Abstract
Stroke rehabilitation is far from meeting patient needs in terms of timing, intensity and quality. This study evaluates the efficacy and safety of an innovative technological tool, combining 3D motion analysis with targeted vibratory feedback, on upper-limb task performance early poststroke (<4 weeks). The study design was a two-sequence, two-period, randomized, crossover trial (NCT01967290) in 44 patients with upper-limb motor deficit (non-plegic) after medial cerebral artery ischemia. Participants were randomly assigned to receive either the experimental session (repetitive motor task under vibratory feedback and 3D motor characterization) or the active comparator (3D motor characterization only). The primary outcome was the number of correct movements per minute on a hand-to-mouth task measured independently. Vibratory feedback was able to modulate motor training, increasing the number of correct movements by an average of 7.2/min (95%CI [4.9;9.4]; P < 0.001) and reducing the probability of performing an error from 1:3 to 1:9. This strategy may improve the efficacy of training on motor re-learning processes after stroke and its clinical relevance deserves further study in longer duration trials.
Similar content being viewed by others
Introduction
Unilateral motor weakness (50–83%) and cognitive impairment (50%) are the most common deficits resulting from stroke and the main causes of disability1,2,3,4,5,6. Early rehabilitation, within the first three to six months, is crucial in increasing the probability of a good functional outcome7,8,9 and currently perceived as an essential part of effective stroke care3,10. However, the type of interventions, when they should begin, how intensive and for how long they should occur all remain unanswered questions and are currently the focus of research in the field11.
Spontaneous recovery or pharmacologically triggered neuroplasticity have shown no significant effect in reducing functional disability after stroke7,8,12 which renders motor recovery largely dependent on rehabilitative interventions mediated by specialized health professionals in institutional environments13. However, the availability of effective poststroke rehabilitation treatments is far from meeting the needs of all stroke patients in due time, intensity, quality or duration3,14,15. Moreover, even patients that achieve functional independence after stroke are at increased risk of developing long-term disability, independently of recurrent stroke or other risk factors16,17.
To overcome these complex problems, new technological tools and rehabilitation approaches are being developed and tested3,11,13,18,19,20,21,22. In addition to the intensity of the rehabilitation23, its quality and timing as well as the dose and monitoring the possible adverse-effects of these interventions are of increasing importance in early poststroke management11.
Over the last few years we have developed a low cost technological tool designated as Stroke Wearable Operative Rehabilitation Device (SWORD)24 that allows for controlled prescription of specific motor tasks in training sessions supervised by a health professional. The SWORD device combines targeted vibratory feedback25, over the major upper or lower limb joints, with wearable 3D vectorial continuous movement quantification analysis26. The training sessions using the device can be controlled at distance and occur complementary to the current institutional-based programs, either at the institution or remotely supervised at home13.
The aim of this study was to explore the efficacy and safety of the SWORD device (vibratory feedback and 3D motor quantification) and to determine its effect on the quality of movement during the first training session of inpatients in an early poststroke phase.
Results
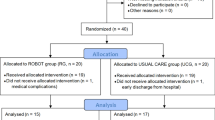
From May to October 2013 a total of 164 patients having had their first stroke were assessed for eligibility (Figure 1). A total of 120 were excluded. Among these, 116 did not meet criteria and 4 declined to participate. The primary reasons for non-inclusion were the type of stroke (n = 56), the severity of the deficits (n = 20) and logistic or acute phase treatment related issues that precluded enrollment within the first 4 weeks (n = 33). A total of 44 participants were included and 22 were randomized to each study arm. All participants were able to complete the cross-over experiment on both sides. On the study arm that performed the active comparator session first, one patient was excluded from the analysis due to technical problems that led to the loss of data during the experiment on the normal side of the body. On the study arm that performed the experimental session first, one patient was excluded after revising the discharge diagnosis given by the stroke unit. The analysis of data was conducted in 43 participants on the paretic side and 42 participants on the normal side (Figure 1).
The mean age of the participants was 66.5 years (SD = 13.1; range 44–92 yrs), 39.5% were female, the average educational level was 4.1 years (SD = 2.4; range 0–9 yrs) and the average time from stroke onset to enrollment was 6.8 days (SD = 7.3; range 3–27 days). Stroke etiology was cardioembolic (30.2%), small vessel disease (20.9%), large vessel disease (11.6%), multiple causes (30.2%) and other causes (7%). The median national institute of health stroke scale (NIHSS) at randomization was 4.0 (IQR [3.0;6.0]), 37.2% had a modified Rankin scale (mRS) score of 1or 2 and 60.5% a mRS score of 3 or 4. The baseline characteristics of each allocation group are presented in Table 1. There were no significant imbalances between groups with respect to gender, education, stroke type, side, severity, frequency of neglect or time from onset.
All patients were able to complete the hand-to-mouth sessions prescribed with no interruption due to fatigue, pain or any medical condition, both in the normal and the paretic side trials. The average duration of each session on the normal side was 3.2 min (SD = 1.6; range 1–10 min) and on the paretic side was 2.9 (SD = 1.7; range 1–10 min). The average amplitude, on the sagittal plane, required to consider the hand-to-mouth movement as correctly done was 31.0 degrees (SD = 10.3; range 12–50°) on the normal side and 26.8 (SD = 11.0; range 12–50°) on the paretic side.
Primary end point
In the trial for the paretic side, the average number of correct movements was 25.7 per min (SD = 11.7; 95% CI [22.1;29.3]) during the experimental session (vibratory feedback on) and 18.5 correct movements/min (SD = 11.4; 95% CI [14.9;22.0]) in the active comparator session (Figure 2A). The number of movements made on the patient's affected side in the experimental session increased by an average of 7.2 correct movements/min (SD = 7.4; 95% CI [4.9;9.4]) compared to the active comparator session (P < 0.001). This difference corresponds to a relative increase average of 2.8 more correct movements per minute in the experimental session (SD = 5.3; 95% CI [1.2;4.4]; P < 0.001). In 93% (40/43) of the patients there was an increase in the number of correct movements performed per unit of time during the experimental session (Figures 2B and 2C).
Primary end point analysis in the paretic and normal sides.
Figure 2A – Comparison of the 95% CI of the number of correct movements/min in the active comparator and vibratory feedback sessions. Figure 2B – Dot plot reporting paired data of the number of correct movements/min in the active comparator and vibratory feedback sessions. Difference between sessions is perceived by comparing the points to the diagonal “line of unity”. Figure 2C – Histogram of the absolute differences in the number of correct movements/min (Vibratory feedback – active comparator). The dashed vertical line indicates no change.
For the trial performed on the normal side, the average number of correct movements was 30.3 correct movements/min (SD = 14.6; 95% CI [25.7;34.9]) during the experimental sessions and 23.6 correct movements/min (SD = 14.2; 95% CI [19.2;27.9]) in the active comparator session (Figure 2A). Analysing the effect size in the experimental session, the number of movements increased an average of 6.7 correct movements/min (SD = 7.8: 95% CI [4.2;9.1]) compared to the active comparator session within each subject (P < 0.001). The relative increase average of correct movements between the two sessions was 2.7 (SD = 7.1; 95% CI [0.5;5.0]; P < 0.001). In 76% (32/42) of the patients an increase in the number of correct movements performed per unit of time was observed during the experimental session (Figures 2B and 2C).
Secondary end-points
Tables 2 and 3 summarize the differences between groups on the additional efficacy outcomes and all the safety outcomes. For the paretic side the total number of movements performed (correct plus incorrect) was significantly higher under vibratory feedback (average within subject difference 4.7 correct movements/min; SD = 1.1; P < 0.001). The average range of motion of correct movements was similar in both sessions (Table 2) with no significant statistical difference (P = 0.13), the same occurred with the number of pauses per minute and per correct movement (P = 0.67). On the normal side (Table 3) there were no significant differences on the total number of movements performed between the sessions (P = 0.16) and the amplitude of correct movements was similar in the two sessions (P = 0.52). On both sides, the range of motion of all correct movements was approximately the double of what was set as the minimum for considering the movement correct.
None of the participants experienced a serious adverse event or other type of distress symptom during the experimental sessions or the 24 h thereafter (Tables 2 and 3). There was also no report of exertion fatigue (Borg score > 13) or pain during any of the sessions although significantly more patients reported the experimental session as moderate intensity activity (Borg's scale 12–13; 25.6% on the paretic side and 19% on the normal side). The perceived quality of movement reported by the patients after each session showed no differences between the active comparator and the experimental conditions. On the normal side, the quality of movement was given higher performance scores (Table 3). There was no correlation between the number of correct movements identified by the device and the perceived quality of movement reported by the patient on either the normal or the paretic side.
Effect of session order on the primary endpoint
Since this was a cross-over trial the possibility of a carry-over effect attributable to a short wash out period was investigated. For the paretic side, the number of correct movements was higher in the experimental than in the comparator session in the two study arms with different order of sessions. The average within subject increase in the comparator - experimental study arm was 6.9 correct movements/min (SD = 8.0: 95% CI [3.4;10.4]; P = 0.001) and in the experimental – active comparator arm 7.4 correct movements/min (SD = 6.9; 95% CI [4.3;10.5]; P < 0.001). There were no significant differences when comparing the absolute increase of correct movements per minute between the two study arms (P = 0.82). A similar pattern was found in the normal side trial. The average within subject increase in the active comparator - experimental study arm was 8.2 correct movements/min (SD = 8.8; 95% CI [4.2;12.2]; P < 0.001) and in the experimental – active comparator arm 5.1 correct movements/min (SD = 6.5; 95% CI [2.2;8.1]; P = 0.02). There were no significant differences when comparing the absolute increase of correct movements per minute between the two study arms (P = 0.21). Performing a comparative analysis of the number of correct movements per minute between the 1st and 2nd sessions, disregarding of the session type, revealed no significant differences in the paretic side (average within subject difference 0.1 correct movements/min; SD = 10.3; P = 0.95) nor in the normal side (average within subject difference -1.5 correct movements/min; SD = 10.2; P = 0.34).
Exploratory analysis of the effects of neglect, motor function and other factors on the primary endpoint
In patients with neglect the average within subject increase in the number of correct movements was 6.0 correct movements/min (SD = 4.2) on the paretic side. This increase was not significantly different (P = 0.45) from the increase observed in patients without neglect (within subject average of 7.5 correct movements/min, SD = 8.1). Regarding the trial in the normal side, the increase in the number of correct movements in patients with neglect also did not differ significantly from patients without neglect (6.0 correct movements/min (SD = 4.3) and 7.5 correct movements/min (SD = 8.1 respectively; P = 0.59).
Although there was a positive univariate correlation between the number of correct movements in the paretic side in the active comparator session and the results of the NIHSS (P = 0.004), motor NIHSS (P = 0.006), NIHSS at admission (P = 0.004) and motor NIHSS at admission (P = 0.004), none of these factors had a significant effect in the variation of correct movements per minute between sessions (P = 0.88; P = 0.93; P = 0.96 and P = 0.98 respectively.) The variation of the primary endpoint between the sessions was also not affected by gender (P = 0.60), age (P = 0.96), educational attainment (P = 0.64) or time since stroke onset (P = 0.25).
Discussion
This study explored the effect of an innovative wearable rehabilitation device (SWORD) on the quality of performance of a specific repetitive upper-limb task. The hand-to-mouth task, extensively prescribed to stroke patients in the course of their rehabilitation programs3,27, is necessary for the completion of several important daily living activities and part of several widely used scales that evaluate upper-limb motor functioning28,29. To our knowledge this cross-over trial was the first to systematically address the effect of targeted vibratory feedback on the modulation of motor performance in the first month after stroke.
The performance in a training session under vibratory feedback was compared with an active comparator session (repetitive task only) within the same subject and under 3D movement characterization. The experimental setting chosen, reproduced a first post-stroke repetitive task session prescribed by a therapist before patient discharge from the stroke unit. For the efficacy outcome (primary end point) this study revealed a 2.8 times increase (95% CI [1.2; 4.4]) in the number of correct hand-to-mouth movements performed per minute of training under vibratory feedback. This corresponded to an absolute increase of 7.2 (95% CI [4.9;9.4]) correct movements per minute. The effect on the quality of the task occurred during a first session on the paretic side, without previous exposure to therapist supervised practice. Additionally, under these particular circumstances and namely within the first month poststroke, vibratory feedback was found safe (no adverse events, exertion fatigue or pain), which is in line with previous results25.
Most importantly, for the primary outcome defined, the size of the study was appropriate and there was no evidence of a carry-over effect on the primary outcome results. No differences were detected between the results of the first and second sessions in each patient, either for the vibratory feedback or active comparator. Additionally, there was no correlation between the number of correct movements identified by the device and the patient' scores of perceived quality of movement. Although it is impossible to blind patients to a proprioceptive stimulus like vibratory feedback, the absence of correlation above reinforces the real nature of the effect measured on the primary outcome. One fifth of the patients were identified as having some kind of neglect, but there was no significant difference on the effect size attributable to this variable. The same happened for total NIHSS, motor NIHSS, age, gender education or time since stroke onset.
One interesting finding was that although the total number of movements (correct and incorrect) was also higher under vibratory feedback, the absolute effect of the vibratory feedback on the number of total movements was smaller than the effect on the number of correct movements (average within subject increase of 4.7 movements/min vs 7.2 correct movements/min). The analysis of the probability of correct movements among total attempts performed per minute under each condition depicted a probability of performing an incorrect movement of 1:3 in the active comparator vs 1:9 under vibratory feedback. This difference in the overall quality accrued with the difference in intensity observed under vibratory feedback. If we hypothesize the long-term use of the SWORD device in an outpatient setting24, these two effects may be relevant for functional network reorganization, adaptation, motor relearning and skill acquisition8,30,31. Their combination may assist the implementation of early poststroke home-based regimens consisting of progressive daily repetitive skills practice while simultaneously reducing error repetition through uninterrupted training programs7,27,31.
Other efficacy secondary outcomes such as range of motion, time between movements and cumulative amplitudes were in line with the findings discussed above. The number of pauses reflected the regularity of the physical effort performed during training and no differences were observed between the experimental and active comparator sessions, in spite of the significant increase in the overall number of correct movements in the experimental session.
The data of the trial performed on the non-paretic side of stroke patients, despite depicting similar results for the absolute and relative differences on outcomes between the two conditions, must be judged with caution. The experiment was conducted first on the non-paretic side, primarily to ensure that all participants understood the dynamic of the trial and for this reason were more susceptible to learning effects in spite of the wash out period. Furthermore, the tasks prescribed for the non-paretic side, although of greater amplitude, were easier to complete if the overall upper-limb functioning of both sides (paretic and non-paretic) was taken into consideration. These limitations also apply for comparisons between paretic and non-paretic side effects.
Although this study strongly suggests that the vibratory feedback with 3D quantification of movement improves the intensity and quality of training, the long-term clinical impact of its use in post-stroke motor rehabilitation is not yet determined. To demonstrate generalization and clinical relevance, longer clinical trials with functional outcomes are required to assess the effect of the continuous use of this method in a parallel group randomized trial (e.g. regular treatment plus up to 30 min/day of several progressive tasks, under vibratory feedback/3D quantification, over 4 weeks). Nevertheless, the distribution of age, gender and level of education of the participants was similar to the characteristics of those patients admitted with a medial cerebral artery infarct in a stroke unit. Ischemic stroke patients with TACI, NIHSS scores > 10, mRS > 4 and Barthel index below 40 are underrepresented in the trial due to the exclusion of patients with severe aphasia or complete upper-limb plegia in the first month poststroke. Posterior circulation and hemorrhagic strokes were excluded. Patients with clinically detectable forms of apraxia were also excluded from this trial and may represent a subgroup amenable to this type of intervention32.
Other limitations of this study deserve comment here. The duration of each hand-to-mouth session was short, 2.87 min on average. This occurred mainly due to the decision to keep the effort of the session below the maximum level of exercise tolerated by the patient and to the poor cardiovascular condition perceived by the clinician during baseline medical assessment. Pre-morbid deconditioning due to sedentary behavior has been recognized as an important problem to address in current neurorehabilitation programs33,34. Clinical application and long-term outpatient trials will implicate a comprehensive approach, with more tasks available for prescription and focused on progressive upper limb and lower limb training. Moreover, despite the selective inclusion criteria, the patients enrolled convey high heterogeneity with one third having subcortical lesions and the other two thirds a varied combination of cortical and subcortical lesions. Subclinical forms of apraxia were not actively searched and may have an effect on the results of the intervention32. All these issues must be addressed in the future planning of research and sample size estimations for the study of the mechanisms behind the effects verified35,36,37.
In the field of poststroke rehabilitation, the findings of this study are in line with the well-recognized need to further characterize the interaction between training and brain plasticity in the first three months after stroke31,35. More specifically, having shown that a wearable device combining 3D motion analysis with targeted vibratory feedback (SWORD) was able to increase the quality and intensity of a specific repetitive task, we may now explore its long-term effects and combination with other neurorehabilitation methods in the most sensitive period after stroke. The study of the combination of error-based and reward-based training methodologies and of the effect of kinematic controlled progressive tasks on the reduction of unlearning and motor forgetting between rehabilitation sessions36,37 is essential for the development of successful neurorehabilitation strategies that lead to improved recovery of function through reacquisition of normal movement patterns31,38. Finally, ongoing efforts to improve the SWORD device for home-based remotely supervised training will also address the growing need for technological tools that open the possibility for large scale cost-effective home-based rehabilitation activities39. These will be important in the near future to guarantee organization of services and the continuum of poststroke care14,15.
In conclusion, vibratory feedback was able to increase the intensity of movement and simultaneously its overall quality. If we consider that contemporary rehabilitation strategies to improve motor function after stroke are centered on high-intensity repetition of progressive skilled tasks3,27 these findings may be of considerable relevance for future research in the field. Furthermore, the combination of intensity, over long periods of time, with quality control and motivation provided by feedback on performance are essential to modulate brain neuroplastic properties8,27,31 and to achieve and maintain good functional outcomes.
Methods
Study design
The study was a two-sequence, two-period, cross-over study, randomized between experiment-active comparator or active comparator-experiment (1:1), non-blind, conducted at a single center in hemiparetic patients having their first stroke within 4 weeks before enrollment. The study was designed to evaluate the efficacy and safety of vibratory feedback, delivered through the SWORD device and to determine if it improves the quality of movement (number of correct) on a repetitive task session performed with the upper extremity (hand-to-mouth). Both sides, normal and paretic, were tested. This study was registered at http://clinicaltrials.gov. under the Unique identifier: NCT01967290.
Participants
The study was conducted in a stroke-unit setting that provides care to 400.000 inhabitants and is based in a Portuguese tertiary hospital institution with clinical and research obligations. Patients over 18 years of age, previously independent, with a mRS 0–140 and admitted for a first-time ischemic stroke were screened for study eligibility between May and October 2013 (Figure 3). Participants were included if they had: 1) clinical symptoms and signs and CT or MRI findings compatible with a lesion in the territory of the medial cerebral artery; 2) persistent motor deficit on the upper limb but not plegia with a score between 0 and 2 on items 5a or 5b of the NIHSS41; 3) no more than 4 weeks after stroke onset; and 4) the ability to sit for more than one hour comfortably and perform two-step commands. Subjects were excluded if they had: 1) no detectable motor deficits at baseline assessment by the neurologist; 2) severe aphasia; 3) clinical dementia or mini mental state examination (MMSE)42 below cutoff; 4) other cognitive or psychiatric comorbidity that impaired communication or compliance with the tasks; 5) severe respiratory or cardiac condition incompatible with more than one minute of continuous mild exercise in a sitting position (e.g. combing hair, brushing teeth); 6) pain or deformity that limited upper limb movement either on the normal or affected side.
Ethical issues
All participants and caregivers were provided with information about the purpose and procedures of the study and gave written informed consent. Approval from the accompanying stroke physician was also obtained to guarantee safety and management of expectations after the trial. The study received a favorable opinion by the hospital ethics committee and by the Portuguese National Data Protection Commission and the methods were conducted in accordance with the approved guidelines.
Baseline measures
Participant characterization included demographics, handedness, antecedent and comorbid conditions, pre-morbid mRS, standard medical and thorough neurological examinations including clinical aphasia, neglect and apraxia assessment, MMSE, stroke characteristics and treatments. Stroke description included: date of onset, type, location, Trial of org 10172 in acute stroke treatment classification (TOAST)43 for etiology, admission NIHSS score and OCSP classification44 as measures of severity and prognosis. The NIHSS score, the modified Asworth scale score45 for muscular tonus, the Barthel index46 and the mRS score for disability and activities of daily living were all used to create a comprehensive motor and functional profile at the time of intervention. All data were collected by a certified stroke neurologist.
Interventions
Experimental - hand-to-mouth task with vibratory feedback
The session occurred under vibratory feedback and 3D movement analysis. The SWORD device was in place over the patient's arm, performing continuous 3D movement analysis and providing vibratory feedback according to quality performance settings established by the clinician after patient assessment. If movement was of lower amplitude or slower than prescribed, a vibratory stimulus was delivered on the patient's wrist (Figure 3).
Active comparator - hand-to-mouth task without vibratory feedback
The setting was the same for the experimental session with the exception of vibratory feedback. The SWORD device was in place over the patient's arm, performing continuous 3D movement analysis.
Technical system used
The version of the SWORD device developed for this trial included two basic modules, one dedicated to 3D movement quantification and analysis (placed at the arm) and the other to direct vibratory feedback (placed at the wrist), connected via Bluetooth with each other and with a laptop computer24,25. The motion quantification module was composed by a three-axis gyroscope, three-axis accelerometer and a three-axis magnetometer. These were assembled in a wearable device that provided continuous 3D vectorial kinematics of the upper-limb and real-time analysis of the quality of movement26. Quality was assessed according to a biomechanical model of the upper limb and by confrontation with the parameters (range of motion, baseline position, rhythm of execution and task duration) set by the clinician for the hand-to-mouth task (Figure 3)26. The vibratory feedback module included two DC motors, with eccentric masses encapsulated in a cylinder 25 mm long and 8.8 mm in diameter47 and was programed to deliver vibratory stimuli at a frequency of 200 Hz and an amplitude of 46 m/s2 at a rated voltage of 2.6 V25. The vibratory feedback was set to trigger every time that the patient did not perform a correct movement, either because the maximum amplitude was not achieved, the baseline was not reassumed after a correct movement in amplitude or the movements were occurring at a slower rate than prescribed. Once triggered, the vibratory stimulus was continuous until a correct movement was performed25.
Randomization
Eligible participants were randomly allocated in a 1:1 ratio to two study arms. Balance between arms was guaranteed using random permuted blocks of two. For one arm the order was an experimental session followed by the active comparator session, for the other it was the reverse. Although motor deficits after stroke are not expected to change significantly within the same day, either spontaneously or due to the intervention, the randomization of the session order was performed to avoid carry-over effects due to fatigue or learning. Based on previous experiments25 an obligatory washout period equal to 20 minutes plus the double the duration of each session was set.
Blinding
Although the investigators and participants were aware of the vibratory stimuli, they were blind to the primary and secondary movement outcomes being measured, as those were automatically recorded by the device and only available at the end of the trial. The statistical analysis was performed blinded for experimental or active comparator status.
Primary outcome definition
The primary outcome with respect to efficacy was the number of correct movements performed per minute within the duration of each hand-to-mouth session, a measure of the quality of movement. This measurement was independent of the investigator and assessed automatically by the device as described elsewhere48.
Secondary outcomes definition
Additional efficacy outcomes were considered: i) the total number of correct and incorrect movements; ii) the average range of motion in degrees (correct movements); iii) the average time between correct movements in seconds; iv) the cumulative amplitude of correct movements in degrees; v) the cumulative amplitude of all movements (correct and incorrect) performed in degrees; and vi) the number of pauses identified, defined as interruptions exceeding the average time between correct movements plus one standard deviation. The SWORD device assessed all these measures automatically.
For the purpose of assessing safety the following outcomes were used: i) patient expresses feeling fatigued using a visual analogical scale adapted from Borg's perceived exertion scale49; ii) patient expresses feeling pain using a visual analogic scale; iii) other distresses identified by the patient or detected through monitoring, at the end of the session and 24 h thereafter; and iv) self-perception of overall quality of movement performed during each session. The need to use adapted visual analogue scales for these safety outcomes (i, ii and iv) resulted from the population's low education.
Sample size estimate
In a pilot study (Bento VF, PhD thesis, 2012), the number of correct movements in the active comparator was estimated at 12.4 per minute (SD = 6.9). Considering a power of 85% and a two-sided 0.05 significance level, 38 patients would be necessary to detect a difference of 60% in the number of correct movements per minute between the active comparator and the experimental sessions for each subject.
Statistical analysis
To assess between-group differences in clinical and demographic variables of the patients allocated to the two study arms, independent samples T test, Mann–Whitney U test, qui-squared test and Fisher exact test were used. To compare the primary and secondary outcomes between the experimental and active comparator sessions on the patient's same side, paired samples T test and McNemar's test were used. To identify possible carry-over effects attributable to the cross-over design two parameters were separately analyzed for the paretic and normal side: i) the differences in the absolute variation of the primary outcome among the two study arms, using independent samples T test; and ii) the differences in the number of correct movements per minute between the first and second sessions, regardless of session type, using related samples T test. To identify possible determinants of the base-line performance and of the improvement in motor outcomes observed during the experimental session, additional analyses were performed using simple and multiple linear regression models. The statistical analysis was performed in SPSS 21.0 considering a two-sided 0.05 significance level.
Additional information
Clinical Trial Registration URL: http://clinicaltrials.gov. Unique identifier: NCT01967290.
Funding statement This work was supported in part by FEDER funds via COMPETE and by Portuguese funds through FCT – Portuguese Foundation for Science and Technology in the project grant PTDC/SAU-NEU/102075/2008. This funding was used for the technological development of SWORD. All design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical approval of research: This study was approved by the local ethics committee at Hospital São Sebastião, Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal (Chair: Rui Carrapato, MD PhD) and the methods were conducted in accordance with the approved guidelines. All patients and caregivers were provided with information about the purpose and procedures of the study and provided written informed consent. Approval from the referring neurologist was also obtained to guarantee that patient and caregiver expectations were properly managed. These procedures were included in the methods section of the manuscript.
References
Rathore, S. S., Hinn, A. R., Cooper, L. S., Tyroler, H. A. & Rosamond, W. D. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke 33, 2718–2721 (2002).
Stinear, C. Prediction of recovery of motor function after stroke. Lancet Neurol 9, 1228–1232 (2010).
Langhorne, P., Bernhardt, J. & Kwakkel, G. Stroke rehabilitation. Lancet 377, 1693–1702 (2011).
Hankey, G. J., Jamrozik, K., Broadhurst, R. J., Forbes, S. & Anderson, C. S. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989-1990. Stroke 33, 1034–1040 (2002).
Douiri, A., Rudd, A. G. & Wolfe, C. D. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 44, 138–145 (2013).
Pinter, M. M. & Brainin, M. Rehabilitation after stroke in older people. Maturitas 71, 104–108 (2012).
Cramer, S. C. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol 63, 549–560 (2008).
Cramer, S. C. et al. Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609 (2011).
Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 25, 457–507 (2008).
Warlow, C. P. et al. Stroke: Practical Management (Wiley, London, 2011).
Bernhardt, J., Indredavik, B. & Langhorne, P. When should rehabilitation begin after stroke? Int J Stroke 8, 5–7 (2013).
Cramer, S. C. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63, 272–287 (2008).
Walker, M. F., Sunnerhagen, K. S. & Fisher, R. J. Evidence-based community stroke rehabilitation. Stroke 44, 293–297 (2013).
World Health Organization. Neurological disorders: public health challenges, (World Health Organization, Geneva, 2006).
Norrving, B. & Kissela, B. The global burden of stroke and need for a continuum of care. Neurology 80, S5–12 (2013).
Dhamoon, M. S. et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke 40, 2805–2811 (2009).
Wolfe, C. D. et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLoS Med 8, e1001033 (2011).
Chollet, F. & Albucher, J. F. Strategies to augment recovery after stroke. Curr Treat Options Neurol 14, 531–540 (2012).
Abo, M. et al. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: the NEURO-VERIFY Study. Int J Stroke Sep 9, 10.1111/ijs.12100 (2013).
Saposnik, G. et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke 41, 1477–1484 (2010).
Subramanian, S. K., Lourenco, C. B., Chilingaryan, G., Sveistrup, H. & Levin, M. F. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehab Neural Re 27, 13–23 (2013).
Wolf, S. L. et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 296, 2095–2104 (2006).
Dromerick, A. W. et al. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): A single-center RCT. Neurology 73, 195–201 (2009).
Bento, V. F., Cruz, V. T., Ribeiro, D. D., Colunas, M. M. & Cunha, J. P. The SWORD tele-rehabilitation system. Stud Health Technol Inform 177, 76–81 (2012).
Bento, V. F., Cruz, V. T., Ribeiro, D. D. & Cunha, J. P. The vibratory stimulus as a neurorehabilitation tool for stroke patients: proof of concept and tolerability test. NeuroRehabilitation 30, 287–293 (2012).
Bento, V. F., Cruz, V. T., Ribeiro, D. D., Branco, C. & Coutinho, P. The potential of motion quantification systems in the automatic evaluation of motor function after stroke. Int J Stroke 8, E37 (2013).
Dobkin, B. H. & Dorsch, A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep 15, 331 (2013).
Wolf, S. L., Lecraw, D. E., Barton, L. A. & Jann, B. B. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol 104, 125–132 (1989).
Fugl-Meyer, A. R., Jaasko, L., Leyman, I., Olsson, S. & Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7, 13–31 (1975).
Krakauer, J. W., Carmichael, S. T., Corbett, D. & Wittenberg, G. F. Getting neurorehabilitation right: what can be learned from animal models? Neurorehab Neural Re 26, 923–931 (2012).
Kitago, T. & Krakauer, J. W. Motor learning principles for neurorehabilitation. Handb Clin Neurol 110, 93–103 (2013).
Buxbaum, L. J., Shapiro, A. D. & Coslett, H. B. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain Apr 27, 10.1093/brain/awu111 (2014).
Billinger, S. A., Coughenour, E., Mackay-Lyons, M. J. & Ivey, F. M. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat 2012, 959120 (2012).
Baert, I. et al. Evolution of cardiorespiratory fitness after stroke: a 1-year follow-up study. Influence of prestroke patients' characteristics and stroke-related factors. Arch Phys Med Rehab 93, 669–676 (2012).
Zeiler, S. R. & Krakauer, J. W. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol 26, 609–616 (2013).
Shmuelof, L. et al. Overcoming motor “forgetting” through reinforcement of learned actions. J Neurosci 32, 14617–14621 (2012).
Kitago, T. et al. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time and removal of errors on motor memory. Front Hum Neurosci 7, 307 (2013).
Carmichael, S. T. & Krakauer, J. W. The promise of neuro-recovery after stroke: introduction. Stroke 44, S103 (2013).
Hillier, S. & Inglis-Jassiem, G. Rehabilitation for community-dwelling people with stroke: home or centre based? A systematic review. Int J Stroke 5, 178–186 (2010).
United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. UK-TIA Study Group. Br Med J (Clin Res Ed) 296, 316–320 (1988).
Brott, T. et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20, 864–870 (1989).
Folstein, M. F., Robins, L. N. & Helzer, J. E. The Mini-Mental State Examination. Arch Gen Psychiatry 40, 812 (1983).
Adams, H. P., Jr et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41 (1993).
Bamford, J., Sandercock, P., Dennis, M., Burn, J. & Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337, 1521–1526 (1991).
Bohannon, R. W. & Smith, M. B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67, 206–207 (1987).
Mahoney, F. I. & Barthel, D. W. Functional Evaluation: The Barthel Index. Md State Med J 14, 61–65 (1965).
Precision Microdrives Limited. “Product Data Sheet - Pico Vibe 9 mm Vibration Motor”, (ed. Canterbury Court, 1-3 Brixton Road, London, UK, 2011).
Bento, V. F., Cruz, V. T., Ribeiro, D. D. & Cunha, J. P. Towards a movement quantification system capable of automatic evaluation of upper limb motor function after neurological injury. Conf Proc IEEE Eng Med Biol Soc 2011, 5456–5460 (2011).
Borg, G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2, 92–98 (1970).
Acknowledgements
This work was supported by FEDER funds via COMPETE and by Portuguese funds through FCT – Portuguese Foundation for Science and Technology in the project grant PTDC/SAU-NEU/102075/2008.
Author information
Authors and Affiliations
Contributions
Study concept and design: V.T.C., V.B., C.B. and P.C. Acquisition of data: V.T.C., D.D.R., M.C., L.R., L.F., R.B., C.M., A.A. and C.B. Analysis and interpretation of data: V.T.C., L.R., V.B., B.C., C.B., N.P.R. and P.C. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: V.T.C., V.B. and P.C. Administrative, technical and material support: V.T.C., D.D.R., L.F., R.B., C.M., L.R., M.C., A.A., C.B. and V.T.C. Study supervision: V.T.C., V.B., M.C., V.T.C., C.B., N.P.R. and P.C.
Ethics declarations
Competing interests
V.T.C., V.B., M.C. and D.D.R. have a shareholder position at EndeavourLab, a spin-Off company of the Aveiro University that develops and commercializes SWORD related products. L.R., L.F., C.M., R.B., A.A., B.C., C.B., N.P.R. and P.C. have no conflicts of interest to report.
Electronic supplementary material
Supplementary Information
Supplementary Material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cruz, V., Bento, V., Ruano, L. et al. Motor task performance under vibratory feedback early poststroke: single center, randomized, cross-over, controled clinical trial. Sci Rep 4, 5670 (2014). https://doi.org/10.1038/srep05670
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05670
This article is cited by
-
Early Rehabilitation After Stroke: a Narrative Review
Current Atherosclerosis Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.