Abstract
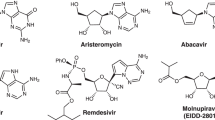
Aminoglycosides (AGs) represent a large group of pseudoglycoside natural products in which several different sugar moieties are harnessed to an aminocyclitol core. AGs constitute a major class of antibiotics that target the prokaryotic ribosome of many problematic pathogens. Hundreds of AGs have been isolated to date, with 1,3-diaminocyclohexanetriol, known as 2-deoxystreptamine (2-DOS), being the most abundant aglycon core. However, due to their diverse and complex architectures, all AG-based drugs are either natural substances or analogues prepared by late-stage modifications. Synthetic approaches to AGs are rare and lengthy; most studies involve semisynthetic reunion of modified fragments. Here we report a bottom-up chemical synthesis of the 2-DOS-based AG antibiotic ribostamycin, which proceeds in ten linear operations from benzene. A key enabling transformation involves a copper-catalysed, enantioselective, dearomative hydroamination, which sets the stage for the rapid and selective introduction of the remaining 2-DOS heteroatom functionality. This work demonstrates how the combination of a tailored, dearomative logic and strategic use of subsequent olefin functionalizations can provide practical and concise access to the AG class of compounds.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The experimental data as well as the characterization data for all the compounds prepared during these studies are provided in the Supplementary Information. Cartesian coordinates of all optimized geometries are provided in a separate file in the.xyz format. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2113321 (30). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
Change history
07 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s44160-023-00357-9
References
Schatz, A., Bugle, E. & Waksman, S. A. Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69 (1944).
Kirst, H. A. & Marinelli, F. in Antimicrobials: New and Old Molecules in the Fight Against Multi-resistant Bacteria (eds Marinelli, F. & Genilloud, O.) 193–209 (Springer, 2014).
Becker, B. & Cooper, M. A. Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 8, 105–115 (2013).
Shomura, T. et al. Studies on antibiotic SF-733, a new antibiotic. I. Taxonomy, isolation and characterization. J. Antibiot. 23, 155–161 (1970).
Waksman, S. A. & Lechevalier, H. A. Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science 109, 305–307 (1949).
Weinstein, M. J. et al. Antibiotic 6640, a new Micromonospora-produced aminoglycoside antibiotic. J. Antibiot. 23, 551–554 (1970).
Magnet, S. & Blanchard, J. S. Molecular Insights into aminoglycoside action and resistance. Chem. Rev. 105, 477–498 (2005).
Borovinskaya, M. A. et al. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nature Struct. Mol. Biol 14, 727–732 (2007).
Garneau-Tsodikova, S. & Labby, J. K. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Med. Chem. Comm 7, 11–27 (2016).
Ramirez, M. S. & Tolmasky, M. E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 13, 151–171 (2010).
Wargo, K. A. & Edwards, J. D. Aminoglycoside-induced nephrotoxicity. J. Pharm. Pract. 27, 573–577 (2014).
Chandrika, N. T. & Garneau-Tsodikova, S. Comprehensive review of chemical strategies for the preparation of new aminoglycosides and their biological activities. Chem. Soc. Rev. 47, 1189–1249 (2018).
Abdul-Mutakabbir, J. C., Kebriaei, R., Jorgensen, S. C. J. & Rybak, M. J. Teaching an old class new tricks: a novel semi-synthetic aminoglycoside, plazomicin. Infect. Dis. Ther. 8, 155–170 (2019).
Umezawa, S. Recent advances in the synthesis of aminoglycoside antibiotics. Pure Appl. Chem. 50, 1453–1476 (1978).
Usui, T. & Umezawa, S. Total synthesis of neomycin B. Carbohydr. Res. 174, 133–143 (1988).
Fukami, H., Kitahara, K. & Nakajima, M. Total synthesis of ribostamycin. Tetrahedron Lett. 17, 545–548 (1976).
Yoshikawa, M., Ikeda, Y., Takenaka, K., Torihara, M. & Kitagawa, I. Synthesis of ribostamycin. an application of a chemical conversion method from carbohydrate to aminocyclitol. Chem. Lett. 13, 2097–2100 (1984).
Busscher, G. F., Rutjes, F. P. J. T. & van Delft, F. L. 2-Deoxystreptamine: central scaffold of aminoglycoside antibiotics. Chem. Rev. 105, 775–792 (2005).
Busscher, G. F., Rutjes, F. P. J. T. & van Delft, F. L. Synthesis of a protected enantiomerically pure 2-deoxystreptamine derivative from d-allylglycine. Tetrahedron Lett. 45, 3629–3632 (2004).
Kitagawa, I., Yoshikawa, M., Ikeda, Y. & Kayakiri, H. Reductive one-step elimination of an acetoxyl residue at β-position of a nitro group: syntheses of (−)-shikimic acid from d-mannose and 2-deoxystreptamine pentaacetate from N-acetyl-d-glucosamine. Heterocycles 17, 209–214 (1982).
Trost, B. M. & Malhotra, S. Asymmetric stereodivergent strategy towards aminocyclitols. Chem. Eur. J. 20, 8288–8292 (2014).
Roche, S. P. & Porco, J. A. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 50, 4068–4093 (2011).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Bryce-Smith, D., Gilbert, A. & Manning, C. 1,2-Photoaddition of primary and secondary amines to benzene. Angew. Chem. Int. Ed. 13, 341–342 (1974).
Southgate, E. H., Pospech, J., Fu, J., Holycross, D. R. & Sarlah, D. Dearomative dihydroxylation with arenophiles. Nature Chem 8, 922–928 (2016).
Liu, R. Y. & Buchwald, S. L. CuH-catalyzed olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 53, 1229–1243 (2020).
Langlois, J.-B. & Alexakis, A. in Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (ed. Kazmaier, U.) 235–268 (Springer, 2012).
Xi, Y. & Hartwig, J. F. Mechanistic studies of copper-catalyzed asymmetric hydroboration of alkenes. J. Am. Chem. Soc. 139, 12758–12772 (2017).
Yang, Y., Shi, S. L., Niu, D., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015).
Lodh, R. S., Borah, A. J. & Phukan, P. Synthesis of bromohydrins using NBS in presence of iodine as catalyst. Indian J. Chem. 53B, 1425–1429 (2014).
Malik, G., Ferry, A., Guinchard, X. & Crich, D. Synthesis of β-hydroxy O-alkyl hydroxylamines from epoxides using a convenient and versatile two-step procedure. Synthesis 45, 65–74 (2013).
Shimomura, N. & Mukaiyama, T. Catalytic synthesis of β-d-ribofuranosides from d-ribofuranose and alcohols. Chem. Lett. 22, 1941–1944 (1993).
Glibstrup, E. & Pedersen, C. M. Scalable synthesis of anomerically pure orthogonal-protected GlcN3 and GalN3 from d-glucosamine. Org. Lett. 18, 4424–4427 (2016).
Dey, R. T. & Sarkar, T. K. On the [3 + 2] annulation of cyclic allylsilanes with N-phenyltriazolinedione: an enantio- and diastereoselective synthesis of cis-1,3-diaminocyclitols. J. Org. Chem. 75, 4521–4529 (2010).
Clique, B. et al. Synthesis of a library of stereo- and regiochemically diverse aminoglycoside derivatives. Org. Biomol. Chem. 3, 2776–2785 (2005).
Acknowledgements
Financial support for this work was provided by the University of Illinois, the University of Pittsburgh, and the NIH/National Institute of General Medical Sciences (GM122891 to D.S. and R35 GM128779 to P.L.). Bristol-Myers Squibb, Amgen, Eli Lilly and FMC are acknowledged for unrestricted research support. We thank D. Olson and L. Zhu for NMR spectroscopic assistance, D. L. Gray and A. S. Shved for X-ray crystallographic analysis assistance and F. Sun for mass spectrometric assistance. Density functional theory calculations were performed at the Center for Research Computing of the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation. We thank S. E. Denmark and C. J. Huck for critical proofreading of this manuscript.
Author information
Authors and Affiliations
Contributions
C.N.U. and D.S. conceived the idea, designed the experiments, analysed the data and prepared the manuscript with the input of all authors. P.G. assisted with optimization and screening efforts involving dearomative hydroamination. Y.Z. carried out the computational studies with P.L. providing guidance. S.L., K.S.L. and J.M.N. assisted with preparation of key intermediates.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Floris Rutjes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Photographs 1 and 2, and Tables 1–5.
Supplementary Data 1
Crystal structure of intermediate compound 30 CCDC (2257524).
Supplementary Data 2
Cartesian coordinates of all optimized geometries.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ungarean, C.N., Galer, P., Zhang, Y. et al. Synthesis of (+)-ribostamycin by catalytic, enantioselective hydroamination of benzene. Nat. Synth 1, 542–547 (2022). https://doi.org/10.1038/s44160-022-00080-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00080-x
This article is cited by
-
Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Nature Communications (2024)
-
The first year of Nature Synthesis
Nature Synthesis (2023)
-
Antibiotics the easy way
Nature Synthesis (2022)