Abstract
Age-related decline in skeletal muscle regenerative capacity is multifactorial, yet the contribution of immune dysfunction to regenerative failure is unknown. Macrophages are essential for effective debris clearance and muscle stem cell activity during muscle regeneration, but the regulatory mechanisms governing macrophage function during muscle repair are largely unexplored. Here, we uncover a new mechanism of immune modulation operating during skeletal muscle regeneration that is disrupted in aged animals and relies on the regulation of macrophage function. The immune modulator mesencephalic astrocyte-derived neurotrophic factor (MANF) is induced following muscle injury in young mice but not in aged animals, and its expression is essential for regenerative success. Regenerative impairments in aged muscle are associated with defects in the repair-associated myeloid response similar to those found in MANF-deficient models and could be improved through MANF delivery. We propose that restoring MANF levels is a viable strategy to improve myeloid response and regenerative capacity in aged muscle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the data generated or analyzed during this study are included in the published article and its Supplementary Information and Source Data files and are available from the corresponding author upon reasonable request. RNA sequencing data generated in this study are available under accession numbers GSE224982 and GSE224983 from the NCBI Gene Expression Omnibus database. Correspondence and requests for materials should be addressed to P.S.-V. and J.N.
References
Neves, J., Sousa-Victor, P. & Jasper, H. Rejuvenating strategies for stem cell-based therapies in aging. Cell Stem Cell 20, 161–175 (2017).
Schultz, M. B. & Sinclair, D. A. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3–14 (2016).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Conboy, I. M., Conboy, M. J. & Rebo, J. Systemic problems: a perspective on stem cell aging and rejuvenation. Aging 7, 754–765 (2015).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Neves, J. & Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 287, 43–52 (2020).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Wosczyna, M. N. & Rando, T. A. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev. Cell 46, 135–143 (2018).
Sousa-Victor, P., Garcia-Prat, L. & Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 23, 204–226 (2022).
Munoz-Canoves, P., Neves, J. & Sousa-Victor, P. Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells. FEBS J. 287, 406–416 (2020).
Tidball, J. G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17, 165–178 (2017).
Chazaud, B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 41, 481–492 (2020).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Perdiguero, E. et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. 195, 307–322 (2011).
Neves, J. et al. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 353, aaf3646 (2016).
Jntti, M. & Harvey, B. K. Trophic activities of endoplasmic reticulum proteins CDNF and MANF. Cell Tissue Res. 382, 83–100 (2020).
Sousa-Victor, P., Jasper, H. & Neves, J. Trophic factors in inflammation and regeneration: the role of MANF and CDNF. Front. Physiol. 9, 1629 (2018).
Lindahl, M., Saarma, M. & Lindholm, P. Unconventional neurotrophic factors CDNF and MANF: structure, physiological functions and therapeutic potential. Neurobiol. Dis. 97, 90–102 (2017).
Tang, Q., Li, Y. & He, J. MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol. Metab. 33, 236–246 (2022).
Sousa-Victor, P. et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat. Metab. 1, 276–290 (2019).
Neves, J. et al. MANF delivery improves retinal homeostasis and cell replacement therapies in ageing mice. Exp. Gerontol. 134, 110893 (2020).
Sousa-Victor, P. et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 (2014).
Rahman, F. A., Angus, S. A., Stokes, K., Karpowicz, P. & Krause, M. P. Impaired ECM remodeling and macrophage activity define necrosis and regeneration following damage in aged skeletal muscle. Int. J. Mol. Sci. 21, 4575 (2020).
Mircescu, M. M., Lipuma, L., van Rooijen, N., Pamer, E. G. & Hohl, T. M. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J. Infect. Dis. 200, 647–656 (2009).
Kowalski, E. A. et al. Monocyte proinflammatory phenotypic control by ephrin type A receptor 4 mediates neural tissue damage. JCI Insight 7, e156319 (2022).
Lehmann, M. L. et al. CCR2 monocytes repair cerebrovascular damage caused by chronic social defeat stress. Brain Behav. Immun. 101, 346–358 (2022).
Varga, T. et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J. Immunol. 196, 4771–4782 (2016).
Wang, Y., Welc, S. S., Wehling-Henricks, M. & Tidball, J. G. Myeloid cell-derived tumor necrosis factor-alpha promotes sarcopenia and regulates muscle cell fusion with aging muscle fibers. Aging Cell 17, e12828 (2018).
Wang, Y. et al. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J. 33, 1415–1427 (2019).
Runyan, C. E. et al. Impaired phagocytic function in CX3CR1+ tissue-resident skeletal muscle macrophages prevents muscle recovery after influenza A virus-induced pneumonia in old mice. Aging Cell 19, e13180 (2020).
Tobin, S. W. et al. Delineating the relationship between immune system aging and myogenesis in muscle repair. Aging Cell 20, e13312 (2021).
Patsalos, A. et al. In vivo GDF3 administration abrogates aging related muscle regeneration delay following acute sterile injury. Aging Cell 17, e12815 (2018).
Summan, M. et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1488–R1495 (2006).
Sahu, A. et al. Age-related declines in alpha-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859 (2018).
Wehling-Henricks, M. et al. Macrophages escape Klotho gene silencing in the mdx mouse model of Duchenne muscular dystrophy and promote muscle growth and increase satellite cell numbers through a Klotho-mediated pathway. Hum. Mol. Genet. 27, 14–29 (2018).
Zhang, C. et al. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J. Cachexia Sarcopenia Muscle 11, 1291–1305 (2020).
Paliwal, P., Pishesha, N., Wijaya, D. & Conboy, I. M. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging 4, 553–566 (2012).
Tidball, J. G., Flores, I., Welc, S. S., Wehling-Henricks, M. & Ochi, E. Aging of the immune system and impaired muscle regeneration: a failure of immunomodulation of adult myogenesis. Exp. Gerontol. 145, 111200 (2021).
Al-Zaeed, N., Budai, Z., Szondy, Z. & Sarang, Z. TAM kinase signaling is indispensable for proper skeletal muscle regeneration in mice. Cell Death Dis. 12, 611 (2021).
Petrova, P. et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 20, 173–188 (2003).
Voutilainen, M. H. et al. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. 29, 9651–9659 (2009).
Lu, J. et al. Photoreceptor protection by mesencephalic astrocyte-derived neurotrophic factor (MANF). eNeuro 5, ENEURO.0109-18.2018 (2018).
Glembotski, C. C. et al. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J. Biol. Chem. 287, 25893–25904 (2012).
Zhang, Y. et al. Hormonal therapies up-regulate MANF and overcome female susceptibility to immune checkpoint inhibitor myocarditis. Sci. Transl. Med. 14, eabo1981 (2022).
He, M. et al. Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism. World J. Gastroenterol. 26, 1029–1041 (2020).
Lindahl, M. et al. MANF is indispensable for the proliferation and survival of pancreatic beta cells. Cell Rep. 7, 366–375 (2014).
Herranen, A. et al. Deficiency of the ER-stress-regulator MANF triggers progressive outer hair cell death and hearing loss. Cell Death Dis. 11, 100 (2020).
Han, D. et al. Mesencephalic astrocyte-derived neurotrophic factor restores blood-brain barrier integrity of aged mice after ischaemic stroke/reperfusion through anti-inflammation via TLR4/MyD88/NF-κB pathway. J. Drug Target. 30, 430–441 (2022).
Yang, F. et al. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J. Stem Cells 12, 633–658 (2020).
Zhang, J. X. et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) prevents the neuroinflammation induced dopaminergic neurodegeneration. Exp. Gerontol. 171, 112037 (2023).
Tonkin, J. et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol. Ther. 23, 1189–1200 (2015).
Zhao, W., Lu, H., Wang, X., Ransohoff, R. M. & Zhou, L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J. 30, 380–393 (2016).
Pakarinen, E. et al. MANF ablation causes prolonged activation of the UPR without neurodegeneration in the mouse midbrain dopamine system. eNeuro 7, ENEURO.0477-19.2019 (2020).
Pakarinen, E., Lindholm, P., Saarma, M. & Lindahl, M. CDNF and MANF regulate ER stress in a tissue-specific manner. Cell. Mol. Life Sci. 79, 124 (2022).
Roy, A. et al. The IRE1/XBP1 signaling axis promotes skeletal muscle regeneration through a cell non-autonomous mechanism. eLife 10, e73215 (2021).
Chen, L. et al. Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway. Sci. Rep. 5, 8133 (2015).
Oh, J. et al. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging 8, 2871–2896 (2016).
Yagi, T. et al. Neuroplastin modulates anti-inflammatory effects of MANF. iScience 23, 101810 (2020).
Ren, H., Xia, X., Dai, X. & Dai, Y. The role of neuroplastin65 in macrophage against E. coli infection in mice. Mol. Immunol. 150, 78–89 (2022).
Sereno, D. et al. An evolutionary perspective on the role of mesencephalic astrocyte-derived neurotrophic factor (MANF): at the crossroads of poriferan innate immune and apoptotic pathways. Biochem. Biophys. Rep. 11, 161–173 (2017).
Doyle, S. E. et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199, 81–90 (2004).
Ortuste Quiroga, H. P., Goto, K. & Zammit, P. S. Isolation, cryosection and immunostaining of skeletal muscle. Methods Mol. Biol. 1460, 85–100 (2016).
Bencze, M., Periou, B., Baba-Amer, Y. & Authier, F. J. Immunolabelling myofiber degeneration in muscle biopsies. J. Vis. Exp. https://doi.org/10.3791/59754 (2019).
Galli, E. et al. Mesencephalic astrocyte-derived neurotrophic factor is upregulated with therapeutic fasting in humans and diet fat withdrawal in obese mice. Sci. Rep. 9, 14318 (2019).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 4, 44–57 (2009).
Acknowledgements
We thank the Flow Cytometry, Comparative Pathology, Bioimaging and Rodent facilities of Instituto de Medicina Molecular João Lobo Antunes for technical support. We thank A. S. Pacheco and E. M. Tranfield from the Electron Microscopy Facility at the Instituto Gulbenkian de Ciência for sample processing, data collection and discussion of the results. This work was supported by EMBO (IG4448 to P.S.V.) and FCT (PTDC/MED-OUT/8010/2020 and EXPL/MED-OUT/1601/2021 to P.S.V. and J.N.). P.S.V. was supported by ‘la caixa’ Foundation Junior Leader Fellowship (LCF/BQ/PI19/11690006). J.N. was supported by an assistant research contract from FCT (2021.03843.CEECIND). P.L. was supported by the Academy of Finland (grant 343299) and by the Jane and Aatos Erkko Foundation.
Author information
Authors and Affiliations
Contributions
J.N. and P.S.V. conceived the study, designed experiments, analyzed and interpreted data, and wrote the manuscript. N.S.S. and M.F.B. designed and performed experiments, and analyzed and interpreted data. I.B.A. performed experiments and analyzed data. P.L. performed the ELISA analysis of muscle extracts. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Matthias Wiens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Regulation of muscle regeneration by MANF.
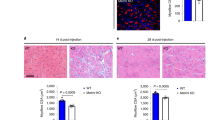
a, Relative levels of MANF mRNA, detected by RT-qPCR, in TA muscles of yg wt (C57BL/6) mice non-injured and at different time points following injury (2, 3, 4, 5, 6, 10 dpi) (n = 3 for 3,4,6,10dpi; n = 4 for n.i.,2,5dpi). b, Illustrative Western blot analysis of MANF levels in protein extracts of TA muscles from yg (2–6 mo) and old (22–25 mo) wt (C57BL/6) mice at 3dpi. Ponceau S-staining of the membrane was used to verify equal protein loading in each sample. c, MANF protein levels, quantified by ELISA, in extracts of TA muscles from yg (2–6 mo) and old (22–25 mo) wt (C57BL/6) mice, at 2 and 3dpi (n = 5 for yg 3dpi; n = 3 for all other conditions). d, Western blot analysis of MANF levels in protein extracts from ManfR26WT and ManfR26Δ mice at 3dpi. Ponceau S-staining of the membrane was used to verify equal protein loading in each sample. In a, p values are from one-way ANOVA with Bonferroni’s multiple comparison post-test. n.i., non-injured; dpi, days post-injury; yg, young.
Extended Data Fig. 2 Effects of aging and MANF-deficiency in the cellular response during muscle regeneration.
a, d, Gating strategy used in flow cytometry analysis of (a) CD45p°s immune cell population, endothelial cells, FAPs and MuSCs; and (d) myeloid cells (CD11bp°s), pro-repair macrophages (F4/80posLy6CLow), pro-inflammatory macrophages (LyC6High), and neutrophils (F4/80negLy6Gpos). b, c, e, f, Quantification, by flow cytometry, of FAPS (b, n = 3/condition), endothelial cells (c, n = 3/condition) and neutrophils (e, n = 7 for ManfR26WT; n = 6 for ManfR26Δ) in regenerating muscles of ManfR26WT and ManfR26Δ mice at 3dpi; and neutrophils (f, n = 4/condition) in regenerating muscles of yg (2–6 mo) and old (22–24 mo) wt (C57BL/6) mice at 3dpi. g–i, Quantification, by flow cytometry, of myeloid cells (CD11bpos, g), pro-repair macrophages (F4/80posLy6CLow, h) and pro-inflammatory macrophages (Ly6CHigh, i) in regenerating muscles of yg (2–6 mo) and old (22–25 mo) wt (C57BL/6) mice at 2 and 3dpi (n = 4 for yg and old 3dpi; n = 5 for old 2dpi; n = 6 for yg 2dpi). Data are represented as average ± s.e.m. and each n represents one animal. p values are from two-tailed Student’s t-test. FAPs, Fibroadipogenic progenitors; MuSCs; Muscle Stem Cells; yg, young.
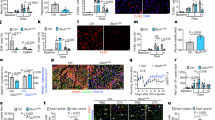
Extended Data Fig. 3 Macrophage-derived MANF in muscle regeneration.
a, d Quantification, by flow cytometry, of macrophages (a), pro-repair macrophages (d, top; F4/80posLy6CLow) and pro-inflammatory macrophages (d, bottom; Ly6CHigh) in regenerating muscles of wt (C57BL/6) mice at different time points following injury (n = 6 for macrophages 2dpi; n = 8 for F4/80posLy6CLow and Ly6CHigh at 2dpi; n = 3 for all other conditions). b, Representative images of cryosections from Tibialis anterior (TA) muscles immunostained against F4/80 (green) and MANF (red). DAPI is used to identify nuclei. Arrowheads indicate cells with high MANF expression co-localized with F4/80. Scale bar: 10μm c, Quantification, by flow cytometry, of macrophages and neutrophils in regenerating quadriceps (QC) muscles of wt (C57BL6/J) mice at 3dpi, treated with clodronate liposomes or control PBS liposomes (n = 6/condition). Data are represented as average ± s.e.m. and each n represents one animal. p values are from two-tailed Student’s t-test. dpi, days post-injury; PBS-Lipo, PBS Liposomes; Clo-Lipo, Clodronate Liposomes.
Extended Data Fig. 4 Macrophage-derived MANF in muscle regeneration.
a–c, Quantification, by flow cytometry, of myeloid cells (CD11bpos, a), pro-repair macrophages (F4/80posLy6CLow, b), and ratio of pro-repair to pro-inflammatory macrophages (LyC6Low/LyC6High, c) in regenerating quadriceps (QC) muscles of tamoxifen treated Manffl/fl and ManfCx3cr1Δ mice at 3dpi (n = 4/condition). d, Representative images of cryosections from Tibialis anterior (TA) muscles of Manffl/fl, ManfCx3cr1WT and ManfCx3cr1Δ mice, at 4 and 14dpi, stained with H&E and immunostained with mouse IgG. Asterisks indicate necrotic myofibres. Scale bars: 50 μm for H&E 4dpi; 20μm for IgG 4dpi; 100μm for H&E 14dpi. e, f, Quantification of the average cross-sectional area of central nucleated new myofibres (f) and frequency distribution of new myofibres by size (e), in regenerating TA muscles from Manffl/fl and ManfCx3cr1Δ mice at 14dpi (n = 4 for Manffl/fl; n = 3 for ManfCx3cr1Δ). g, Quantification of the average cross-sectional area of myofibers in non-injured TA muscles from ManfCx3cr1WT and ManfCx3cr1Δ mice (n = 5 for ManfCx3cr1WT; n = 3 for ManfCx3cr1Δ). Data are represented as average ± s.e.m. and each n represents one animal. p values are from two-tailed Student’s t-test. dpi, days post-injury; H&E, Hematoxylin and Eosin; msIgG, mouse Immunoglobulin; CSA, cross-sectional area.
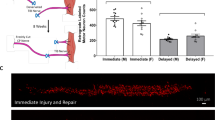
Extended Data Fig. 5 Effects of MANF ablation in Cx3cr1pos cells.
a, Experimental timeline for analysis of animals with conditional ablation of MANF only in Cx3cr1-expressing cells existing prior to muscle injury. b–d, Quantification, by flow cytometry, of myeloid cells (CD11bpos, b), pro-repair macrophages (F4/80posLy6CLow, c), and pro-inflammatory macrophages (LyC6High, d) in 3dpi regenerating QC muscles of ManfCx3cr1WT and ManfCx3cr1Δ mice, treated with tamoxifen prior to the injury (n = 3/condition). e, f, Quantification, by flow cytometry, of macrophages (CD11bposF4/80pos, e) and neutrophils (CD11bposF4/80negLy6Gpos, f) non-injured QC muscles of tamoxifen treated Manffl/fl and ManfCx3cr1Δ mice (n = 4/condition). g, Representative density plots from flow cytometry analysis of myeloid blood cell populations gated on CD11bposF4/80neg population, identifying neutrophils (Ly6Gpos), Ly6Cpos classical monocytes (Ly6GnegLy6Cpos) and Ly6Cneg non-classical monocytes (Ly6GnegLy6CnegCx3cr1pos). h-i, Quantification, by flow cytometry, of myeloid cells (CD11bpos), Ly6Cpos classical monocytes, Ly6Cneg non-classical monocytes and neutrophils in the blood of ManfCx3cr1WT and ManfCx3cr1Δ mice prior to muscle injury (h, n = 6 for ManfCx3cr1WT; n = 4 for ManfCx3cr1Δ), and at 3dpi (i, n = 4/ condition). j, l, Representative density plots from flow cytometry analysis of macrophage populations from ManfCx3cr1WT and ManfCx3cr1Δ animals at 3dpi, showing EdU signal (j) and Apopxin-Green signal (l). For both stains the FMO density plot is shown to define the positive population. k, Quantification, by flow cytometry, of EdUpos macrophages as a percentage of pro-repair and pro-inflammatory populations, in ManfCx3cr1WT and ManfCx3cr1Δ animals at 3dpi (n = 3 for ManfCx3cr1WT; n = 4 for ManfCx3cr1Δ). m, Quantification, by flow cytometry, of Apopxinpos macrophages as a percentage of pro-repair the population, in ManfCx3cr1WT and ManfCx3cr1Δ animals at 3dpi (n = 7 for ManfCx3cr1WT; n = 5 for ManfCx3cr1Δ). Data are represented as average ± s.e.m. and each n represents one animal. p values are from two-tailed Student’s t-test. FSC, Forward Scatter; Edu, 5-ethynyl-2’-deoxyuridine; FMO, Fluorescence Minus One Control.
Extended Data Fig. 6 MANF-deficiency affects macrophage phenotypic transition and inflammatory status.
a, Experimental timeline for analysis of animals with ablation of MANF in macrophages. b, Western blot analysis of MANF levels in protein extracts from F4/80pos cells FACS-isolated from QC muscles of Manffl/fl and ManfLysMΔ mice at 3dpi. Vinculin was used to verify equal protein loading in each sample. c–e, Quantification, by flow cytometry, of myeloid cells (CD11bpos, c), pro-repair macrophages (F4/80posLy6CLow, d) and pro-inflammatory macrophages (LyC6High, e) in regenerating QC muscles of Manffl/fl and ManfLysMΔ mice at 3dpi (n = 4/condition). f, Relative levels of Manf, Il1β and TNFα mRNA, detected by RT-qPCR, in BMDMs generated from Manffl/fl and ManfR26Δ mice in control conditions or 3 h after stimulation with Fibrinogen (n = 6 for ManfWT and n = 8 for ManfKO). Data are represented as average ± s.e.m. and each n represents one animal or one cell culture derived from one independent animal. p values are from two-tailed Student’s t-test. BMDMs, Bone marrow-derived macrophages; Fgn, Fibrinogen.
Extended Data Fig. 7 Defects of MANF-deficient macrophages.
a, GO categories of biological processes showing significant enrichment in the dataset of genes differentially expressed in macrophages (CD45posF4/80pos) FACS-isolated at 3dpi from quadriceps muscles of ManfCx3cr1Δ mice compared to ManfCx3cr1WT mice (fold change < 0.75 or >1.5 and p ≤ 0.05, p values from two-tailed Student’s t-test, n = 3/condition). b, Representative histogram of the Fluoresbrite® 641 signal in BMDMs generated from Manffl/fl (grey) and ManfR26Δ (pink) mice, 3 h after stimulation with opsonized Fluoresbrite® 641 Carboxylate beads. Signal in non-stimulated BMDMs is shown in blue. BMDMs, Bone marrow-derived macrophages.
Extended Data Fig. 8 Transmission electron microscopy analysis MANF-deficient and macrophages.
Representative images of pro-repair macrophages (F4/80posLy6CLow) FACS-isolated at 3dpi from QC muscles of ManfCx3cr1WT and ManfCx3cr1Δ mice, analyzed by TEM. Scale bars: 2 μm Quantifications of these images, for independent cells, are shown in Fig. 6c–e. The quantification includes 54 macrophages isolated from ManfCx3cr1WT mice and 42 macrophages isolated from ManfCx3cr1Δ mice. Macrophages were sorted from n = 3 animals/condition in 2 independent experiments (experiment 1: n = 1/condition; experiment 2: n = 2/condition, pooled). Samples obtained in each experiment were processed independently for analysis.
Extended Data Fig. 9 Defects of aged macrophages.
a, GO categories of biological processes with relevance within the context of tissue regeneration showing significant enrichment in the dataset of genes down-regulated in pro-repair macrophages (CD11bposF4/80posLy6CLow) FACS-isolated at 3dpi from quadriceps muscles of old (22–24 mo) mice compared to yg (2–6 mo) mice (fold change < 0.75 and p ≤ 0.05, p values from two-tailed Student’s t-test, n = 3/condition). e, Representative histograms of the FITC signal derived from lysosomal hydrolysis of a self-quenched substrate in basal conditions (light) and after stimulation with Apop-necro debris (dark), in BMDMs generated from yg (grey) and old (brown) mice, and in old BMDMs cultured in the presence of rMANF (blue), 3 h after stimulation. GO, Gene Ontology; BP, Biological process; BMDMs, Bone marrow-derived macrophages.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sousa, N.S., Brás, M.F., Antunes, I.B. et al. Aging disrupts MANF-mediated immune modulation during skeletal muscle regeneration. Nat Aging 3, 585–599 (2023). https://doi.org/10.1038/s43587-023-00382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00382-5