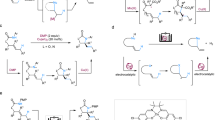
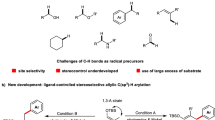
Abstract
Transforming C(sp3)–H bonds efficiently and selectively into C(sp3)–C(sp3) or C(sp3)–X bonds is a highly relevant task. The direct arylation of allylic C(sp3)–H bonds provides an elegant method for the formation of unconjugated aryl-substituted olefins. Although both ionic- and radical-based transition metal catalysis has been applied to achieve this transformation, numerous challenges remain. The requirement for persistent radical coupling partners, moderate selectivity and the need for tri- or tetrasubstituted olefins have limited the generality of existing methods. Now we report a ternary catalytic method that combines organic photoredox, hydrogen atom transfer and nickel catalysis, and can directly arylate allylic C(sp3)–H bonds of readily available olefins. This process operates under mild conditions and exhibits a remarkable reaction scope in both aryl halide and olefin coupling partners. Mechanistic experiments, coupled with density functional theory calculations of Ni-oxidation states and reaction energetics allowed the elucidation of a ternary catalytic cycle and the origin of regioselectivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Materials and methods, detailed optimization studies, experimental procedures, mechanistic studies and copies of the NMR spectra are available in the Supplementary Information. NMR data in a mnova file format and gas chromatography–mass spectroscopy data for KIE analysis are available at Zenodo at https://zenodo.org/record/5614753#.Yaq8DN8kGUk, under the Creative Commons Attribution 4.0 International license.
References
Trost, B. The atom economy—a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Trost, B. M. & Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 103, 2921–2944 (2003).
Cheng, Q. et al. Iridium-catalyzed asymmetric allylic substitution reactions. Chem. Rev. 119, 1855–1969 (2019).
Dutta, S., Bhattacharya, T., Werz, D. B. & Maiti, D. Transition-metal-catalyzed C–H allylation reactions. Chem 7, 555–605 (2021).
Wang, P.-S. & Gong, L.-Z. Palladium-catalyzed asymmetric allylic C-H functionalization: mechanism, stereo- and regioselectivities, and synthetic applications. Acc. Chem. Res. 53, 2841–2854 (2020).
Bayeh, L. & Tambar, U. K. Catalytic asymmetric intermolecular allylic functionalization of unactivated internal alkenes. ACS Catal. 7, 8533–8543 (2017).
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2, 1016–1026 (2019).
Huang, C. et al. Quantum dots enable direct alkylation and arylation of allylic C(sp3)–H bonds with hydrogen evolution by solar energy. Chem 7, 1244–1257 (2021).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Lei, H. & Rovis, T. A site-selective amination catalyst discriminates between nearly identical C–H bonds of unsymmetrical disubstituted alkenes. Nat. Chem. 12, 725–731 (2020).
Ni, G., Zhang, Q.-J., Zheng, Z.-F., Chen, R.-Y. & Yu, D.-Q. 2-arylbenzofuran derivatives from Morus cathayana. J. Nat. Prod. 72, 966–968 (2009).
Knölker, H. J. & Reddy, K. R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 102, 4303–4427 (2002).
Sekine, M., Ilies, L. & Nakamura, E. Iron-catalyzed allylic arylation of olefins via C(sp3)–H activation under mild conditions. Org. Lett. 15, 714–717 (2013).
Lerchen, A. et al. Non-directed cross-dehydrogenative (hetero)arylation of allylic C(sp3)−H bonds enabled by C−H activation. Angew. Chem. Int. Ed. 57, 15248–15252 (2018).
Knecht, T., Pinkert, T., Dalton, T., Lerchen, A. & Glorius, F. CpRhIII-catalyzed allyl–aryl coupling of olefins and arylboron reagents enabled by C(sp3)–H activation. ACS Catal. 9, 1253–1257 (2019).
Pal, S., Cotard, M., Gérardin, B., Hoarau, C. & Schneider, C. Cu-catalyzed oxidative allylic C–H arylation of inexpensive alkenes with (hetero)aryl boronic acids. Org. Lett. 23, 3130–3135 (2021).
Luo, H., Hu, G. & Li, P. Sulfur-mediated allylic C–H arylation, epoxidation, and aziridination. J. Org. Chem. 84, 10569–10578 (2019).
Wang, G.-W., Zhou, A.-X., Li, S.-X. & Yang, S.-D. Regio- and stereoselective allylic C–H arylation with electron-deficient arenes by 1,1′-bi-2-naphthol–palladium cooperation. Org. Lett. 16, 3118–3121 (2014).
Jiang, H., Yang, W., Chen, H., Li, J. & Wu, W. Palladium-catalyzed aerobic oxidative allylic C–H arylation of alkenes with polyfluorobenzenes. Chem. Commun. 50, 7202–7204 (2014).
Zhang, M.-M., Wang, Y.-N., Lu, L.-Q. & Xiao, W.-J. Light up the transition metal-catalyzed single-electron allylation. Trends Chem. 2, 764–775 (2020).
Huang, H.-M., Bellotti, P. & Glorius, F. Transition metal-catalysed allylic functionalization reactions involving radicals. Chem. Soc. Rev. 49, 6186–6197 (2020).
Borg, R. M., Arnold, D. R. & Cameron, T. S. Radical ions in photochemistry. 15. The photosubstitution reaction between dicyanobenzenes and alkyl olefins. Can. J. Chem. 62, 1785–1802 (1984).
Hoshikawa, T. & Inoue, M. Photoinduced direct 4-pyridination of C(sp3)–H bonds. Chem. Sci. 4, 3118–3123 (2013).
Cuthbertson, J. D. & MacMillan, D. W. C. The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis. Nature 519, 74–77 (2015).
Tanaka, H., Sakai, K., Kawamura, A., Oisaki, K. & Kanai, M. Sulfonamides as new hydrogen atom transfer (HAT) catalysts for photoredox allylic and benzylic C–H arylations. Chem. Commun. 54, 3215–3218 (2018).
McAtee, R. C., McClain, E. J. & Stephenson, C. R. J. Illuminating photoredox catalysis. Trends Chem. 1, 111–125 (2019).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Tellis, J. C. et al. Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp3–sp2 cross-coupling. Acc. Chem. Res. 49, 1429–1439 (2016).
Zhu, C., Yue, H., Chu, L. & Rueping, M. Recent advances in photoredox and nickel dual-catalyzed cascade reactions: pushing the boundaries of complexity. Chem. Sci. 11, 4051–4064 (2020).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Shields, B. J. & Doyle, A. G. Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Kariofillis, S. K. & Doyle, A. G. Synthetic and mechanistic implications of chlorine photoelimination in nickel/photoredox C(sp3)–H cross-coupling. Acc. Chem. Res. 54, 988–1000 (2021).
Huang, L. & Rueping, M. Direct cross-coupling of allylic C(sp3)−H bonds with aryl- and vinylbromides by combined nickel and visible-light catalysis. Angew. Chem. Int. Ed. 57, 10333–10337 (2018).
Milligan, J. A., Phelan, J. P., Badir, S. O. & Molander, G. A. Alkyl carbon–carbon bond formation by nickel/photoredox cross-coupling. Angew. Chem. Int. Ed. 58, 6152–6163 (2019).
Sancheti, S. P., Urvashi, Shah, M. P. & Patil, N. T. Ternary catalysis: a stepping stone toward multicatalysis. ACS Catal. 10, 3462–3489 (2020).
Shaw, M. H., Shurtleff, V. W., Terrett, J. A., Cuthbertson, J. D. & MacMillan, D. W. C. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 352, 1304–1308 (2016).
Kato, S. et al. Hybrid catalysis enabling room-temperature hydrogen gas release from N-heterocycles and tetrahydronaphthalenes. J. Am. Chem. Soc. 139, 2204–2207 (2017).
Fuse, H., Kojima, M., Mitsunuma, H. & Kanai, M. Acceptorless dehydrogenation of hydrocarbons by noble-metal-free hybrid catalyst system. Org. Lett. 20, 2042–2045 (2018).
Fuse, H., Mitsunuma, H. & Kanai, M. Catalytic acceptorless dehydrogenation of aliphatic alcohols. J. Am. Chem. Soc. 142, 4493–4499 (2020).
Tanabe, S., Mitsunuma, H. & Kanai, M. Catalytic allylation of aldehydes using unactivated alkenes. J. Am. Chem. Soc. 142, 12374–12381 (2020).
Pitzer, L., Sandfort, F., Strieth-Kalthoff, F. & Glorius, F. Carbonyl–olefin cross-metathesis through a visible-light-induced 1,3-diol formation and fragmentation sequence. Angew. Chem. Int. Ed. 57, 16219–16223 (2018).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Petruncio, G., Shellnutt, Z., Elahi-Mohassel, S., Alishetty, S. & Paige, M. Skipped dienes in natural product synthesis. Nat. Prod. Rep. https://doi.org/10.1039/D1NP00012H (2021).
Song, F. et al. Visible-light-enabled stereodivergent synthesis of E- and Z-configured 1,4-dienes by photoredox/nickel dual catalysis. Angew. Chem. Int. Ed. 59, 177–181 (2020).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic vinylation. J. Am. Chem. Soc. 135, 994–997 (2013).
Kim, S., Goldfogel, M. J., Gilbert, M. M. & Weix, D. J. Nickel-catalyzed cross-electrophile coupling of aryl chlorides with primary alkyl chlorides. J. Am. Chem. Soc. 142, 9902–9907 (2020).
Kattamuri, P. V. & West, J. G. Hydrogenation of alkenes via cooperative hydrogen atom transfer. J. Am. Chem. Soc. 142, 19316–19326 (2020).
Isrow, D. & Captain, B. Synthesis and reactivity of a transition metal complex containing exclusively TEMPO Ligands: Ni(η2-TEMPO)2. Inorg. Chem. 50, 5864–5866 (2011).
Kavarnos, G. J. & Turro, N. J. Photosensitization by reversible electron transfer: theories, experimental evidence, and examples. Chem. Rev. 86, 401–449 (1986).
Rand, A. W. et al. Dual Catalytic Platform for enabling sp3 α C–H arylation and alkylation of benzamides. ACS Catal. 10, 4671–4676 (2020).
Shen, Y., Gu, Y. & Martin, R. sp3 C–H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc. 140, 12200–12209 (2018).
Börjesson, M. et al. Remote sp2 C–H carboxylation via catalytic 1,4-Ni migration with CO2. J. Am. Chem. Soc. 142, 16234–16239 (2020).
Cong, F., Lv, X., Day, C. S. & Martin, R. Dual catalytic strategy for forging sp2–sp3 and sp3–sp3 architectures via β-scission of aliphatic alcohol derivatives. J. Am. Chem. Soc. 142, 20594–20599 (2020).
Mega, R. S., Duong, V. K., Noble, A. & Aggarwal, V. K. Decarboxylative conjunctive cross-coupling of vinyl boronic esters using metallaphotoredox catalysis. Angew. Chem. Int. Ed. 59, 4375–4379 (2020).
Tellis, J. C., Primer, D. N. & Molander, G. A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 345, 433–436 (2014).
Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014).
Gutierrez, O., Tellis, J. C., Primer, D. N., Molander, G. A. & Kozlowski, M. C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 137, 4896–4899 (2015).
Nakajima, K., Nojima, S. & Nishibayashi, Y. Nickel- and photoredox-catalyzed cross-coupling reactions of aryl halides with 4-alkyl-1,4-dihydropyridines as formal nucleophilic alkylation reagents. Angew. Chem. Int. Ed. 55, 14106–14110 (2016).
Yuan, M., Song, Z., Badir, S. O., Molander, G. A. & Gutierrez, O. On the nature of C(sp3)–C(sp2) bond formation in nickel-catalyzed tertiary radical cross-couplings: a case study of Ni/photoredox catalytic cross-coupling of alkyl radicals and aryl halides. J. Am. Chem. Soc. 142, 7225–7234 (2020).
Oderinde, M. S. et al. Highly chemoselective iridium photoredox and nickel catalysis for the cross-coupling of primary aryl amines with aryl halides. Angew. Chem. Int. Ed. 55, 13219–13223 (2016).
Oderinde, M. S., Frenette, M., Robbins, D. W., Aquila, B. & Johannes, J. W. Photoredox mediated nickel catalyzed cross-coupling of thiols with aryl and heteroaryl iodides via thiyl radicals. J. Am. Chem. Soc. 138, 1760–1763 (2016).
Zhu, C. et al. A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat. Catal. 2, 678–687 (2019).
Cavallo, L. et al. Mechanistic insight into the photoredox–nickel–HAT triple catalyzed arylation and alkylation of α-amino Csp3–H bonds. J. Am. Chem. Soc. 142, 16942–16952 (2020).
Marcus, R. A. On the theory of oxidation‐reduction reactions involving electron transfer. I. J. Chem. Phys. 24, 966–978 (1956).
Acknowledgements
This work was generously supported by the Alexander von Humboldt Foundation (H.-M.H.) and the Deutsche Forschungsgemeinschaft (Leibniz Award, SBF 858), the National Science Foundation (CHE-1764328 to K.N.H.) and Zhejiang University for support of P.-P.C. Calculations were performed on the IDRE Hoffman2 cluster at the University of California, Los Angeles, and the Vienna Scientific Cluster. We thank J. Cornella (Max-Planck-Institut für Kohlenforschung) for the generous donation of a sample of Ni(Fstb)3 and X. Zhang, T. Dalton, J. Li, J. Ma, H. Wang and T. Hu (all University of Münster) for helpful discussions and experimental support.
Author information
Authors and Affiliations
Contributions
H.-M.H and F.G. conceived the project. H.-M.H and P.B. performed all of the experiments and analysed all the data. P.-P.C. performed the DFT calculations. H.-M.H, P.B., P.-P.C., K.N.H. and F.G. supervised the research and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Gabriel dos Passos Gomes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Thomas West was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary text and discussion
Supplementary Data 1
Computational data.
Supplementary Data 2
UV-vis data.
Rights and permissions
About this article
Cite this article
Huang, HM., Bellotti, P., Chen, PP. et al. Allylic C(sp3)–H arylation of olefins via ternary catalysis. Nat Synth 1, 59–68 (2022). https://doi.org/10.1038/s44160-021-00006-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-021-00006-z
This article is cited by
-
Radical thioesterification via nickel-catalysed sensitized electron transfer
Nature Synthesis (2023)
-
Site- and enantioselective cross-coupling of saturated N-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis
Nature Communications (2023)
-
Photochemical diversification of strong C(sp3)–H bonds enabled by allyl bromide and sodium fluoride
Nature Synthesis (2023)
-
Modulating stereoselectivity in allylic C(sp3)-H bond arylations via nickel and photoredox catalysis
Nature Communications (2023)
-
Catalytic multicomponent reaction involving a ketyl-type radical
Nature Synthesis (2022)