Abstract
Cutaneous vaccination with microneedle patches offers several advantages over more frequently used approaches for vaccine delivery, including improved protective immunity. However, the involvement of specific APC subsets and their contribution to the induction of immunity following cutaneous vaccine delivery is not well understood. A better understanding of the functions of individual APC subsets in the skin will allow us to target specific skin cell populations in order to further enhance vaccine efficacy. Here we use a Langerin-EGFP-DTR knock-in mouse model to determine the contribution of langerin+ subsets of skin APCs in the induction of adaptive immune responses following cutaneous microneedle delivery of influenza vaccine. Depletion of langerin+ cells prior to vaccination resulted in substantial impairment of both Th1 and Th2 responses and decreased post-challenge survival rates, in mice vaccinated cutaneously but not in those vaccinated via the intramuscular route or in non-depleted control mice. Our results indicate that langerin+ cells contribute significantly to the induction of protective immune responses following cutaneous vaccination with a subunit influenza vaccine.
Similar content being viewed by others
Introduction
Cutaneous immunization has gained increasing interest due to its ability to induce robust host immune responses1. Although the cornerstone of influenza prevention is vaccination, the current conventional method of annual influenza vaccination is intramuscular injection of inactivated trivalent subunit or split vaccine which can only provide moderate protection against influenza2. Microneedle technology platform takes the advantage of the immunological potential of skin and relies on controlled and rapid delivery of the antigen to epidermal and dermal layers3. The length of the microneedles is 600–700 μM which is appropriate for both mouse and human skin despite their difference in thickness4. In the process of skin insertion the needles span both the epidermis and the dermis delivering the vaccine to both layers5.
We and others have previously shown that cutaneous immunization with influenza vaccines, delivered via metal or polymer microneedles, elicits long-lived and robust mucosal and systemic immune responses6 and confers improved protection against lethal virus challenge in mice as compared to intramuscular immunization5,7,8,9,10,11,12,13,14. In addition to the induction of improved immune responses, microneedle technology offers other significant advantages such as increased safety due to the elimination of biohazard sharps, lack of pain and distress at the site of immunization, ease of administration by minimally trained personnel and independence from refrigerated transport and storage3.
Skin is the largest immunological organ in the body. In addition to harboring a large number of T lymphocytes, it is densely populated by antigen presenting cells (APC) which are important sentinels against pathogens15. The epidermis is populated by Langerhans cells (LCs), which are specialized APCs characterized by the expression of langerin (CD207), a type II transmembrane C-type lectin16 and MHCII molecules15,17. Although langerin expression was initially thought to be unique for LCs, it is also expressed in subpopulations of DCs and migrating LCs in the dermis and within skin-draining lymph nodes (LN)17,18,19. Several subsets of dermal DCs (dDc) are observed in both human and mouse dermis. In mice, the dermis contains at least five different DC subsets which can be differentiated based on their expression of langerin, CD11b, CD103 and CD8α markers. Most antigens delivered to the skin are captured by APCs which migrate to skin-draining lymph nodes, although some can move to draining lymph nodes via a cell-independent mechanism20. Among lymph node-resident DCs, langerin+/CD8+ cells constitute about 20%15 and are reportedly superior to other dermal DC in promoting T-helper type 1 (Th1) cell differentiation.
A few studies have investigated the induction of adaptive immune responses in mice following gene gun delivery of OVA or β-galactosidase21,22,23 or microneedle delivery of recombinant human adenovirus encoding HIV-1 gag24. The contribution though of individual APC subsets in protective immunity to microneedle immunization with influenza subunit vaccines has not been completely elucidated. In previous studies we have shown that following microneedle vaccination with Alexa 488 labeled influenza vaccine the majority of the influenza antigen-positive cell emigrating from auricular explants in the medium were CD11c+ whereas the numbers of CD11c-negative cells were approximately 3-fold lower. FACS analysis showed that more than 50% were activated and mature25. The findings were in agreement with earlier reports26. Based on our preliminary observations and on other reported studies that dermal langerin+ DCs migrate from the skin to the LNs after inflammation and in the steady state and represent the majority of langerin+ DCs in skin draining LNs27, we decided to investigate the role of langerin+ cells in influenza-induced adaptive immune responses following skin immunization, since these cells are abundant in the epidermis and constitute a minor part of the APC in the dermis. In this study we used a knock-in mouse model expressing enhanced GFP (EGFP) fused with a diphtheria toxin (DT) receptor (DTR), under the control of the langerin promoter (Langerin-DTR/EGFP) on the C57BL/6 background. Administration of DT leads in 24 h to the elimination of all langerin+ cells, including LCs, without affecting the langerin− DC compartment and without skin or systemic toxicity28,29.
We found that depletion of langerin+ cells impacted the immune response following microneedle vaccination though it had no effect on the response to intramuscular vaccination.
Results
Depletion of langerin+ cells reduces humoral immune responses following skin immunization with microneedles
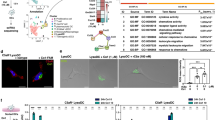
To define the role of langerin+ cells in the immune response to microneedle vaccination, we coupled the Langerin-EGFP-DTR mouse model with an established influenza vaccination protocol using metal microneedles. The langerin-EGFP-DTR model enables depletion of LCs and langerin+ dDCs within 24–48 h after a single injection of DT28. No langerin+ cells were detectable in the skin two days after DT treatment as seen with immunofluorescence microscopy (Figure 1A). Subsequently, three days are required before langerin+ dDCs are once again detectable within the dermis and 2–3 weeks for LCs to reappear within the epidermis22,30. We confirmed these prior findings using our experimental system. Quantitation of vaccine content in the skin of mice not treated with DT showed that 6% of the antigen was present 24 h after microneedle insertion, suggesting uptake by either cellular elements of the skin or by a cell-independent process. In contrast, the depletion of langerin+ cells resulted in 34% retention of the vaccine in the skin (Figure 1B and 1C, Supplementary Figure 1). Supporting this notion, in vitro incubation of the vaccine with skin extract in the presence and absence of protease inhibitors showed no decrease of detectable vaccine due to vaccine degradation (Figure 1D).
Kinetics of antigen loss from the site of immunization.
(A) Cross sections of murine dorsal skin were stained with anti-GFP Alexa 488 antibody to demonstrate selective ablation of langerin+ cells after DT treatment. DT− (24 h) DT+ (24 h). (B) Dorsal skin lysates from mice treated with DT or PBS and vaccinated with 5 μg influenza HA (3 per group) were analyzed 24 h later for vaccine content with SDS-PAGE and Western blot. Vaccine protein bands were visualized with HA-specific sheep antibody and HRP-conjugated rabbit anti-sheep antibody. V: vaccine stock, 1–3: unvaccinated skin, 4–6: vaccinated skin at 0 h, 7–9: vaccinated, DT-treated skin at 24 h, 10–12: vaccinated, PBS treated skin at 24 h. The panel shows combination of two blots performed with the same samples and developed with the same exposure time. The full length blots are presented in Supplementary Figure 1. (C) The intensity of the bands was quantified from the original films presented in Supplementary Figure 1 using ImageJ software. (D) Vaccine was incubated with skin lysate for 3, 6, 12 and 24 h at 37°C with or without protease inhibitors (n = 3) and binding activity was measured by ELISA. The values were normalized to the values of control at 0.25 time point.
Mice treated with DT for depletion of langerin+ cells immunized with vaccine-coated microneedles and control groups were compared for systemic humoral immune responses (Figure 2). We first measured levels of influenza-specific IgG and IgG1 antibody titers and found that they were not significantly different between the DT+ and DT− groups (Figure 3A and 3B). In contrast, IgG2c antibody production was reduced 3 to 5-fold in the DT+ mice compared to the DT− cohort on days 14 (p = 0.027) and 28 (p = 0.004) post-immunization (Figure 3C). This reduction resulted in increased IgG1/IgG2c ratios, indicating a shift towards Th2 responses (Figure 3D). To ensure that the observed differences were not due to non-specific toxic effects caused by the DT treatment, control C57BL/6 mice were similarly injected with DT prior to immunization. No significant difference in IgG and IgG1 antibody titers was observed between the DT-treated Langerin-EGFP-DTR mice and DT-treated C57BL/6 mice (Supplementary Figure 2A, B). However, the IgG2c antibody levels in wild type mice treated with DT were still significantly higher (p = 0.035) than those observed in Langerin-DTR mice treated with DT which indicates that langerin+ cells play a role in the induction IgG2c antibody titers follow microneedle vaccination (Supplementary Figure 1C). There was no statistical significance between levels of IgG2c in the PBS-treated Langerin-EGFP-DTR group (Figure 3C) and the DT-treated C57BL/6 group (Supplementary Figure 2C), p = 0.4629 by Student unpaired 2-tailed t-test. There was no difference between PBS and DT treated C57BL/6 mice in influenza-specific binding antibody titers, ensuring that diphtheria toxin treatment targets specifically DT receptors and does not cause a systemic toxic effect (data not shown)28.
Humoral immune responses are reduced in mice depleted of langerin+ cells prior to skin vaccination.
Anti-influenza binding antibodies were determined by ELISA in sera collected from DT+ and DT− mice 14 and 28 days after immunization. (A) IgG, (B) IgG1 and (C) IgG2c antibody titers. (D) IgG1/IgG2c antibody ratios. Values are expressed as mean +/− SEM (n ≥ 15).
We next determined how the loss of langerin+ cells impacts the generation of antibodies against influenza hemagglutinin using the hemagglutination inhibition (HAI) assay or the microneutralization assay (NT) both of which are considered correlates of vaccine-induced protective immunity31. The DT+ group had significantly lower serum HAI titers than the DT− group on both days 14 (p = 0.009) and 28 (p = 0.016) (Figure 4A). A two-fold decrease in the neutralizing antibody titers (NT) was also observed in LC-depleted mice (Figure 4B). These results indicate that functional antibody responses to cutaneous vaccination are impaired in LC-depleted mice.
Depletion of langerin+ cells prior to skin vaccination results in decreased functional antibody titers and fewer antigen-specific IgM and IgG secreting cells in LNs.
(A) Hemagglutination inhibition (HAI) and (B) neutralizing antibody (NT) titers in sera collected 14 and 28 days after immunization. Values are expressed as geometric mean with a ±95% confidence interval (n ≥ 15). ELISPOT assay of (C) IgM and (D) IgG secreting cells from spleens and inguinal lymph nodes on day 7 and 14 after vaccination. Values are expressed as mean +/− SEM (n = 5). Plasma cell numbers of vaccinated mice were considered positive if the numbers of spots were higher than the sum of naïve infected group spots + 3 × SDev.
Virus-specific antibody secreting cells (ASC) in draining lymph nodes and spleens are decreased following langerin+ depletion
Since we observed reduced humoral immune responses in the DT-treated mice, cutaneous lymph nodes and spleens were analyzed for IgM and IgG-secreting cells at 0, 7 and 14 days after immunization. We chose these time points because we have observed that between days 7 and 14 peak influenza-specific IgM and IgG ASC peak responses occur. At least a twelve-fold higher frequency of IgM-secreting cells was observed in lymph nodes of control mice (DT−) at day 7 compared to the DT+ mice (Figure 4C). At day 14, despite a decline of IgM-secreting cells in the DT− group, the numbers remained two-fold higher than those in the DT+ mice (p ≤ 0.001). Mice treated with DT had also lower numbers of IgG-secreting cells at both days 7 and 14 post-vaccination (Figure 4D). Overall influenza specific IgM and IgG secreting cells in spleens were significantly lower than those observed in lymph nodes. Thus, the numbers of IgM- secreting cells in spleens were already reduced by 50% in DT+ mice 7 days after immunization when compared to the controls (p = 0.005). Despite lower numbers of IgG-secreting cells in spleens of DT+ mice as compared to the controls the differences were not as significant as in lymph nodes, indicating that the effect of LC depletion is more pronounced at sites proximal to antigen delivery.
Langerin+ cell depletion reduces IFN-γ and IL-4 secreting cells in lymph nodes
Naïve T cell differentiation into Th1 or Th2 cells following antigen presentation depends on the cytokine environment. Th2 cells produce IL-4 and mediate humoral immune responses, while Th1 cells secrete IFN-γ and mediate cellular immune responses32. We found that the numbers of IL-4 secreting cells collected on day 7 and 14 from the lymph nodes of DT-treated Langerin-EGFP-DTR mice were reduced by at least 90% (Figure 5A). Since IL-4 is involved in isotype class switch33, this result is consistent with the delayed kinetics of IgM and IgG secreting plasma cells observed in lymph nodes of DT-treated mice at day 14. Similarly, significantly lower numbers of IFN-γ secreting cells were detected in lymph nodes of the DT+ group as compared to control mice (Figure 5B), consistent with the decreased levels of IgG2c. Although influenza specific IL-4 and IFN-γ secreting cells were detected in spleens of the DT+ and DT− mice, no difference was observed between groups in these cell numbers (Figure 5A and 5B). Thus depletion of langerin+ cells alters the generation of cells able to produce cytokines relevant to class switching and cellular immune responses in lymph nodes proximal to the site of immunization.
Depletion of langerin+ cells prior to skin immunization results in reduced T-cell responses in LNs.
Cutaneous lymph nodes (LN) and spleens (S) were collected on day 7 and 14 after immunization of Langerin-EGFP DTR mice. (A) IL-4 and (B) IFN-γ secreting cells were quantified by ELISPOT. Values are expressed as mean +/− SEM (n = 5).
Protective immunity is compromised in langerin+-depleted mice following cutaneous vaccination with microneedles
To investigate the effect of langerin+ depletion prior to cutaneous immunization on protective immunity, DT-treated mice were initially challenged with 5 × LD50 of mouse-adapted A/California/07/09 (H1N1) virus five weeks after vaccination. Greater weight losses (7–8%) were observed in langerin+-depleted mice when compared to control (DT−) mice (3–4%) at 6 to 8 days post-infection (Figure 6A). Both DT+ and DT− groups survived this challenge, whereas the unvaccinated (naïve) mice died by day 8 (Figure 6B). When the challenge infection was repeated with a four-fold higher dose of virus, the DT+ group incurred 12–15% weight losses while the DT− group lost only about of 8–10% of the initial body weight at 6–8 days (Figure 6C). Although there was no statistical significance of differences between survival curves likely due to relatively small numbers of mice per group (10 per group), it is worth noting that the DT+ group showed a 25% higher mortality than the DT− cohort (Figure 6D). Consistent with these results, analysis of virus load in lungs of mice infected with the 20 × LD50 dose showed about 1.5-log lower titers in control mice (DT−) than the naive uninfected control. In contrast the DT+ mice exhibited only about 0.5-log lower virus lung titers than the naive mice (Figure 6E). The average reduction of virus load correlated the average serum HAI titers measured on day 28 in each vaccinated group and the unvaccinated control. Thus, the virus load (expressed in PFU/g tissue) of the DT− group with an average serum HAI titer of 24 was eight times lower than the virus load of DT+ mice with an HAI titer of 16 and twenty one times lower than the lung titers of naive mice with HAI titer at the detection limit.
Protective immunity after skin vaccination is impaired against lethal challenge with homologous virus in mice depleted of langerin+ cells.
Immunized groups were challenged with mouse adapted A/California 07/09 (H1N1) virus 35 days post immunization. (A) Body weight changes and (B) survival rates after challenge with 5 × LD50 of virus were monitored for 14 days (5 mice/group). (C) Body weight changes and (D) survival rates after challenge with 20 × LD50 of virus were monitored for 14 days (8–10 mice/group) (E) Titers of virus in lungs collected 4 days after challenge with 20 × LD50 as calculated by plaque assay (4–5 mice/group) Values are expressed as mean +/− SEM.
In contrast to cutaneous immunization with vaccine coated microneedles, similar levels of influenza-specific binding and functional antibodies (Figure 7A–E) were observed in DT− or DT+ mice vaccinated via the intramuscular route which, when challenged with 20 × LD50 homologous virus, demonstrated about 1.5 log reduction of viral lung titers when compared to naïve mice (Figure 7F). These results support the conclusion that langerin+ cells play an important role in protective immunity elicited by cutaneous vaccination.
Depletion of langerin+ cells prior to intramuscular immunization does not affect immune responses.
(A) IgG, (B) IgG1, (C) IgG2c, (D) HAI and (E) NT antibody titers were measured in sera collected on days 14 and 28 after intramuscular immunization of Langerin-EGFP-DTR mice. (F) Mice were challenged with 20 × LD50 of homologous virus and titers of virus in lungs 4 days after challenge were assessed by plaque assay (4–5 mice/group). IgG titers and PFU/g lung tissue are expressed as the mean +/− SEM. HAI and NT values are expressed as geometric mean with a ±95% confidence interval.
Discussion
Microneedle technology has become one of the most promising novel vaccine delivery platforms. From the immunological point of view, epidermal and dermal layers of skin are densely populated in antigen presenting cells, hence skin is a very attractive site for vaccine delivery15. In this study metal microneedles coated with subunit influenza vaccine were used to deliver subunit influenza vaccine antigen to mouse epidermis and dermis skin.
We observed that 95% of the vaccine is eliminated from the intact skin within 24 hours, whereas only 65% of the vaccine is eliminated from langerin+ cell-depleted skin. The depletion of langerin+ cells prior to cutaneous vaccination resulted in impaired humoral and cellular immune responses. Influenza-specific functional antibody titers were reduced and the Th1 and Th2 profiles in the draining lymph nodes were altered. Absence of langerin+ cells affected isotype switching and resulted in a major reduction of IgG2c antibodies. This reduction is particularly significant because these antibodies have high affinity for complement proteins34 and Fc receptors35, mediating opsonization and presentation of antigens to cells. IgG2a antibodies from BALB/c mice (corresponding to IgG2c in C57BL6)36,37 have been associated with increased efficacy of influenza vaccination38, virus neutralization39, protection against infection40 and immunity to heterologous viruses39,41. Consistent with the decrease of IgG2c titers, the frequency of IFN-γ secreting lymphocytes in DT-treated mice was markedly reduced, further supporting the role of langerin+ cells in stimulation of Th1 immune responses42. Significant decrease and altered kinetics of induction of influenza-specific IgM and IgG antibody secreting cells were mainly observed in lymph nodes proximal to site of vaccination and to a lesser degree in the spleens of DT+ and DT− groups. The mechanism for the slow switch is not yet known but we will follow up in subsequent studies. Interestingly the significantly reduced numbers of IgM and IgG ASC correlated well with the neutralizing antibody titers in the DT+ group. It is likely that the intensity of ASC responses are less pronounced in the spleen because systemic passage of antigens through the vascular supply of the skin, hair follicles and lymphatics23 is limited, although in humans antigens may have preferential interaction with local cell subsets because the numbers of hair follicles are greatly reduced when compared to mice43. However, the qualitative and quantitative differences in antibody secreting cell numbers in local lymph nodes and significant decrease in IgG2c titers in sera suggest that lack of langerin+ cells in mice vaccinated using microneedles results in impaired clonal expansion of B cells to ASCs.
Although it was reported that the bulk of langerin+ (including CD8+) DCs are present in splenic marginal zones around white pulp nodules and hence ideally placed to take up antigens delivered by the blood stream, the langerin+ CD8+ DCs in spleen inefficiently take up antigen from blood44. Consistently with these observations, we found that depletion of langerin+ cells did not alter host immunity following intramuscular immunization. These findings provided further evidence that skin langerin+ cells are more important in the induction of immune responses to cutaneous vaccination as they are in close proximity to antigen delivery site.
The role of epidermal LCs in immunomodulation has been re-assessed in recent studies, indicating that langerin+ cells may play an important role in the induction of adaptive immune responses21,24,45. In one study the authors suggested that langerin+ cells are not required for CD8 T-cell priming following delivery of live adenovirus HIV-1 Gag vaccine via dissolvable microneedles24. Although our results also showed that langerin+ cells were dispensable for the induction of immune responses in spleen, they are important for the induction of immune responses in skin draining lymph nodes following vaccination with microneedle patches coated with influenza vaccine. There are several possible reasons for these differences between their study and ours. First, influenza immunization relies on robust humoral immune responses, whereas live adenovirus vaccines induce robust CD8 T-cell responses46 hence the role of APC subsets may be vaccine-dependent. Secondly, the delivery technology was different as we used metal microneedle arrays vs. polymer needles. Thirdly, the site of immunization was different as we applied microneedles to dorsal flank skin and determined the frequencies of APCs in inguinal lymph nodes as opposed to dorsal surface of the foot to analyze popliteal lymph nodes. Differences in the contribution of langerin+ cells to the induction of immune responses were also observed when the antigen was applied to the ear versus flank skin22, suggesting that use of different sites of immunization will impact the results obtained.
We did not distinguish the role of LCs versus langerin+ dDCs in the induction of immune responses to cutaneous vaccination because with the microneedle platform vaccine is delivered to both the epidermal and dermal skin layers. Previous studies using gene gun vaccination for protein or DNA vaccines reached differing conclusions on the role of LCs and langerin+ dDCs. It has been postulated that LCs are not required for the induction of humoral or cellular immune responses21 or that they may facilitate IgG1 production, a Th2-dependent process23 or may play an immunosuppressive role in unperturbed skin47. In the case of microneedle insertion, an event that perturbs the skin architecture and initiates a sequence of inflammatory events due to chemokine and cytokine secretion, the involvement of LCs may upregulate the immune responses instead22. As for the involvement of langerin+ dDCs in immune responses it has been suggested that they may only mediate differentiation of CD8+ T cells21 or that they may participate in the initiation of humoral immune responses and optimal production of IgG2a/c and IgG2b antibodies early in the response23,48 thus promoting Th1 cell differentiation as compared to other DCs. Our findings are in agreement with reports on the importance of both langerin+ dDCs and LCs in the induction of optimal CD8+ T cell responses following skin vaccination49,50,51 since we showed that depletion of langerin+ cells led to a dramatic reduction of IgG2c antibodies and decrease of IFN-γ secreting T cells.
While we found that langerin+ cells contribute significantly to the induction of immune responses in regional lymph nodes following cutaneous immunization, the survival rates of langerin+ cell depleted animals in the challenge study indicated that they only play a partial role in the protective efficacy of this vaccination approach. The reduction of protection against a lethal dose of influenza virus demonstrated a correlation with the functional antibody titers as well as the percent of vaccine retention in murine skin treated with DT.
It will be of interest to further dissect the role of other APC populations using additional transgenic mouse models following cutaneous delivery. Also, the approach used for antigen delivery (metal versus polymer microneedles) may affect the recruitment of various cell subsets and antigen-APC interaction due to different physicochemical properties affecting vaccine diffusion rate. More detailed understanding of the immunological mechanisms involved in cutaneous immunization and the identification of specific subsets of APCs that are important for capturing antigens and for induction of immune responses could enable further improvements in vaccine efficacy by including adjuvants that target the skin APCs or by targeting antigens to selected DC subsets52,53,54.
Methods
Cells and virus stocks
Madin-Darby canine kidney (MDCK) cells (CCL 34, ATCC, Manassas, VA) were maintained in Dulbecco's Modified Eagle's Medium (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Hyclone, Thermo Scientific, Rockford, IL). Influenza virus stocks (A/California/07/09 (H1N1)) were propagated in MDCK cells. The hemagglutination (HA) activity was determined using turkey blood cells (LAMPIRE, Pipersville, PA)55. Mouse-adapted virus was obtained by serial passage in lungs of C57BL/6 mice and titers were determined by plaque assay56. The LD50 was determined using Reed-Munch formula57.
Animals
Female Langerin-EGFP-DTR mice were kindly provided by Dr. Bernard Malissen (Centre d'Immunologie de Marseille-Luminy, France). Six- to eight-week-old female wild type C57BL/6 mice were purchased from Harlan Laboratories (Tampa, FL). All mice were bred and housed at Emory University. All experiments were performed in accordance to Emory University's Institutional Animal Care and Use Committee guidelines.
Depletion of Langerin+ cells
For systemic depletion, Langerin-EGFP-DTR and C57BL/6 mice (6–8 weeks old) were injected intraperitoneally with 1 μg of DT (Sigma, St. Louis, MO) two days prior22 and on the day of vaccination. Depletion was confirmed by either direct fluorescence staining with anti-GFP Alexa 488 antibody (Molecular Probes, Inc.) of transversal skin cryosections (7 μm thick). The skin was taken from the site of microneedle insertion. No depletion was observed in Langerin-EGFP-DTR mice injected with PBS (DT− group).
Microneedle preparation
Metal microneedles were coated with influenza vaccine as previously described58. H1N1 A/California/07/09 subunit vaccine (261 μg HA/ml stock concentration) was provided by Novartis Vaccines and Diagnostics (Cambridge, MA). The vaccine was concentrated to 7.9 mg HA/ml using a Sartorius Concentrator (Thermo Scientific, Waltham, MA) and combined with an equal volume of solution of 2% w/v of carboxymethylcellulose and 30% w/v of trehalose dehydrate (Sigma Aldrich, St. Louis, MO)59 for coating.
Vaccine stability in skin
Vaccine was labeled with biotin using EZ-link Sulfo-NHS-LC-Biotin (Thermo Scientific, Waltham, MA). The vaccine was concentrated using an Eppendorf Vacufuge (Eppendorf, New York, NY) and delivered to skin using microneedle patches. Mice were euthanized as indicated and 1 cm2 pieces of skin from the site of vaccination were excised and stored in RNAse later solution (Life Technologies, Carlsbad, CA) overnight at 4°C. Skin was washed in cold PBS and homogenized using Lysing Matrix D tubes and Fast Prep-24 homogenizer (MP Biomedicals, Santa Ana, CA). An equal volume of 2× RIPA buffer (2% NP-40, 1% Na-deoxycholate, 0.2% SDS, 100× Halt protease inhibitor cocktail (Thermo Scientific) was added for overnight rotation at 4°C. Supernatants were collected after centrifugation at 14,000 rpm for 20 min at 4°C. To quantify vaccine contents in skin, lysates were analyzed using an ELISA60 with the following modifications. Immunoplates were coated overnight at 4°C with anti-A/California/07/09 HA (CBER, Kensington, MD) diluted 1:5000 in PBS-T followed by 1 h incubation with streptavidin-HRP (BD Biosciences, San Jose, CA) diluted 1:4000 in PBS-T.
Vaccine retention in murine skin in the absence of langerin+ cells
Microneedles coated with 5 μg influenza hemagglutin were applied to dorsal flank skin of mice treated with 1 μg DT for langerin+ cell depletion. Mice untreated with DT and further immunized with vaccine-coated microneedles were used as positive control; mice untreated with DT and unvaccinated were the negative control group. Twenty four hours later the mice were sacrificed and the skin at the site of microneedle insertion was excised and processed as described above. Skin lysates were subjected to denaturing SDS-PAGE and Western blot according to standard protocols. Vaccine protein bands were visualized with HA-specific sheep antibody (CBER) and HRP-conjugated rabbit anti-sheep antibody (ICL Lab, Portland, OR). The intensity of the bands was quantified using ImageJ software.
Immunizations, challenge and sample collection
Langerin-EGFP-DTR mice (25 per group) or C57BL/6 controls were anesthetized by intraperitoneal injection of xylazine/ketamine cocktail to remove dorsal hair. Arrays of five microneedles coated with 5 μg of influenza subunit vaccine were inserted into the skin for 5 min. For comparison, mice from both strains were immunized with the same dose of vaccine intramuscularly (IM). All animals were bled retro-orbitally 14 and 28 days post-immunization under systemic anesthesia. Five animals per immunized group were euthanized on days 7 and 14 post-immunization and spleens and inguinal lymph nodes were collected for evaluation of cellular immune responses5. The remaining animals were challenged on day 30 by intranasal instillation of 5 × LD50 (15 PFU) or 20 × LD50 (60 PFU) mouse adapted virus under isoflurane anesthesia. Fifteen animals were monitored over 14 days for body weight changes, fever, hunched posture and mortality. Weight loss exceeding 25% was used as the experimental end point, at which mice were euthanized according to IACUC guidelines. All studies were approved by Emory University's Institutional Animal Care and Use Committee. The remaining animals were euthanized on day 4 post-infection and lungs were collected for determination of viral titers. All tissues were processed into single cell suspensions in complete RPMI 1640 or DMEM.
Humoral immune responses
Virus-specific antibody levels were determined by ELISA60. Hemagglutination inhibition titers (HAI) were assessed using the WHO protocol55. Neutralizing antibody titers were determined in heat inactivated sera by microneutralization assay using 100 TCID50/well of A/California/07/2009 virus10.
Cellular immune responses
Freshly isolated splenocytes and lymphocytes (1.0 × 106/200 μl cRPMI) were cultured for 36–48 h in the presence of 4 μg/ml influenza subunit vaccine to enumerate IL-4 and IFN-γ secreting cells. ELISPOT reagents were purchased from BD-PharMingen (San Jose, CA). Enumeration of virus-specific ASC (antibody secreting cells) was carried out by ELISPOT assay60 using Millipore plates (Danvers, MA) coated with 5 μg/ml influenza subunit vaccine and counted using an ELISPOT reader and counter (Cellular Technologies, Shaker Heights, OH).
Post-challenge lung titers
Lung homogenates were prepared in DMEM and viral titers were assessed per gram of tissue by plaque assay56.
Statistics
The statistical significance of differences was calculated by two-tailed unpaired Student's t-test and one-way ANOVA including Bonferronis's multiple comparison test. The statistical significance of differences between survival curves was calculated with Mantel-Cox Test and Gehan-Breslow-Wilcoxon Test. A p value less than 0.05 was considered significant. Means and standard deviations were calculated from quadruplicate runs and at least two independent animal experiments.
References
Kupper, T. S. Old and new: recent innovations in vaccine biology and skin T cells. J Invest Dermatol. 132, 829–834, 10.1038/jid.2011.400 (2012).
Adalja, A. A. in Clinician's Biosecurity News, Analysis of Advances and Challenges in Clinical Biosecurity (UPCM Center for Health Security 2013 http://issuu.com/centerforbiosecurity/docs/upmc_web_2013).
Kim, Y. C., Park, J. H. & Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 64, 1547–1568, 10.1016/j.addr.2012.04.005 (2012).
Pasparakis, M., Haase, I. & Nestle, F. O. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 14, 289–301, 10.1038/nri3646 (2014).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 16, 915–920, nm.2182 [pii] 10.1038/nm.2182 (2010).
Koutsonanos, D. G., Compans, R. W. & Skountzou, I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 785, 121–132, 10.1007/978-1-4614-6217-0_13 (2013).
Zhu, Q. et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 106, 7968–7973, 10.1073/pnas.0812652106 (2009).
Quan, F. S., Kim, Y. C., Compans, R. W., Prausnitz, M. R. & Kang, S. M. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release 147, 326–332, 10.1016/j.jconrel.2010.07.125 (2010).
Gill, H. S., Soderholm, J., Prausnitz, M. R. & Sallberg, M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 17, 811–814, 10.1038/gt.2010.22 (2010).
Koutsonanos, D. G. et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2, 357, 10.1038/srep00357 (2012).
Fernando, G. J. et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release 159, 215–221, 10.1016/j.jconrel.2012.01.030 (2012).
Matsuo, K. et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria and influenza. J Control Release 160, 495–501, 10.1016/j.jconrel.2012.04.001 (2012).
Kommareddy, S. et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 31, 3435–3441, 10.1016/j.vaccine.2013.01.050 (2013).
Vrdoljak, A. et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release 159, 34–42, 10.1016/j.jconrel.2011.12.026 (2012).
Henri, S. et al. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 88, 366–375, 10.1038/icb.2010.34 (2010).
Stoitzner, P., Tripp, C. H., Douillard, P., Saeland, S. & Romani, N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol 125, 116–125, 10.1111/j.0022-202X.2005.23757.x (2005).
Romani, N., Clausen, B. E. & Stoitzner, P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev 234, 120–141, 10.1111/j.0105-2896.2009.00886.x IMR886 [pii] (2010).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 8, 935–947, 10.1038/nri2455 nri2455 [pii] (2008).
Henri, S. et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 207, 189–206, 10.1084/jem.20091964 jem.20091964 [pii] (2010).
Mahe, B. et al. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J Invest Dermatol 129, 1156–1164, 10.1038/jid.2008.356 (2009).
Stoecklinger, A. et al. Epidermal langerhans cells are dispensable for humoral and cell-mediated immunity elicited by gene gun immunization. J Immunol. 179, 886–893, 179/2/886 [pii] (2007).
Wang, L. et al. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 180, 4722–4727 (2008).
Nagao, K. et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 106, 3312–3317, 10.1073/pnas.0807126106 0807126106 [pii] (2009).
Bachy, V. et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A. 110, 3041–3046, 10.1073/pnas.1214449110 (2013).
del Pilar Martin, M. et al. Local response to microneedle-based influenza immunization in the skin. mBio 3, e00012–00012, 10.1128/mBio.00012-12 (2012).
Poulin, L. F. et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 204, 3119–3131, jem.20071724 [pii] 10.1084/jem.20071724 (2007).
Bursch, L. S. et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 204, 3147–3156, jem.20071966 [pii] 10.1084/jem.20071966 (2007).
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654, S1074-7613(05)00131-7 [pii] 10.1016/j.immuni.2005.04.004 (2005).
Bennett, C. L. et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 169, 569–576, 10.1083/jcb.200501071 (2005).
Liard, C. et al. Intradermal Immunization Triggers Epidermal Langerhans Cell Mobilization Required for CD8 T-Cell Immune Responses. J Invest Dermatol. 132, 615–625, Doi 10.1038/Jid.2011.346 (2012).
Couch, R. B. et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 207, 974–981, 10.1093/infdis/jis935 (2013).
Constant, S. L. & Bottomly, K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 15, 297–322, 10.1146/annurev.immunol.15.1.297 (1997).
Purkerson, J. & Isakson, P. A two-signal model for regulation of immunoglobulin isotype switching. FASEB J. 6, 3245–3252 (1992).
Seino, J. et al. Activation of human complement by mouse and mouse/human chimeric monoclonal antibodies. Clin Exp Immunol 94, 291–296 (1993).
Heusser, C. H., Anderson, C. L. & Grey, H. M. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 145, 1316–1327 (1977).
Martin, R. M., Brady, J. L. & Lew, A. M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 212, 187–192 (1998).
Gerth, A. J., Lin, L. & Peng, S. L. T-bet regulates T-independent IgG2a class switching. Int Immunol 15, 937–944 (2003).
Weldon, W. C. et al. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PloS one 7, e41501, 10.1371/journal.pone.0041501 (2012).
Gerhard, W., Mozdzanowska, K., Furchner, M., Washko, G. & Maiese, K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev 159, 95–103 (1997).
Coutelier, J. P., van der Logt, J. T., Heessen, F. W., Warnier, G. & Van Snick, J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 165, 64–69 (1987).
Moran, T. M., Park, H., Fernandez-Sesma, A. & Schulman, J. L. Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 180, 579–585 (1999).
Snapper, C. M. & Paul, W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947 (1987).
Calabro, K., Curtis, A., Galarneau, J. R., Krucker, T. & Bigio, I. J. Gender variations in the optical properties of skin in murine animal models. J Biomed Opt. 16, 011008, 10.1117/1.3525565 (2011).
Idoyaga, J., Suda, N., Suda, K., Park, C. G. & Steinman, R. M. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci U S A. 106, 1524–1529, 10.1073/pnas.0812247106 (2009).
Romani, N. et al. Targeting skin dendritic cells to improve intradermal vaccination. Curr Top Microbiol Immunol. 351, 113–138, 10.1007/82_2010_118 (2012).
Barouch, D. H. et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–93, 10.1038/nature10766 (2012).
Stary, G. et al. Glucocorticosteroids modify Langerhans cells to produce TGF-beta and expand regulatory T cells. J Immunol. 186, 103–112, 10.4049/jimmunol.1002485 (2011).
King, I. L., Kroenke, M. A. & Segal, B. M. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 207, 953–961, jem.20091844 [pii] 10.1084/jem.20091844 (2010).
Stoitzner, P. et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 103, 7783–7788, 10.1073/pnas.0509307103 (2006).
Liard, C. et al. Intradermal immunization triggers epidermal langerhans-cell mobilization required For CD8 T-cell immune responses. J Invest Dermatol. 131, S101–S101 (2011).
Elnekave, M. et al. Directly transfected langerin+ dermal dendritic cells potentiate CD8+ T cell responses following intradermal plasmid DNA immunization. J Immunol. 185, 3463–3471, 10.4049/jimmunol.1001825 (2010).
Steinman, R. M. Dendritic cells in vivo: a key target for a new vaccine science. Immunity 29, 319–324, 10.1016/j.immuni.2008.08.001 (2008).
Idoyaga, J. et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 180, 3647–3650 (2008).
Caminschi, I., Lahoud, M. H. & Shortman, K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol 39, 931–938, 10.1002/eji.200839035 (2009).
WHO/CDS/CSR/NCS. WHO Manual of Animal Influenza Diagnosis and Surveillance. Department of Communicable Disease Surveillance and Response (2002).
Sha, Z. & Compans, R. W. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol 74, 4999–5005. (2000).
Reed, L. J. & Muench, H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497. (1938).
Gill, H. S. & Prausnitz, M. R. Coated microneedles for transdermal delivery. J Control Release 117, 227–237, S0168-3659(06)00583-9 [pii] 10.1016/j.jconrel.2006.10.017 (2007).
Gill, H. S. & Prausnitz, M. R. Coating formulations for microneedles. Pharm Res 24, 1369–1380, 10.1007/s11095-007-9286-4 (2007).
Koutsonanos, D. G. et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 204, 582–591, 10.1093/infdis/jir094 (2011).
Acknowledgements
The work was supported by U.S. National Institute of Health grant NIH EB012495. We thank Dahnide Taylor-Williams for valuable laboratory technical support and thank Derek O'Hagan, Sushma Kommareddy and their colleagues at Novartis Vaccines and Diagnostics for providing influenza vaccine.
Author information
Authors and Affiliations
Contributions
J.P.P. designed the majority of the study. E.V. designed part of the study. I.S. designed part of the study. E.S.E. and J.P.P. maintained the animal colony and carried out all the animal work. J.P.P., E.S.E., E.V., I.S. and M.T.T. performed experiments, analyzed data and prepared the figures. J.W.L. prepared the vaccine coated microneedles. B.P.P. consulted in the in vitro skin experiments and contributed in manuscript editing and relevant citations. J.P.P., M.R.P., R.W.C. and I.S. wrote the manuscript. All authors discussed the results and commented on the manuscript. All experiments were performed in accordance to Emory University's Institutional Animal Care and Use Committee guidelines.
Ethics declarations
Competing interests
M.R.P. is an inventor on patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of such companies. This possible conflict of interest is being managed by Georgia Institute of Technology and Emory University.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Pulit-Penaloza, J., Esser, E., Vassilieva, E. et al. A protective role of murine langerin+ cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep 4, 6094 (2014). https://doi.org/10.1038/srep06094
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06094
This article is cited by
-
Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine
Scientific Reports (2017)
-
Enhanced Stability of Inactivated Influenza Vaccine Encapsulated in Dissolving Microneedle Patches
Pharmaceutical Research (2016)
-
Modulation of influenza vaccine immune responses using an epidermal growth factor receptor kinase inhibitor
Scientific Reports (2015)
-
Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature
Drug Delivery and Translational Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.