Abstract
Understanding how declining seawater pH caused by anthropogenic carbon emissions, or oceanacidification, impacts Southern Ocean biota is limited by a paucity of pH time-series. Here,we present the first high-frequency in-situ pH time-series in near-shore Antarctica fromspring to winter under annual sea ice. Observations from autonomous pH sensors revealed aseasonal increase of 0.3 pH units. The summer season was marked by an increase in temporalpH variability relative to spring and early winter, matching coastal pH variability observedat lower latitudes. Using our data, simulations of ocean acidification show a future periodof deleterious wintertime pH levels potentially expanding to 7–11 months annually by 2100.Given the presence of (sub)seasonal pH variability, Antarctica marine species have anexisting physiological tolerance of temporal pH change that may influence adaptation tofuture acidification. Yet, pH-induced ecosystem changes remain difficult to characterize inthe absence of sufficient physiological data on present-day tolerances. It is thereforeessential to incorporate natural and projected temporal pH variability in the design ofexperiments intended to study ocean acidification biology.
Similar content being viewed by others
Introduction
The extensive effects of ocean acidification, the systematic reduction of ocean pH due to theabsorption of anthropogenic carbon dioxide (CO2) by surface oceans1,are predicted to be first observed in high-latitude seas2. Cold waters of theSouthern Ocean are naturally rich with CO2, which results in low carbonate(aragonite and calcite) saturation states2. As ocean acidification progresses,pH and aragonite saturation state (Ωarag) will decrease and facilitate thedissolution of marine calcium carbonate. From a biological perspective, evolution in theabsence of shell-crushing predators in the near-shore Antarctic has left many benthic biogeniccalcifiers with relatively brittle shells3 that may be vulnerable to oceanacidification. Shell dissolution in live Southern Ocean pteropods, Limacina helicinaantarctica, has already been observed in CO2-rich upwelled waters(Ωarag ≈ 1)4. Antarctic marine biota is hypothesized to be highlysensitive to ocean acidification5 and predicting the impact of thisanthropogenic process and the potential for future organismal adaptation is a researchpriority6.
To predict how future ocean acidification will affect any marine ecosystem, it is firstnecessary to understand present-day pH variability. In the Southern Ocean, there are strongseasonal cycles in carbonate chemistry7,8,9 due to the temporal partitioningof summertime primary production and wintertime heterotrophy10. Summertimephytoplankton blooms regularly drive the partial pressure of CO2 in seawater(pCO2) well below atmospheric equilibrium and are the primary source forpCO2 variability in the Southern Ocean9. This seasonal carbonatechemistry cycle corresponds to a summertime pH increase of 0.06 units on a regional scale inthe Southern Ocean11 and as much as 0.6 units locally in Prydz Bay7 and the Ross Sea12. The summertime pH increase (e.g. 0.6) can thusexceed the 0.4 pH unit magnitude of ocean acidification predicted for 210013.
Future ocean carbonate chemistry remains challenging to predict due to other environmentalprocesses and biological feedbacks14. Southern Ocean aragonite undersaturation(approx. pH ≤ 7.9) is predicted to occur first during the winter season in the next 20years11. However, seasonal ice cover may delay the onset of oceanacidification thresholds by a few decades due to reduced air-sea gas exchange12. Likewise, decreasing seasonal ice cover, due to changes in wind and air temperature, areestimated to yield at least a 14% increase in primary production in the Ross Sea by 210015. This could potentially increase pH and Ωarag in summer. Furthermore,increased stratification in the future may result in phytoplankton community shifts15. As an example, diatom communities dominate periods of highly stratified watersin the Ross Sea but drawdown less CO2 compared to the dominant bloom algaePhaeocystis antarctica that proliferate in deeply mixed waters16.Thus, seasonal changes in carbonate chemistry (for example, from primary production) may yieldalternative scenarios for ocean acidification outcomes17. Currently,projections of ocean acidification for near-shore Antarctica are largely based on discretesampling11, which may not have detected sub-seasonal (e.g. daily, weekly) pHvariability that could be important for biological processes.
Although ocean acidification is generally predicted to be deleterious to marine life, not alltaxa and species respond similarly to future conditions18. There is emergingevidence that an organism's pH-exposure history can influence its tolerance of oceanacidification. For example, Ref. 19 showed that an Arctic copepodspecies that experienced varied depth-dependent pH exposure was more tolerant ofCO2-acidified seawater treatments compared to another Arctic copepod species thatexperiences a smaller range in pH. Comprehensive characterization of the ‘pH-seascape’ is thusnecessary to link CO2-perturbation experiments with present-day and futureorganismal performance in the field. Such field time-series are sparse in near-shoreAntarctica and are either extremely short20,21 or low in samplingfrequency7,8,12.
In this study, our main goal was to describe pH variability experienced by organisms innear-shore Antarctica across seasonal transitions in an area with annual sea ice cover. Inaddition, we use the data to explore how pH variability and changes in seasonal CO2drawdown (as a proxy for changes in primary production) may impact future trajectories ofocean acidification in our study region.
Results
pH data
To collect high-resolution pH data, we deployed autonomous SeaFET pH sensors22 in the austral spring at two sites in near-shore McMurdo Sound, Jetty andCape Evans, on subtidal moorings in separate years (Fig. 1). Boththe Jetty and Cape Evans showed four general sequences of pH variation during the observedperiod (Fig. 2a, 2b; Table 1). First, pH(reported on the total hydrogen ion scale for all measurements) rapidly increased fromapproximately 8.0 to 8.3 units in the early austral summer from December to January.Second, monthly pH variability from December through April (s.d. ± 0.03 to 0.08 units) washigher than that observed in November (s.d. ± 0.01 units). The increase in short-term pHvariability remained after removing low-frequency pH variability that was inherentlyincluded in the monthly standard deviations listed in Table 1.Standard deviation of the 10-day moving average of high-pass filtered pH data was greaterduring the summer months relative to November, May and June and peaked in January at bothsites (<0.05 pH units, Fig. 3a, 3b). Third, following peak pHin January, pH and short-term pH variability generally declined to the end of April, butremained higher than November and early-December conditions. Fourth, around the onset of24 h darkness at the end of April and during stabilized temperature pH declined and wasfollowed by lower mean monthly pH and variability in May (s.d. ± 0.02 units) and in June(s.d. ± 0.01 units), relative to summer months. The initial pH increase from fall to peakJanuary conditions corresponded to a decline in the calculated dissolved inorganic carbon(DIC) of 167 and 137 µmol kgSW−1 at the Jetty (54 days) and CapeEvans (31 days), respectively.
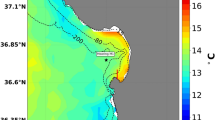
Map of pH sensor deployments in McMurdo Sound, Antarctica.
Sensors were deployed at the Jetty (J) in 2011 and at Cape Evans (CE) in 2012. Annualsea ice contour (marble color) approximates November conditions for 2011 (RISCORapidIce Viewer). Mapping data are courtesy of the Scientific Committee onAntarctic Research, Antarctic Digital Database. Map was constructed in QGIS (Version2.0.1) and sea ice contour was added using GIMP (Version 2.6.11).
pH and temperature cycles in McMurdo Sound, Antarctica.
Time-series pH (a, b) and temperature (c, d) at the Jetty and Cape Evans as recorded bySeaFET pH sensors (grey line). A 10-day low-pass filter (10-d LPF) was applied to the pHand temperature observations (blue line). Daylight is noted by colored x-axis bars where‘sunsets’ indicates decreasing day length. Arrows indicate anecdotal events ofphytoplankton blooms as observed by United States Antarctic Program SCUBA divers.Calibration samples are noted (circle). Ticks on x-axes denote the first day of themonth.
The seasonal pH range was 0.30 and 0.33 pH units for the Jetty and Cape Evans,respectively (Fig. 1a, 1b), based on a 10-day low pass filter.Short-term pH variability contributed to a total range of observed pH from summer towinter conditions of 0.40 and 0.42 units, at the Jetty and Cape Evans, respectively.Maximum pH was observed in January and minimum pH was observed in May at both sites. MeanpH differed between the Jetty and Cape Evans when comparing pH observations of the samedate range (8.15 ± 0.08 at the Jetty; 8.08 ± 0.09 at Cape Evans; Mann-Whitney Wilcoxontest, p < 0.001, W = 3481992, n = 2103). In general,summertime sub-seasonal (Fig. 2a, 2b) and short-term pH variability(Fig. 3a, 3b) was greater at Cape Evans in 2013 compared to theJetty in 2012. Changes in pH of ± 0.13 units occurred various times over the course ofhours to a day at Cape Evans. The largest pH change over a relatively short period was−0.27 units over 5.5 days in March at Cape Evans.
Within the same site, temperature data showed similar patterns in variability as pH:temperature increased from the start of the recording period, peaked in January, afterwhich it declined and stabilized in early April to similar temperatures observed inNovember and early December (Fig. 2c, 2d). Low-pass filtered datashow a seasonal warming of 1.33°C and 1.55°C at the Jetty and Cape Evans, respectively.Like pH, high-pass filtered temperature data showed a seasonal increase in short-termvariability from January through April (Fig. 3d, 3d). Absoluteseasonal temperature change was 1.8°C and 1.7°C at the Jetty and Cape Evans,respectively.
At both the Jetty and Cape Evans, temperature was significantly and positively correlatedwith pH over the deployment period (p < 0.001; Table2), opposing the thermodynamic relationship. High-pass filtered temperature wassignificantly correlated with pH at both sites (Table 2), but thedirection of this relationship was different at both sites and explains little of theoverall pH variation (< 5%, Table 2).
When used for carbonate calculations (DIC, pCO2, Ωarag), pH dataindicate that McMurdo Sound is currently supersaturated with respect to aragonite (Table 1). Monthly mean Ωarag in late fall and early winterapproached 1. Conditions may have actually reached undersaturation (Ωarag<1) for brief periods at Cape Evans in May and June (minimum of Ωarag0.96), depending on the error in pH measurements (see Methods).
Ocean acidification scenarios
McMurdo Sound regional ocean acidification trajectories were made using averaged pHobservations from 2011–2013 and forced with the Representative Concentration Pathway 8.5(RCP8.5) CO2 emission scenario23. Due to the potential offset inpH measurements associated with use of unpurified m-cresol dye (~0.03 pH units, seeMethods), our results may slightly overestimate acidification trends. The equilibriumscenario12, which represents an increase in seawater pCO2 thattracks atmospheric levels, predicted more extreme acidification than the disequilibriumscenario12, which represents a 65% reduced CO2 uptake due toseasonal ice cover (Fig. 4, 5).
Present-day and end-century pH and aragonite saturation state.
Present–day (circle) and end-century monthly mean pH (a) and aragonite saturationstate, Ωarag (b), in McMurdo Sound, Antarctica, using a disequilibrium andequilibrium scenario (solid line). Within each scenario, a simulated 20% increase (upperdashed lines) and decrease (lower dashed lines) in seasonal DIC amplitude is used tosimulated changes in net community production. Dotted lines reference pH 7.9 andΩarag of 1.
Annual changes in pH and aragonite saturation state ranges.
Projections of yearly changes in pH and aragonite saturation state, Ωarag,in McMurdo Sound, Antarctica, using a disequilibrium (a, c) and equilibrium (b, d)scenario. Annual range in pH increases and Ωarag decreases with futureacidification. End-century maximum pH and Ωarag remain above acidificationthresholds of pH 7.9 and Ωarag of 1. Projections are based on field datacollected in 2011–2013 (circle). January and June monthly means represent mid-summer andwinter conditions, respectively. The overall mean represent mean values from spring intowinter conditions. Onset of aragonite undersaturation (triangles) is marked for eachparameter and additionally for November monthly mean conditions.
In both scenarios, CO2 forcing increased the seasonal pH amplitude andreflects the process of reduced ocean buffer capacity as CO2 is absorbed24. For example, present-day range of observed monthly mean pH from January toJune was 0.28 units and increased to 0.31 and 0.35 units under the disequilibrium andequilibrium scenario, respectively. For all scenarios, wintertime pH of ~7.9 (approximatearagonite undersaturation) occurred by the end of the century (Fig.4, 5). Assuming that pH < 7.9 persists for theperiod that we lack data for (July through October), the disequilibrium and equilibriummodels suggest a 7- and 11-month annual duration of pH conditions < 7.9 units andundersaturation by 2100, respectively.
As a proxy for simulating changes in net community production, DIC amplitude wasperturbed by ± 20% (Fig. 4). A 20% increase in seasonal DICamplitude raised pH and Ωarag during the summer and fall but failed to raise pHand Ωarag to present-day levels. For example, under the equilibrium model, a20% increase in seasonal DIC amplitude marginally extended end-century duration ofsummertime pH > 7.9 from January (pH 7.93, Ωarag 1.07) to January (pH7.99, Ωarag 1.21) and February (pH 7.93, Ωarag 1.05).
Any reduction in the amplitude of seasonal DIC will exacerbate the effects of oceanacidification. For example, during the month of peak pH, mean January pH remained above7.9 units in all scenarios, except under the equilibrium scenario with a simulated 20%reduction in seasonal DIC amplitude (January pH 7.87, Fig. 4). Thislatter scenario was the only scenario that exhibited permanent aragonite undersaturationin McMurdo Sound by 2100.
Due to the increase in pH variability observed during summer months (Fig.3), organisms at our study sites will likely still periodically experience pH> 7.9 and Ωarag > 1 by 2100 (Fig. 5). Forinstance, under the equilibrium scenario, maximum pH was pH 8.19 and 0.47 units abovemean January conditions (pH 7.72). Acidification thresholds (pH ~7.9 and Ωarag< 1) were crossed earlier under the equilibrium model compared to thedisequilibrium model (Fig. 5). Here, onset of June (i.e. winter)undersaturation was projected to occur by 2018, a decade earlier than under thedisequilibrium scenario. November (i.e. spring) aragonite undersaturation was predicted tofirst occur by 2045 in the equilibrium model, 46 years earlier than predicted by thedisequilibrium model. Timing of the threshold crossings may be delayed given the potentialoffset in Ωarag associated with the pH measurement error.
Discussion
The observed pH regime in McMurdo Sound can be grouped into two seasonal patterns: (1)stable pH with low variability during the winter and spring and (2) elevated pH with highvariability during the summer and fall. While our pH sensors did not record data from Julythrough October, previous studies of pH (in October21) and temperature25 in this region support our hypothesis of low environmental variability duringthe winter. Note, observations from Prydz Bay8 (68°S) suggest that pH maydecline slightly (<0.1 units) from June to September.
The amplitude of summertime pH elevation (0.3–0.4 units) observed in McMurdo Sound is amongone of the greatest observed in the ocean and matches pH cycles at a northern coastal sitein Prydz Bay, Antarctica7,8. In McMurdo Sound, the intense summertime DICdrawdown started in December and matched the timing of the annually recurringPhaeocystis sp. phytoplankton blooms, which are well-described and typicallycentered on 10 December (R. Robbins pers. comm.)26. The initial pH increaseat Cape Evans (pH 8.01 to 8.12) occurred within 24 h on 9 December 2012 during which SCUBAdivers noted sudden increase in phytoplankton presence in McMurdo Sound (R. Robbins pers.comm.).
Given that (1) the sudden increase in pH at our study sites followed a period of extremelystable pH conditions20,21, (2) maximum observed pH corresponded topCO2 ~200 μatm below atmospheric equilibrium and (3) productive watersfrom the Ross Sea are advected south into east McMurdo Sound10, the initialrapid pH increase in December is likely the signature of phytoplankton blooms thatoriginated in the Ross Sea and reached our coastal sites. Calculated DIC drawdown from fallto summer at the Jetty and Cape Evans (167 and 137 µmol kgSW−1 DIC)matches the timing and magnitude of CO2 cycles observed at similar depths in theRoss Sea27 and Prydz Bay (~135–200 μatm kgSW−1DIC)7,8.
Following the peak pH in January, pH steadily declined to pre-summer conditions by the endof April. A recent study of autonomous pCO2 measurements on incoming seawater atPalmer Station from Arthur Harbor (64°S) observed a summertime increase in primaryproduction, starting in November28. Here, a phytoplankton bloom was capturedwith peak production corresponding to an observation of 50 μatm pCO2.Contrary to the slow return of carbonate chemistry to pre-summer conditions observed inMcMurdo Sound over 4–5 months, pCO2 at Arthur Harbor rapidly returned toatmospheric equilibrium in December and persisted to the end of the study in March. Theauthors attributed the crash of the bloom to physical mixing and zooplankton grazing, whichwould control phytoplankton density and contribute respiratory CO2. Depending onthe year-to-year pH variability on the Antarctic Peninsula, the season of high pH in ArthurHarbor may potentially be much shorter compared to that in McMurdo Sound. For example,interannual carbonate chemistry variability in the Weddell Sea is linked to the timing ofsea-ice melt and phytoplankton productivity in the mixed layer29. The declinein pH observed at McMurdo is likely a combination of reduced primary production, increasedheterotrophy and deepening of the mixed layer, as has been suggested to occur in PrydzBay7 and observed in other notable bloom regions such as the NorthAtlantic30.
Calculated pCO2 at Cape Evans in April (403 ± 44 μatm) nears observationsfrom the Ross Sea made in April 1997 (320–400 μatm)9. A stabilizationof pH in May and June at Cape Evans corresponded to ~500 μatm pCO2. Wewere unable to collect validation samples during this period, however, biofouling was not anissue at our sites and SeaFET pH sensors have been shown to maintain stability over>9 months31. Similar observations have been made elsewhere innear-shore Antarctica. For example, high pCO2 (~490 μatm) was observednear the Dotson Ice Shelf in the Amundsen Sea Polynya in summer and was correlated with thedeepening of the mixed layer relative to the surrounding area32. In addition,the range of Ωarag from mean summer (January) to winter (May) conditions was 0.70and 0.75 at the Jetty and Cape Evans, respectively, matching the latest observations fromPrydz Bay (0.738) and the Weddell Sea (0.7729). The lowpCO2 recorded in May and June at Cape Evans may thus be a combination of watercolumn mixing and heterotrophy, as well as a potential 37 μatm pCO2overestimation associated with the offset of our pH measurement.
The observed ~0.3 unit summertime increase in pH in McMurdo Sound is much larger than thatof northern high-latitudes33. While primary productivity in Antarctic watersis comparable to that of the high-latitude North Atlantic and Pacific9, theobserved < 2°C annual temperature variation is typical of McMurdo Sound25 and plays almost no role in the seasonal amplitude of pH (1.8°C warmingcorresponds to a pH decrease of 0.03 units). In contrast, at locations such as the NorthPacific the temperature cycle can be ~5 times greater than the observed range oftemperatures in this study9. At our sites, the seasonal temperature forcingon pH counteracts seasonal forcing by primary production. As a result, the absence of asignificant temperature forcing in near-shore Antarctica leads to a more pronounced seasonalpH cycle with greater amplitude compared to other bloom regions in the world33.
As captured in our dataset, the summer season in McMurdo Sound is marked by an increase insub-seasonal and short-term pH variability from December through April. In terms of s.d. ofunfiltered (monthly s.d.) and high-pass filtered (10-day s.d.) pH, pH variability in McMurdoSound is of similar magnitude to that observed in temperate kelp forests (e.g. ± 0.043 −0.111) and tropical coral reefs (e.g. ± 0.022) over 30 days34. This issurprising due to absence of large temperature forcing and structural macrophytes andholobionts, which induce diurnal pH cycles at lower latitudes. On a Hawaiian reef,variability in pH was correlated with environmental parameters such as wave and height, windspeed and solar radiation35, suggesting a combination of influential abioticand biotic drivers on coastal seawater pH variability.
We did not directly measure abiotic and biotic factors that influence carbonate chemistryin our study region and more measurements would be needed to quantify the sources ofvariability over different frequencies. For instance, air-sea gas exchange contributes to pHon a seasonal timeframe, where summertime CO2 uptake by the ocean during ice-freeperiods masks the total contribution of net community production to DIC drawdown7. Likewise, summer meltwater dilutes DIC and AT7,8and may contribute to short-term pH variability in summer. The timing of sea ice melt onsetmay impact the duration and magnitude of carbonate chemistry seasonality where early meltingenhances phytoplankton production under optimal mixed layer depths, as has been observed inthe Weddell Sea29. Small pH variability (8.009 ± 0.015) observed from lateOctober through November in McMurdo Sound may be explained by algal photosynthesis, althoughtides may play a small role as well36. Tidal exchanges of shallow and deeperwater masses could play a larger role in summer pH variability, compared to spring36, when the water column is highly stratified37. Low pHvariability observed in winter and spring could also stem from a decrease in respiratoryCO2 contributions to DIC due to metabolic depression during periods of low foodavailablity, as has been observed to occur in pteropods38. In contrast,increased pH variability during the summer and fall is potentially influenced by thedominant biological forcing on the carbonate system in the Ross Sea at that time9. Such phytoplankton blooms create large spatial differences inpCO232 that could lead to sub-seasonal and short-term pHvariability through bloom patchiness across water mass movement. Quantification of abioticand biotic parameters described above would improve estimations for future oceanacidification when incorporated into sensitivity models17.
We explored how seasonal pH variability may influence future ocean acidification in ourstudy region in order to provide guidelines for biological experiments assessing futurespecies' and ecosystem responses. The equilibrium and disequilibrium models provideboundaries for potential worst- and best-case acidification under a CO2 emissionscenario that does not account for climate mitigation efforts23. Within allmodel parameters we employed, marine biota at our study sites are anticipated to experiencechanges beyond the envelope of current conditions, as has been predicted for lower latitudemarine ecosystems as well17. As atmospheric CO2 continues toincrease, (1) pH and duration of summertime high pH (> 7.9) will decrease and (2) themagnitude of seasonal and short-term pH variability may increase.
Previous studies of ocean acidification in the Southern Ocean and the Ross Sea identify theimportance of seasonality and predict onset of wintertime aragonite undersaturation(Ωarag < 1) between 2030 and 2050 under Intergovernmental Panel onClimate Change emissions scenario IS92a2,11,12. Our calculations ofΩarag show wintertime undersaturation in McMurdo Sound occurring within thissame timeframe, despite the higher CO2 emission scenario and high-resolution dataused in our study and potential over estimation of acidification trends associated with theoffset in pH measurements. Given that pH and Ωarag may decrease slightly fromJune through September8 and the lack of pH observations during these months,it is possible that periodic aragonite undersaturation may occur sooner than our predictionsbased on June observations. For context, the consequences of such periodic undersaturationcould lead to calcium carbonate dissolution of live animals, as was observed for L.helicina antarctica at Ωarag ≈ 14. Likewise, studies onAntarctic sea urchin, Sterechinus neumayeri, early development conducted during theperiod of stable spring pH and urchin spawning in McMurdo Sound, suggest that persistingconditions of pH < 7.9 (approximate aragonite undersaturation) may to impair larvalgrowth39 and calcification (G. E. Hofmann and P. C. Yu, unpubl.). Suchconditions could occur in the latter half of this century during the sea urchin spawningseason. Future carbonate chemistry conditions will ultimately depend on the rate at whichanthropogenic CO2 is released to the atmosphere and any future changes in localphysical and biological processes that our model does not account for (e.g. changes intemperature, meltwater, wind, mixing and stratification, upwelling, gas-exchange, andphytoplankton blooms).
Despite the dominant biological footprint in pH seasonality in the Southern Ocean, a 20%increase in seasonal DIC amplitude (simulating an increase in net community production)failed to raise pH to present-day levels at our study site. This suggests that relativelylarge changes in seasonal primary productivity may have a small effect on the pH exposure ofcoastal organisms relative to the changes induced by ocean acidification. Phytoplanktonblooms, as a food source however, may impact species responses to ocean acidification. Forexample, a study of L. helicina antarctica collected in McMurdo Sound found that (1)feeding history (e.g. weeks, months, seasons) impacted oxygen consumption rates and (2)metabolic suppression due to low pH exposure was a masked during periods of foodlimitation38. This study highlights the importance of incorporatingenvironmental history when interpreting experimental results. As the feeding history islikely correlated with pH exposure in the bloom, parsing out the effects of pH history andfood availability will present a challenge for Antarctic physiology.
In Antarctic ocean acidification biology, ‘control’ conditions used in experiments areoften ∼pH 8.0 (e.g. Ref. 39, 40)and represent current spring conditions in McMurdo Sound. Based on our future projections,this ‘control’ treatment will only occur during summer months if at all. Regardless of theexact rate of ocean acidification, the seasonal window of pH > 7.9 andΩarag > 1 will likely shorten in the future. This shrinking andseasonally shifting window of high pH may lead to unpredictable ecological consequencesthrough changes in physiological and seasonally dependent biological processes (e.g. seaurchin larval development). It remains largely unknown how summertime pH levels currentlycontribute to animal physiology and whether or not a reduction in future peak pH andduration of high pH exposure influences physiological recovery following 7–11 monthsunprecedented low pH conditions. As an example, oxygen consumption and gene expression ofheat shock protein 70 in the Antarctic bivalve Laternula elliptica increased whenadults were exposed to experimental conditions near the habitat maxima (pH 8.32, categorizedas ‘glacial levels’ by the authors) and below their current pH exposure (pH 7.77), relativeto performance at ∼pH 8.040. These results suggest that summer exposures mayinduce stress similar to conditions predicted with ocean acidification. Understanding howorganisms are adapted to their present-day exposures will help elucidate how they willrespond to future conditions.
As the exposure period of pH > 7.9 shrinks under simulated ocean acidification, themagnitude of annual pH variability increases. These changes suggest that calcifying marinebiota of Antarctic coastal regions will experience larger seasonal pH cycles in addition toexposure to lower environmental pH. Due to the reduced buffer capacity of the ocean underhigh CO2, it is likely that the short-term pH variability in McMurdo Sound willbe amplified in the future as well24. This has been predicted for coral reefsunder ocean acidification scenarios17 and shown experimentally in pelagicfield mesocosms41 where primary production drives diurnal pH cycles.
Our results provide guidance for the design of biological experiments aimed to address thepotential for Antarctic species to adapt to a seasonally shrinking window of future high pHconditions. Although ocean acidification is likely to create an unprecedented marineenvironment, the existing presence of high pH variability in near-shore Antarctica may havebeneficial implications for biological tolerance of ocean acidification. The distinctsummertime increase in pH and pH variability in near-shore McMurdo Sound suggests thatmarine biota here have some capacity to deal with large fluctuations in the carbonatesystem, as has also been suggested by Ref. 42 in relation tothe seasonal pH cycle. Unlike temperate upwelling regions where pH variability frequentlydrops below pH 8.043, elevation of summer pH in McMurdo Sound opposes thedirection of future ocean acidification. Future studies are necessary to describe how thispH-seascape may select for physiological tolerances of ocean acidification. For example, arenatural positive (e.g. near-shore Antarctica) or negative deviations (e.g. temperateupwelling systems43) from pH 8.0 important for tolerance of futureacidification? Will high summertime pH prepare organisms for low pH conditions in thewinter? What frequency of pH variability promotes acidification tolerance?
A few recent studies have tackled such questions in temperate regions with mixed results.For example, Ref. 44 found that larval growth of musselMytilus galloprovincialis veligers was reduced under low static pH but recoveredunder similar conditions of low mean pH when semi-diurnal pH variability was introduced.However, congener M. californianus did not exhibit this ‘rescued’ response withdiurnal cycles44. Although the Southern Ocean does not experience year-rounddiurnal photoperiods, a similar experimental approach can be used to guide studies on theimpact of pH seasonality on ocean acidification tolerance45 and ultimately,adaptation.
We highlight a coupled oceanography and biology research strategy for studying oceanacidification biology in the Southern Ocean. Studying physiological tolerance and localadaptation to variable seawater chemistry ideally requires large differences in spatial andtemporal pH variability34,43. If patterns of pH variability differ spatiallyaround the Antarctic continent (e.g. McMurdo Sound vs. Arthur Harbor28), wecan begin to investigate possible levels of adaptation to local pH regimes as a proxy forevolutionary adaptions to future conditions46. In other words, evidence ofadaptation in space suggests that animals may be able to adapt in time, as the capacity todo so is linked directly to standing genetic diversity in populations47. Asillustrated in the Southern Ocean, population level differences (e.g. Ross Sea vs. WesternAntarctic Peninsula biota) and local adaptation in tolerance of future anthropogenicstressors may be possible due to different rates in regional warming48. Somestudies have shown genetic structure across the biogeographic boundary of the Drake Passage(reviewed by Ref. 49). Studies regarding population differencesin pH tolerances and exposures in circum-Antarctic species can be accomplished withstrategic placement of oceanographic sensors and design of biological experiments withenvironmentally relevant pH treatments43,50. In addition, use of autonomouspH sensors would address the need for pH observations at high-latitudes5,38.
Methods
Study sites and deployment
Autonomous SeaFET pH sensors containing Honeywell DuraFET® electrodes22were deployed in the austral spring at two sites in separate years on subtidal moorings innear-shore east McMurdo Sound (Fig. 1). Two SeaFETs were deployedside-by-side in December 2011 at a site near McMurdo Station (the Jetty, -77.85115,166.66425) and one SeaFET was deployed during November 2012 at Cape Evans (-77.634617,166.4159). Cape Evans is located 25 km north of the Jetty and is a highly productive sitewith an abundance of fish, macrophytes and marine invertebrates, including the sea urchinS. neumayeri. This site has previously been important for ocean acidificationbiology20,39. Subtidal moorings were anchored at approximately 27 m withsensor depth of 18 m. SeaFETs sampled on a two-hour frequency.
Calibration
All reported pH is on a total hydrogen ion scale and listed as ‘pH’. Raw voltage recordedby the SeaFETs was converted to pH using one discrete seawater sample per sensordeployment following methods from Ref. 31. Calibrationsamples were collected via SCUBA following sensor conditioning to seawater within thefirst two weeks of each deployment, using a 5 L GO-FLO sampling bottle. Ideally,additional validation samples are collected throughout a sensor deployment. However, theremoteness of our sites restricted this work to one discrete sample per sensordeployment.
Calibration samples were preserved with saturated mercuric chloride according to StandardOperating Procedure (SOP) 151. Spectrophotometric pH was determined at 25°Cfollowing SOP 6b51 using m-cresol purple from Sigma-Aldrich®. Totalalkalinity (AT) was measured via open-cell titration with a Mettler-Toledo T50(SOP 3b51). Salinity was measured using a calibrated YSI 3100 ConductivityInstrument. Certified Reference Materials of seawater (CRMs) and acid titrant weresupplied by Dr. Andrew G. Dickson (University of California San Diego, Scripps Institutionof Oceanography). pH at in situ temperature, as recorded by SeaFETs, was calculated fromspectrophotometric measurements of pH25°C and AT and salinity on thebottle sample using the program CO2Calc [Version 1.0.1, 2010, U.S. Geological Survey] withCO2 constants from Ref. 52 refit by Ref.53. All reported carbonate system calculation wereconducted according to these constants.
Data processing and analysis
Raw data from the SeaFETs were cropped based on battery exhaustion, which occurred beforesensor recovery. One of the two sensors deployed at the Jetty failed quality controlanalyses and data from this instrument are not reported. Inspections of raw voltagesrecorded by the functional SeaFETs confirmed that the calibration samples were collectedafter the period of sensor conditioning to seawater. In the absence of biofouling (as wasthe case for our sensors), sensor stability has been demonstrated over similar deploymenttimes31 thereby generating high-quality pH datasets. A comparison of pHfrom each site was conducted using a Mann-Whitney Wilcoxon test as pH values were notnormally distributed (Minitab® 16, Kolmogorov-Smirnov test, p < 0.10, foreach site). All time is reported as UTC.
Time-series carbonate parameters were calculated from pH measurements using CO2calc for adepth of 18 m. Monthly mean salinity data was used from prior measurements in McMurdoSound54 (Table 3). AT was calculatedfrom the empirical relationship between sea surface salinity (SSS) and sea surfacetemperature (SST, as measured by SeaFETs) for the Southern Ocean as reported by Ref.55:

AT measurements on SeaFET calibration samples matched the calculatedAT within the accuracy of titrator (AT and salinity were 2342µmol kgSW−1 and 34.3 for the Jetty; 2351 µmolkgSW−1 and 34.6 for Cape Evans, respectively). Monthly mean nutrientconcentrations were estimated from the literature for McMurdo Sound and various Ross Seastations in close proximity following the directions of ocean currents (max measurementsmonth−1 = 4). Due to the lack of published phosphate measurements forthis region, the Redfield ratio was applied to estimate phosphate from nitrate and silicicacid concentrations, in some cases (W. O. Smith, Jr. pers. comm.).
Summertime decrease in DIC was calculated for both sites from stable fall mean DICconditions to minimum DIC observed in summer. Temperature and pH data were analyzed forevent-scale to seasonal (10-day low-pass filter) and short-term (10-day high-pass filter)trends. Standard deviation of a 10-day moving average window on high-pass filtered datawas calculated to describe seasonal changes in short-term pH and temperature variability.Unfiltered and 10-day high-pass filtered pH and temperature data from the duration of theentire deployment was investigated for each site using a linear correlation analysis(Matlab R2012b, Minitab® 16).
Error estimates
SeaFET thermistors were not individually calibrated resulting in a maximum estimatedtemperature error of ~0.3°C. The estimate of the combined standard uncertainty associatedwith the pH measurement of the calibration samples is ± 0.026 pH units (quadratic sum ofpartial uncertainties). The quantified sources in pH error are: use of unpurified m-cresoldye (0.0256), spatio-temporal mismatch of the calibration sample (±0.01531), user differences (± 0.006) and calibration of the SeaFETthermistor (± 0.005). Measurements of spectrophotometric pH on CRMs, although notspecified by the SOP, suggest that our benchtop methods may underestimatepH25°C by 0.032 (± 0.006, n = 18, across different users and days)relative to theoretical CRM pH calculated from DIC, AT and salinity. It ishoped that, in the future, purified indicator dye will become widely available to theoceanography community in order to improve accuracy of pH measurements. The estimateduncertainty for the pH of calibration samples does not impact the relative changes in pHrecorded by the SeaFET on hourly to monthly time scales, which in the absence ofbiofouling can be resolved to better than 0.001. Thermistors provide a stable temperaturereading with resolution of better than 0.01°C. Based on replicate analyses of CRMs, theprecision of the titration system used for calibration samples is ± ≤10 µmolkgSW−1 and did not impact the pH calculation of our calibration samplesat in situ temperatures. Errors in salinity were not quantified. Instead, calculations ofDIC, pCO2 and Ωarag from the pH time-series were conducted usingmonthly estimates of AT and salinity (Table 3). Forreference, a +0.026 pH error corresponds to errors under November (January) conditions of-9 (-11) µmol kgSW−1 DIC, -27 (-17) µatm pCO2, and+0.07 (+0.10) Ωarag.
Ocean acidification scenarios
RCP8.5, which predicts atmospheric CO2 to reach 935.87 ppm by 210023, was used to generate four ocean acidification scenarios. The equilibriumscenario assumes an increase in DIC at the same rate as would be expected if seawaterpCO2 tracks the atmospheric value (~100 µmol kgSW−1increase in DIC by 2100) and (2) the disequilibrium scenario assumes a DIC increase at a65% slower rate due to seasonal ice cover12. Secondary simulations of a ±20% change in the observed seasonal amplitude of DIC are included along with theCO2 forcing scenarios. The disequilibrium model likely overestimates pH andΩarag as horizontal advection of northern ice-free water masses with longersurface residence times was not accounted for Ref. 12.
First, November was used as a baseline for CO2 forcing scenarios because it isa period of stable pH and has been measured for three consecutive years at Cape Evans20,34,36. Based on these prior studies and data collected in November 2012during this study, mean November pH from 2010–2012 was pH 8.01. Calculated mean Novemberseawater pCO2 was then forced with pCO2 from the RCP8.5 emissionscenario assuming air-sea equilibrium and annual changes in pCO2 were used tocalculate annual changes in November DIC up to 2100.
Second, monthly mean pH and temperature observations from the Jetty and Cape Evans from2011–2013 were averaged to calculate a partial (8-month), present-day, regional DICclimatology. Calculations were performed in CO2calc following methods listed above, withthe exception that monthly mean temperature in June at Cape Evans was corrected from−2.0°C up to −1.9°C to match previous long-term observations25. Inputvariables are listed in Table 3. Starting from the Novemberbaseline, present-day changes in DIC where calculated by month (December – June) and forthe maximum and minimum observed DIC and overall mean. Monthly changes in DIC were assumedconstant for future projections and were applied to end-century November DIC to generate aDIC climatology for 2100. Annual DIC trajectories were modeled for observed minimum andmaximum DIC, November, January, June and overall mean. For simulations of ± 20% change inseasonal DIC amplitude, monthly changes in DIC were increased or decreased by 20%. Owingto the lack of projections of future warming for coastal Antarctica, the effects of futuretemperature change were not included in our simulation.
Additional information
Accession codes: Temperature and pH data are deposited on Biological and ChemicalOceanography Data System. Hofmann, GE. ‘‘pH temp sal.’’ BCO DMO, WHOI. iPub: 13 March2015.
Change history
02 July 2015
A correction has been published and is appended to both the HTML and PDF versionsof this paper. The error has been fixed in the paper.
References
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192, 10.1146/annurev.marine.010908.163834 (2009).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686, 10.1038/nature04095 (2005).
Aronson, R. B. et al. Climate change and invasibility of the Antarctic benthos. Annu. Rev. Ecol. Evol. Syst. 38, 129–154, 10.1146/annurev.ecolsys.38.091206.095525 (2007).
Bednaršek, N. et al. Extensive dissolution of live pteropods in the Southern Ocean. Nat. Geosci. 5, 881–885 (2012).
Fabry, V. J., McClintock, J. B., Mathis, J. T. & Grebmeier, J. M. Ocean acidification at high latitudes: The bellweather. Oceanography 22, 160–171 (2009).
Kennicutt, M. C. et al. Six priorities for Antarctic science. Nature 512, 23–25 (2014).
Gibson, J. A. & Trull, T. W. Annual cycle of fCO2 under sea-ice and in open water in Prydz Bay, East Antarctica. Mar. Chem. 66, 187–200 (1999).
Roden, N. P., Shadwick, E. H., Tilbrook, B. & Trull, T. W. Annual cycle of carbonate chemistry and decadal change in coastal Prydz Bay, East Antarctica. Mar. Chem. 155, 135–147 (2013).
Takahashi, T. et al. Global sea-air CO2 flux based on climatological surface ocean pCO2 and seasonal biological and temperature effects. Deep-Sea Res. II 49, 1601–1622 (2002).
Rivkin, R. B. Seasonal patterns of planktonic production in McMurdo Sound, Antarctica. Am. Zool. 31, 5–16 (1991).
McNeil, B. I. & Matear, R. J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2 . Proc. Natl. Acad. Sci. 105, 18860–18864, 10.1073/pnas.0806318105 (2008).
McNeil, B. I., Tagliabue, A. & Sweeney, C. A multi-decadal delay in the onset of corrosive ‘acidified’ waters in the Ross Sea of Antarctica due to strong air-sea CO2 disequilibrium. Geophys. Res. Lett. 37, L19607, 10.1029/2010gl044597 (2010).
IPCC. . Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Stocker T. F.,, D. Qin G.-K., Plattner M., Tignor S. K., Allen J., Boschung A., Nauels Y., Xia V., Bex and Midgley P. M., eds. (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp. (2013).
Riebesell, U., Körtzinger, A. & Oschlies, A. Sensitivities of marine carbon fluxes to ocean change. Proc. Natl. Acad. Sci. 106, 20602–20609 (2009).
Smith, W. O. J., Dinniman, M. S., Hofmann, E. E. & Klinck, J. M. The effects of changing winds and temperatures on the oceanography of the Ross Sea in the 21st century. Geophys. Res. Lett. 41, 1624–1631 (2014).
Arrigo, K. R. et al. Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 283, 365–367 (1999).
Shaw, E. C., McNeil, B. I., Tilbrook, B., Matear, R. & Bates, M. L. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Global Change Biol. 19, 1632–1641 (2013).
Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434, 10.1111/j.1461-0248.2010.01518.x (2010).
Lewis, C. N., Brown, K. A., Edwards, L. A., Cooper, G. & Findlay, H. S. Sensitivity to ocean acidification parallels natural pCO2 gradients experienced by Arctic copepods under winter sea ice. Proc. Natl. Acad. Sci. 110, E4960–E4967 (2013).
Kapsenberg, L. & Hofmann, G. E. Signals of resilience to ocean change: high thermal tolerance of early stage Antarctic sea urchins (Sterechinus neumayeri) reared under present-day and future pCO2 and temperature. Polar Biol. 37, 967–980, 10.1007/s00300-014-1494-x (2014).
Matson, P. G., Martz, T. R. & Hofmann, G. E. High-frequency observations of pH under Antarctic sea ice in the southern Ross Sea. Antarct. Sci. 23, 607–613, 10.1017/s0954102011000551 (2011).
Martz, T. R., Connery, J. G. & Johnson, K. S. Testing the Honeywell Durafet® for seawater pH applications. Limnol. Oceanogr. Methods 8, 172–184 (2010).
Riahi, K., Grübler, A. & Nakicenovic, N. Scenarios of long-term socio-economic and environmental development under climate stabilization. Technol. Forecast. Soc. Change 74, 887–935 (2007).
Egleston, E. S., Sabine, C. L. & Morel, F. M. Revelle revisited: Buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Global Biogeochem. Cycles 24, GB1002 (2010).
Cziko, P. A., DeVries, A. L., Evans, C. W. & Cheng, C.-H. C. Antifreeze protein-induced superheating of ice inside Antarctic notothenioid fishes inhibits melting during summer warming. Proc. Natl. Acad. Sci. 111, 14583–14588 (2014).
Putt, M., Miceli, G. & Stoecker, D. K. Association of bacteria with Phaeocystis sp. in McMurdo Sound, Antarctica. Mar. Ecol. Prog. Ser. 105, 179–189 (1994).
Sweeney, C. The annual cycle of surface water CO2 and O2 in the Ross Sea: A model for gas exchange on the continental shelves of Antarctica. Antarct. Res. Ser. 78, 295–312 (2003).
Tortell, P. D. et al. Metabolic balance of coastal Antarctic waters revealed by autonomous pCO2 and ΔO2/Ar measurements. Geophys. Res. Lett. 41, 6803–6810 (2014).
Weeber, A., Swart, S. & Monteiro, P. Seasonality of sea ice controls interannual variability of summertime ΩA at the ice shelf in the Eastern Weddell Sea – an ocean acidification sensitivity study. Biogeosci. Disc. 12, 1653–1687, 10.5194/bgd-12-1653-2015 (2015).
Körtzinger, A. et al. The seasonal pCO2 cycle at 49°N/16.5°W in the northeastern Atlantic Ocean and what it tells us about biological productivity. J. Geophys. Res. 113, C04020 (2008).
Bresnahan, P. J. J., Martz, T. R., Takeshita, Y., Johnson, K. S. & LaShomb, M. Best practices for autonomous measurement of seawater pH with the Honeywell Durafet. Methods Oceangr. 9, 44–60 (2014).
Mu, L., Stammerjohn, S., Lowry, K. & Yager, P. Spatial variability of surface pCO2 and air-sea CO2 flux in the Amundsen Sea Polynya, Antarctica. Elementa: Sci. Anthrop. 2, 000036 (2014).
Shadwick, E., Trull, T., Thomas, H. & Gibson, J. Vulnerability of polar oceans to anthropogenic acidification: comparison of Arctic and Antarctic seasonal cycles. Sci. Rep. 3, 2339 (2013).
Hofmann, G. E. et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. Plos One 6, e28983, 10.1371/journal.pone.0028983 (2011).
Lantz, C., Atkinson, M., Winn, C. & Kahng, S. Dissolved inorganic carbon and total alkalinity of a Hawaiian fringing reef: chemical techniques for monitoring the effects of ocean acidification on coral reefs. Coral Reefs 33, 105–115, 10.1007/s00338-013-1082-5 (2014).
Matson, P. G., Washburn, L., Martz, T. R. & Hofmann, G. E. Abiotic versus biotic drivers of ocean pH variation under fast sea ice in McMurdo Sound, Antarctica. Plos One 9, e107239 (2014).
Barry, J. Hydrographic patterns in McMurdo Sound, Antarctica and their relationship to local benthic communities. Polar Biol. 8, 377–391 (1988).
Seibel, B. A., Maas, A. E. & Dierssen, H. M. Energetic plasticity underlies a variable response to ocean acidification in the pteropod, Limacina helicina antarctica. Plos One 7, e30464 (2012).
Yu, P. C. et al. Growth attenuation with developmental schedule progression in embryos and early larvae of Sterechinus neumayeri raised under elevated CO2 . Plos One 8, e52448 (2013).
Cummings, V. et al. Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. Plos One 6, e16069, 10.1371/journal.pone.0016069 (2011).
Schulz, K. G. & Riebesell, U. Diurnal changes in seawater carbonate chemistry speciation at increasing atmospheric carbon dioxide. Mar. Biol. 160, 1889–1899 (2013).
McNeil, B. I., Sweeney, C. & Gibson, J. A. E. Short Note Natural seasonal variability of aragonite saturation state within two Antarctic coastal ocean sites. Antarct. Sci. 23, 411–412 (2011).
Hofmann, G. E. et al. Exploring local adaptation and the ocean acidification seascape – studies in the California Current Large Marine Ecosystem. Biogeosciences 11, 1053–1064 (2014).
Frieder, C. A., Gonzalez, J. P., Bockmon, E. E., Navarro, M. O. & Levin, L. A. Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Global Change Biol. 20, 754–764, 10.1111/gcb.12485 (2014).
Murray, C. S., Malvezzi, A., Gobler, C. J. & Baumann, H. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11, 10.3354/meps10791 (2014).
Sanford, E. & Kelly, M. W. Local adaptation in marine invertebrates. Ann. Rev. Mar. Sci. 3, 509–535, 10.1146/annurev-marine-120709-142756 (2011).
Sunday, J. M. et al. Evolution in an acidifying ocean. Trends Ecol. Evol. 29, 117–125 (2014).
Steig, E. J. et al. Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature 457, 459–462 (2009).
Kaiser, S. et al. Patterns, processes and vulnerability of Southern Ocean benthos: a decadal leap in knowledge and understanding. Mar. Biol. 160, 2295–2317 (2013).
McElhany, P. & Busch, D. S. Appropriate pCO2 treatments in ocean acidification experiments. Mar. Biol. 160, 1807–1812 (2013).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3, 191 pp. (2007).
Mehrbach, C., Culberso, C. H., Hawley, J. E. & Pytkowic, R. M. Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973).
Dickson, A. G. & Millero, F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. I 34, 1733–1743, 10.1016/0198-0149(87)90021-5 (1987).
Littlepage, J. L. Oceanographic investigations in McMurdo sound, Antarctica. Antarct. Res. Ser. 5, 1–37 (1965).
Lee, K. et al. Global relationships of total alkalinity with salinity and temperature in surface waters of the world's oceans. Geophys. Res. Lett. 33, L19605 (2006).
Liu, X., Patsavas, M. C. & Byrne, R. H. Purification and characterization of meta-cresol purple for spectrophotometric seawater pH measurements. Environ. Sci. Technol. 45, 4862–4868 (2011).
Gordon, L. et al. Seasonal evolution of hydrographic properties in the Ross Sea, Antarctica, 1996–1997. Deep-Sea Res. II 47, 3095–3117 (2000).
Noble, A. E., Moran, D. M., Allen, A. & Saito, M. A. Dissolved and particulate trace metal micronutrients under the McMurdo Sound seasonal sea ice: basal sea ice communities as a capacitor for iron. Front. Chem. 1, 25 (2013).
Smith, W. O. J., Dinniman, M. S., Klinck, J. M. & Hofmann, E. E. Biogeochemical climatologies in the Ross Sea, Antarctica: seasonal patterns of nutrients and biomass. Deep-Sea Res. II 50, 3083–3101 (2003).
Acknowledgements
We thank Dr. Paul G. Matson for sensor preparation in 2011 and United States AntarcticProgram staff members, Rob Robbins and Steven Rupp, for SCUBA diving support. We thank Dr.Craig A. Carlson for insightful discussions. This research was supported by U.S. NationalScience Foundation (NSF) grants ANT-0944201 and PLR-1246202 to GEH. LK was supported by aNSF Graduate Research Fellowship and ALK was supported by the NSF Postdoctoral Fellowshipin Polar Regions Research, award number ANT-1204181.
Author information
Authors and Affiliations
Contributions
G.E.H. and L.K. conceived deployment strategy. L.K. conducted sensor deployment in 2012 andall data processing. L.K. and T.R.M. determined data quality. A.L.K. assisted with sensorrecovery in 2013 and conducted regression analysis. L.K. and E.C.S. conducted modelprojections. All authors reviewed and contributed to writing the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 InternationalLicense. The images or other third party material in this article are included in thearticle's Creative Commons license, unless indicated otherwise in the credit line; if thematerial is not included under the Creative Commons license, users will need to obtainpermission from the license holder in order to reproduce the material. To view a copy ofthis license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kapsenberg, L., Kelley, A., Shaw, E. et al. Near-shore Antarctic pH variability has implications for the design of oceanacidification experiments. Sci Rep 5, 9638 (2015). https://doi.org/10.1038/srep09638
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09638
This article is cited by
-
A comparative and ontogenetic examination of mitochondrial function in Antarctic notothenioid species
Journal of Comparative Physiology B (2022)
-
In situ response of Antarctic under-ice primary producers to experimentally altered pH
Scientific Reports (2019)
-
Thermal windows and metabolic performance curves in a developing Antarctic fish
Journal of Comparative Physiology B (2018)
-
Southern Ocean pteropods at risk from ocean warming and acidification
Marine Biology (2018)
-
Transcriptomic response of the Antarctic pteropod Limacina helicina antarctica to ocean acidification
BMC Genomics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.