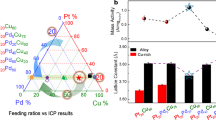
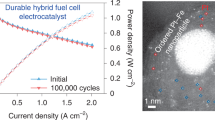
Abstract
A critical technological roadblock to the widespread adoption of proton-exchange membrane fuel cells is the development of highly active and durable platinum-based catalysts for accelerating the sluggish oxygen reduction reaction, which has largely relied on anecdotal discoveries so far. While the oxygen binding energy ∆EO has been frequently used as a theoretical descriptor for predicting the activity, there is no known descriptor for predicting durability. Here we developed a binary experimental descriptor that captures both the strain and Pt transition metal coupling contributions through X-ray absorption spectroscopy and directly correlated the binary experimental descriptor with the calculated ∆EO of the catalyst surface. This leads to an experimentally validated Sabatier plot to predict both the catalytic activity and stability for a wide range of Pt-alloy oxygen reduction reaction catalysts. Based on the binary experimental descriptor, we further designed an oxygen reduction reaction catalyst wherein high activity and stability are simultaneously achieved.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The atomic coordinates of the DFT calculation data and simulated XANES data are available in the Supplementary Data. The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Wu, J. & Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 46, 1848–1857 (2013).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Peng, Z. & Yang, H. Designer platinum nanoparticles: control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 4, 143–164 (2009).
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Kodama, K., Nagai, T., Kuwaki, A., Jinnouchi, R. & Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 16, 140–147 (2021).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Rossmeisl, J., Karlberg, G. S., Jaramillo, T. & Nørskov, J. K. Steady state oxygen reduction and cyclic voltammetry. Faraday Discuss. 140, 337–346 (2009).
Zhang, L. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015).
Kitchin, J. R., Nørskov, J. K., Barteau, M. A. & Chen, J. G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 120, 10240–10246 (2004).
Zhao, Z. et al. Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 31, 1808115 (2019).
Toda, T., Igarashi, H., Uchida, H. & Watanabe, M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 146, 3750–3756 (1999).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007).
Stephens, I. E. L. et al. Tuning the activity of Pt(111) for oxygen electroreduction by subsurface alloying. J. Am. Chem. Soc. 133, 5485–5491 (2011).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2, 454–460 (2010).
Kitchin, J. R., Nørskov, J. K., Barteau, M. A. & Chen, J. G. Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Phys. Rev. Lett. 93, 156801 (2004).
Liu, Z., Zhao, Z., Peng, B., Duan, X. & Huang, Y. Beyond extended surfaces: understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 142, 17812–17827 (2020).
Chen, Y., Cheng, T. & Goddard, W. A. III Atomistic explanation of the dramatically improved oxygen reduction reaction of jagged platinum nanowires, 50 times better than Pt. J. Am. Chem. Soc. 142, 8625–8632 (2020).
Bligaard, T. & Nørskov, J. K. Ligand effects in heterogeneous catalysis and electrochemistry. Electrochim. Acta 52, 5512–5516 (2007).
Calle-Vallejo, F. et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science 350, 185–189 (2015).
Cui, C., Gan, L., Heggen, M., Rudi, S. & Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 12, 765–771 (2013).
Cherevko, S., Kulyk, N. & Mayrhofer, K. J. J. Durability of platinum-based fuel cell electrocatalysts: dissolution of bulk and nanoscale platinum. Nano Energy 29, 275–298 (2016).
Calle-Vallejo, F. et al. Why conclusions from platinum model surfaces do not necessarily lead to enhanced nanoparticle catalysts for the oxygen reduction reaction. Chem. Sci. 8, 2283–2289 (2017).
Chattot, R. et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 17, 827–833 (2018).
Bu, L. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Tian, X. et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366, 850–856 (2019).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Zhang, C., Hwang, S. Y. & Peng, Z. Size-dependent oxygen reduction property of octahedral Pt–Ni nanoparticle electrocatalysts. J. Mater. Chem. A 2, 19778–19787 (2014).
Wang, C. et al. Correlation between surface chemistry and electrocatalytic properties of monodisperse PtxNi1-x nanoparticles. Adv. Funct. Mater. 21, 147–152 (2011).
Huang, X. et al. A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts. Energy Environ. Sci. 7, 2957–2962 (2014).
Fortunelli, A. et al. The atomistic origin of the extraordinary oxygen reduction activity of Pt3Ni7 fuel cell catalysts. Chem. Sci. 6, 3915–3925 (2015).
Gong, M. et al. Optimizing PtFe intermetallics for oxygen reduction reaction: from DFT screening to in situ XAFS characterization. Nanoscale 11, 20301–20306 (2019).
Wang, T. et al. Sub-6 nm fully ordered L10-Pt–Ni–Co nanoparticles enhance oxygen reduction via Co doping induced ferromagnetism enhancement and optimized surface strain. Adv. Energy Mater. 9, 1803771 (2019).
Dutta, I. et al. Electrochemical and structural study of a chemically dealloyed PtCu oxygen reduction catalyst. J. Phys. Chem. C 114, 16309–16320 (2010).
Hwang, B. J. et al. An investigation of structure−catalytic activity relationship for Pt−Co/C bimetallic nanoparticles toward the oxygenreduction reaction. J. Phys. Chem. C 111, 15267–15276 (2007).
Moraweck, B., Renouprez, A. J., Hlil, E. K. & Baudoing-Savois, R. Alloying effects on X-ray absorption edges in nickel-platinum single crystals. J. Phys. Chem. 97, 4288–4292 (1993).
Hlil, E. K., BaudoingSavois, R., Moraweck, B. & Renouprez, A. J. X-ray absorption edges in platinum-based alloys. 2. Influence of ordering and of the nature of the second metal. J. Phys. Chem. 100, 3102–3107 (1996).
Chen, J. et al. Elucidating the many-body effect and anomalous Pt and Ni core level shifts in X-ray photoelectron spectroscopy of Pt–Ni alloys. J. Phys. Chem. C 124, 2313–2318 (2020).
Mukerjee, S., Srinivasan, S., Soriaga, M. P. & Mcbreen, J. Role of structural and electronic-properties of Pt and Pt alloys on electrocatalysis of oxygen reduction: an in-situ XANES and EXAFS investigation. J. Electrochem. Soc. 142, 1409–1422 (1995).
Rehr, J. J., Kas, J. J., Vila, F. D., Prange, M. P. & Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 12, 5503–5513 (2010).
Pápai, Z. & Pap, T. L. Analysis of peak asymmetry in chromatography. J. Chromatogr. A 953, 31–38 (2002).
Kuhn, M. & Sham, T. K. Charge redistribution and electronic behavior in a series of Au-Cu alloys. Phys. Rev. B 49, 1647–1661 (1994).
Jia, Q. et al. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: in situ observation of the linear composition–strain–activity relationship. ACS Nano 9, 387–400 (2015).
Cao, L. et al. Differential surface elemental distribution leads to significantly enhanced stability of PtNi-based ORR catalysts. Matter 1, 1567–1580 (2019).
Li, J. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule 3, 124–135 (2019).
Barcaro, G., Sementa, L. & Fortunelli, A. A grouping approach to homotop global optimization in alloy nanoparticles. Phys. Chem. Chem. Phys. 16, 24256–24265 (2014).
Hu, J. et al. Increasing stability and activity of core–shell catalysts by preferential segregation of oxide on edges and vertexes: oxygen reduction on Ti–Au@Pt/C. J. Am. Chem. Soc. 138, 9294–9300 (2016).
Jennings, P. C., Aleksandrov, H. A., Neyman, K. M. & Johnston, R. L. A DFT study of oxygen dissociation on platinum based nanoparticles. Nanoscale 6, 1153–1165 (2014).
Arán-Ais, R. M. et al. Elemental anisotropic growth and atomic-scale structure of shape-controlled octahedral Pt–Ni–Co alloy nanocatalysts. Nano Lett. 15, 7473–7480 (2015).
Zhao, Z. et al. Composition tunable ternary Pt–Ni–Co octahedra for optimized oxygen reduction activity. Chem. Commun. 52, 11215–11218 (2016).
Huang, X. et al. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Huang, J. et al. PtCuNi tetrahedra catalysts with tailored surfaces for efficient alcohol oxidation. Nano Lett. 19, 5431–5436 (2019).
Arruda, T. M., Shyam, B., Ziegelbauer, J. M., Mukerjee, S. & Ramaker, D. E. Investigation into the competitive and site-specific nature of anion adsorption on Pt using in situ X-ray absorption spectroscopy. J. Phys. Chem. C 112, 18087–18097 (2008).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Newville, M., Līviņš, P., Yacoby, Y., Rehr, J. J. & Stern, E. A. Near-edge X-ray-absorption fine structure of Pb: a comparison of theory and experiment. Phys. Rev. B 47, 14126–14131 (1993).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Mattheiss, L. F. Energy bands for solid argon. Phys. Rev. 133, A1399–A1403 (1964).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Condens. Matter Phys. 21, 395502 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Wang, Z., Guo, X., Montoya, J. & Nørskov, J. K. Predicting aqueous stability of solid with computed Pourbaix diagram using SCAN functional. npj Comput. Mater. 6, 160 (2020).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Gong, M. et al. One-nanometer-thick Pt3Ni bimetallic alloy nanowires advanced oxygen reduction reaction: integrating multiple advantages into one catalyst. ACS Catal. 9, 4488–4494 (2019).
Beermann, V. et al. Rh-doped Pt–Ni octahedral nanoparticles: understanding the correlation between elemental distribution, oxygen reduction reaction, and shape stability. Nano Lett. 16, 1719–1725 (2016).
Lim, J. et al. Ga–doped Pt–Ni octahedral nanoparticles as a highly active and durable electrocatalyst for oxygen reduction reaction. Nano Lett. 18, 2450–2458 (2018).
Bu, L. et al. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 7, 11850 (2016).
Acknowledgements
Y.H., Q.J., W.A.G. and X.D. gratefully acknowledge the support of the Office of Naval Research (award N000141812155). The XAS data were collected at beamlines 6-BM, 7-BM and 8-ID of the National Synchrotron Light Source II, a US Department of Energy Office of Science User Facility operated for the Department of Energy Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. We acknowledge the use of facilities and instrumentation at the University of California Irvine Materials Research Institute, supported in part by the National Science Foundation Materials Research Science and Engineering Center programme through the University of California Irvine Center for Complex and Active Materials (DMR-2011967). We also thank the Electron Imaging Center of Nanomachines at the California NanoSystems Institute (CNSI) for TEM support. A.F. and W.A.G. received support from the National Science Foundation (CBET-1805022 and CBET-2005250). A.F., G.B. and L.S. gratefully acknowledge the contribution of the International Research Network on Nanoalloys Centre national de la recherche scientifique (CNRS) and computational support from the CINECA supercomputing centre within the Italian SuperComputing Resource Allocation (ISCRA) programme.
Author information
Authors and Affiliations
Contributions
J.H., M.F., M.L., Y.L., C.W., S.-J.L., B.P. and Z.L. conducted the synthesis of electrocatalysts, structural characterization and electrochemical experiments. M.X. and J.H. conducted the TEM and EDX characterizations. Q.J., E.L., L.J. and D.L. conducted the XAS studies. A.F., L.S., G.B., Q.J., J.H. and W.A.G. performed the modelling and data analyses. The project was conceived by Y.H. and supervised by Y.H. (project design, syntheses and evaluation of the catalysts); Q.J. (XAS studies); and A.F. and W.A.G. (computational studies). J.H., Y.H., Q.J. and A.F. wrote the original draught. J.H., Y.H., Q.J., A.F., W.A.G. and Z.L. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Janis Timoshenko and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Figs. 1–34, Tables 1–6 and refs. 1–12.
Supplementary Data
Coordination of DFT models.
Source data
Source Data Fig. 2
Source data of the XANES simulation.
Rights and permissions
About this article
Cite this article
Huang, J., Sementa, L., Liu, Z. et al. Experimental Sabatier plot for predictive design of active and stable Pt-alloy oxygen reduction reaction catalysts. Nat Catal 5, 513–523 (2022). https://doi.org/10.1038/s41929-022-00797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00797-0
This article is cited by
-
Identifying the distinct roles of dual dopants in stabilizing the platinum-nickel nanowire catalyst for durable fuel cell
Nature Communications (2024)
-
Coordinatively unsaturated single Co atoms immobilized on C2N for efficient oxygen reduction reaction
Nano Research (2023)
-
Spectroscopy predicts catalyst functionality
Nature Catalysis (2022)