29 (CCDC 1999595).
Rights and permissions
About this article
Cite this article
Hong, S.Y., Kim, D. & Chang, S. Catalytic access to carbocation intermediates via nitrenoid transfer leading to allylic lactams. Nat Catal 4, 79–88 (2021). https://doi.org/10.1038/s41929-020-00558-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00558-x