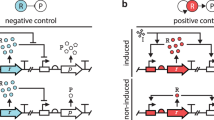
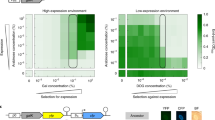
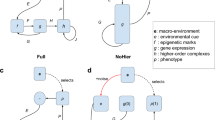
Abstract
In response to severe genetic and environmental perturbations, wild-type organisms can express hidden alternative phenotypes adaptive to such adverse conditions. While our theoretical understanding of the population-level fitness advantage and evolution of phenotypic switching under variable environments has grown, the mechanism by which these organisms maintain phenotypic switching capabilities under static environments remains to be elucidated. Here, using computational simulations, we analyzed the evolution of gene circuits under natural selection and found that different strategies evolved to increase the gene expression stability near the optimum level. In a population comprising bistable individuals, a strategy of maintaining bistability and raising the potential barrier separating the bistable regimes was consistently taken. Our results serve as evidence that hidden bistable switches can be stably maintained during environmental stasis—an essential property enabling the timely release of adaptive alternatives with small genetic changes in the event of substantial perturbations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The simulation data along with the data analysis scripts associated with the current submission and the source data for Figs. 1–4 are available in the Zenodo repository48.
Code availability
The simulator source code and data analysis scripts have been deposited in GitHub, and they can be accessed at https://github.com/hkuwahara/evo-hidden-switch. The tool package has also been deposited in Zenodo48.
References
Waddington, C. H. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (1942).
Waddington, C. H. Genetic assimilation of an acquired character. Evolution 7, 118–126 (1953).
Schmalhausen I. I., Dordick I. & Dobzhansky T. Factors of Evolution: The Theory of Stabilizing Selection (Univ. Chicago Press, 1987).
Gibson, G. & Wagner, G. Canalization in evolutionary genetics: a stabilizing theory? Bioessays 22, 372–380 (2000).
West-Eberhard, M. J. Toward a modern revival of Darwin’s theory of evolutionary novelty. Phil. Sci. 75, 899–908 (2008).
Félix, M. A. & Barkoulas, M. Pervasive robustness in biological systems. Nat. Rev. Genet. 16, 483–496 (2015).
West-Eberhard, M. J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (1989).
Suzuki, Y. & Nijhout, H. F. Evolution of a polyphenism by genetic accommodation. Science 311, 650–652 (2006).
Eldar, A. et al. Partial penetrance facilitates developmental evolution in bacteria. Nature 460, 510–514 (2009).
Raj, A., Rifkin, S. A., Andersen, E. & van Oudenaarden, A. Variability in gene expression underlies incomplete penetrance. Nature 463, 913–918 (2010).
Specchia, V. et al. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 463, 662–665 (2010).
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford Univ. Press, 2003).
Masel, J., King, O. D. & Maughan, H. The loss of adaptive plasticity during long periods of environmental stasis. Am. Nat. 169, 38–46 (2007).
Kærn, M., Elston, T., Blake, W. & Collins, J. Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 6, 451–464 (2005).
Raser, J. M. & O’Shea, E. K. Noise in gene expression: origins, consequences, and control. Science 309, 2010–2013 (2005).
Losick, R. & Desplan, C. Stochasticity and cell fate. Science 320, 65–68 (2008).
Raj, A. & van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226 (2008).
Acar, M., Mettetal, J. T. & van Oudenaarden, A. Stochastic switching as a survival strategy in fluctuating environments. Nat. Genet. 40, 471–475 (2008).
Capp, J. P. Noise-driven heterogeneity in the rate of genetic-variant generation as a basis for evolvability. Genetics 185, 395–404 (2010).
Johnston, R. J. Jr & Desplan, C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu. Rev. Cell Dev. Biol. 26, 689–719 (2010).
Kuwahara, H. & Soyer, O. S. Bistability in feedback circuits as a byproduct of evolution of evolvability. Mol. Syst. Biol. 8, 564 (2012).
Becskei, A., Séraphin, B. & Serrano, L. Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. EMBO J. 20, 2528–2535 (2001).
Thattai, M. & Van Oudenaarden, A. Stochastic gene expression in fluctuating environments. Genetics 167, 523–530 (2004).
Salathé, M., Van Cleve, J. & Feldman, M. W. Evolution of stochastic switching rates in asymmetric fitness landscapes. Genetics 182, 1159–1164 (2009).
Balázsi, G., van Oudenaarden, A. & Collins, J. J. Cellular decision making and biological noise: from microbes to mammals. Cell 144, 910–925 (2011).
Liberman, U., Van Cleve, J. & Feldman, M. W. On the evolution of mutation in changing environments: recombination and phenotypic switching. Genetics 187, 837–851 (2011).
Bedford, T. & Hartl, D. L. Optimization of gene expression by natural selection. Proc. Natl Acad. Sci. USA 106, 1133–1138 (2009).
Ozbudak, E. M., Thattai, M., Kurtser, I., Grossman, A. D. & van Oudenaarden, A. Regulation of noise in the expression of a single gene. Nat. Genet. 31, 69–73 (2002).
Lachmann, M. & Jablonka, E. The inheritance of phenotypes: an adaptation to fluctuating environments. J. Theor. Biol. 181, 1–9 (1996).
Meyers, L. A., Ancel, F. D. & Lachmann, M. Evolution of genetic potential. PLoS Comput. Biol. 1, 236–243. (2005).
Zhang, Z., Qian, W. & Zhang, J. Positive selection for elevated gene expression noise in yeast. Mol. Syst. Biol. 5, 299 (2009).
Bintu, L. et al. Transcriptional regulation by the numbers: models. Curr. Opin. Genet. Dev. 15, 116–124 (2005).
Kuwahara, H. et al. in Transactions on Computational Systems Biology VI (eds Priami, C. & Plotkin, G.) 150–175 (Springer, 2006).
Gunawardena, J. Time-scale separation—Michaelis and Menten’s old idea, still bearing fruit. FEBS J. 281, 473–488 (2014).
Acar, M., Becskei, A. & van Oudenaarden, A. Enhancement of cellular memory by reducing stochastic transitions. Nature 435, 228–232 (2005).
Xiong, W. & Ferrell, J. E. Jr A positive-feedback-based bistable’memory module’ that governs a cell fate decision. Nature 426, 460–465 (2003).
Lestas, I., Vinnicombe, G. & Paulsson, J. Fundamental limits on the suppression of molecular fluctuations. Nature 467, 174–178 (2010).
Thattai, M. & van Oudenaarden, A. Intrinsic noise in gene regulatory networks. Proc. Natl Acad. Sci. USA 98, 8614–8619 (2001).
Kuwahara, H., Arold, S. T. & Gao, X. Beyond initiation-limited translational bursting: the effects of burst size distributions on the stability of gene expression. Integr. Biol. 7, 1622–1632 (2015).
Duveau, F. et al. Fitness effects of altering gene expression noise in Saccharomyces cerevisiae. eLife 7, e37272 (2018).
Gibson, G. & Dworkin, I. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5, 681–690 (2004).
Nevozhay, D., Adams, R. M., Van Itallie, E., Bennett, M. R. & Balázsi, G. Mapping the environmental fitness landscape of a synthetic gene circuit. PLoS Comput. Biol. 8, e1002480 (2012).
González, C. et al. Stress-response balance drives the evolution of a network module and its host genome. Mol. Syst. Biol. 11, 827 (2015).
Kheir Gouda, M., Manhart, M. & Balázsi, G. Evolutionary regain of lost gene circuit function. Proc. Natl Acad. Sci. USA 116, 25162–25171 (2019).
Pujadas, E. & Feinberg, A. P. Regulated noise in the epigenetic landscape of development and disease. Cell 148, 1123–1131 (2012).
Gillespie, D. T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81, 2340–2361 (1977).
Friedman, N., Cai, L. & Xie, X. S. Linking stochastic dynamics to population distribution: an analytical framework of gene expression. Phys. Rev. Lett. 97, 168302 (2006).
Kuwahara, H. Simulation tool and data analysis scripts for the study of stable maintenance of bistable switch under static environments (version 0.16). Zenodo https://doi.org/10.5281/zenodo.4120179 (2020).
Acknowledgements
We thank O. Soyer and T. Gojobori for their comments on an earlier version of the manuscript. X.G. was supported by the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under award numbers BAS/1/1624-01, URF/1/3412-01, URF/1/3450-01, FCC/1/1976-18, FCC/1/1976-23, FCC/1/1976-25, FCC/1/1976-26 and FCS/1/4102-02.
Author information
Authors and Affiliations
Contributions
H.K. conceived and designed the study, developed tools, performed analysis and wrote the paper. X.G. oversaw the project and wrote the paper. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Computational Science thanks Gábor Balázsi and Xiaojun Tian for their contribution to the peer review of this work. Fernando Chirigati was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–4 and Figs. 1–18.
Rights and permissions
About this article
Cite this article
Kuwahara, H., Gao, X. Stable maintenance of hidden switches as a strategy to increase the gene expression stability. Nat Comput Sci 1, 62–70 (2021). https://doi.org/10.1038/s43588-020-00001-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-020-00001-y
This article is cited by
-
Discovering evolutionary hidden treasures
Nature Computational Science (2021)