Abstract
Kelp forests on coastal rocky shores are negatively impacted by sudden sediment loads that can occur with storms and floods. Using laboratory experiments, we studied the effects of sediment deposition on the survival of the large brown alga Eisenia bicyclis juveniles (zoospores and gametophytes) to quantify the potential impacts of particulate matter on kelp forests. The zoospore adhesion rate and the gametophyte survival and growth rates all declined markedly with increasing sediment load, particularly with smaller particle diameter. Using experimental results, we derived an equation to calculate the rate of initial kelp depletion with sediment load based on the quantity and size distribution of sediment particles. The equation enabled the estimation of E. bicyclis depletion rates in the field by measurement of particle quantity and diameter distribution of sediments on the reef substrate.
Similar content being viewed by others
Introduction
Kelp forests are marine communities established by brown macrophytes such as Eisenia bicyclis and Ecklonia cava that serve as valuable feeding and nursery grounds for a diverse range of coastal life1,2. However, kelp forests are affected by various factors including water temperature3,4, nutrients3,5,6, the strong currents associated with storms and waves2, grazing by herbivorous organisms7,8,9 and competition from other algae2,10, which result in a cycle of expansion and reduction of the forest area. Changes in turbidity and seabed sediments also affect kelp forests. Pulsed sediment loads occurring from natural phenomena, e.g., river floods11,12, cliff erosion and runoff13, resuspension after storms2,14,15 and artificial processes including construction and solid waste disposal16,17,18 can inflict serious harm on these macrophyte species.
Increased seawater turbidity and/or sediments can impact marine kelp communities either by indirectly reducing sunlight or by the direct physical influence of the particulate matter. Studies have examined the effects of reduced sunlight on seaweed beds inhabiting coastal areas19,20,21. The direct impacts of particulate matter have been studied on several species inhabiting temperate reefs22,23,24,25. For example, sediments may directly influence the canopy-forming macrophyte Macrocystis by inhibiting sporophyte adhesion22. Growth of Undaria pinnatifida26, another brown macrophyte species, is also inhibited by sediment particles. Studies have shown that the rate of brown macrophyte zoospore adhesion is greatly reduced by the accumulation of particulate matter on the substrate and sediment settlement atop zoospores and gametophytes of these species reduces growth and survival27,28,29. However, these studies examined one particle size in the laboratory and did not encompass the wide range of particle sizes occurring in the field. In contrast, field experiments on turf-forming algae have shown that increasing sediment quantities promote turf algae while inhibiting canopy-forming macrophytes23,24,25.
Using laboratory experiments, we determined the effects of different sizes of sediment particles on zoospore adhesion and gametophyte growth and survival of the canopy-forming macrophyte E. bicyclis. We used this data to develop an equation estimating the rate of initial E. bicyclis depletion under sediment loads that more accurately estimate the effects of reef sediment deposition on kelp forests.
Results
Effects of sediment particles on E. bicyclis zoospore adhesion
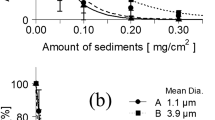
Relative to the control, the E. bicyclis zoospore adhesion rates in the presence of 15.0-μm particles were 31.8 ± 1.6%, 19.2 ± 2.5% and 1.2 ± 0.6% under the 1, 2 and 5 mg sediment cm−2, respectively. Thus, adhesion declined as a logarithmic function of increasing particle quantity (Fig. 1). The adhesion rate in treatments with 48.2, 164 and 599-μm particles also declined logarithmically with increasing sediment quantity. With the 10 mg cm−2 sediment, the adhesion rates were 14.4 ± 6.6%, 35.4 ± 11.9% and 51.9 ± 9.0% in the 48.2, 164 and 599-μm particle size treatments, respectively, indicating that smaller particles have greater negative effects for a given volume of sediment.
Based on the exponential approximation of the adhesion rate in the absence of sediment (defined as 100%), the relationship between the E. bicyclis zoospore adhesion rate and sediment quantity for each particle diameter was defined as:




where, Ar and Q denote the relative E. bicyclis zoospore adhesion rate (%) and the quantity of sediment particles (mg cm−2), respectively.
Effects of sediment particles on gametophyte growth and survival
Survival of E. bicyclis gametophytes declined markedly as sediment quantity increased (Fig. 2). Relative to the controls with no sediment, gametophyte survival rates under 15.0-μm diameter particles of kaolinite were 64.2 ± 13.2%, 22.3 ± 18.7% and 0% in the 1, 10 and 25 mg cm−2 treatments, respectively. Survival rates under 48.2, 164 and 599-μm particles also declined as a logarithmic function of particle quantity. Additionally, smaller particle sizes had stronger negative effects on survival. With 25 mg cm−2 sediment, the survival rates were 38.3 ± 9.2%, 48.8 ± 1.4% and 65.7 ± 8.1% in the 48.2, 164 and 599-μm particle size treatments, respectively.
Exponential approximation of the relative gametophyte survival rates for different particle diameters gave the following relationships:




where, Sr and Q denote the relative E. bicyclis gametophyte survival rate (%) and the quantity of sediment particles (mg cm−2), respectively.
The total length of E. bicyclis gametophytes on day 12 is shown for each particle size in Tables 1 and 2. In the absence of sediment, mean gametophyte length was 28.3 ± 2.0 μm on day 6 and 144.9 ± 3.9 μm for male and 164.8 ± 3.9 μm for female gametophytes on day 12. Analysis of covariance (ANCOVA) revealed that the negative effects of sediment on gametophyte growth increased both with increasing sediment quantity and decreasing particle size (P < 0.001). Additionally, gametophyte length differed significantly between males and females (P < 0.001). However, there were no interactions between either, particle size and sex (P = 0.900) or sediment quantity and sex (P = 0.274), indicating no difference in the effects of sediment on gametophyte growth between males and females.
Estimation of field rates of E. bicyclis depletion under varying sediment loads
The present study revealed the relationships between the E. bicyclis zoospore adhesion rate and the quantity of sediments with different particle diameters [equations (1, 2, 3, 4)], as well as the relationships between the E. bicyclis gametophyte survival rate and the quantity of sediments with different particle diameters [equations (5, 6, 7, 8)]. Here, we used these equations to estimate the expected fields rates of E. bicyclis zoospore adhesion and gametophyte survival based on the quantity and diameter of sediment particles deposited on the substrate.
Equations (1, 2, 3, 4) and (5, 6, 7, 8) describe the effects of uniformly sized particles. Arakawa et al.30 showed that the inhibitory effect of sediment on Gelidium elegans spore adhesion was greater under a range of particle sizes than under those of a uniform diameter, even when both sets of particles had an identical mean diameter. Because particulate matter on the seabed is composed of particles of varying diameter, equations estimating the effects of a wide range of particle diameters are needed. Based on the relationship between the E. bicyclis zoospore adhesion rate and sediment quantity, the relationship between the inverse of particle diameter (x = 1/D) and the coefficient (y) in equations (1, 2, 3, 4) was determined to be (Fig. 3a):
Relationship between the reciprocal of particle diameter and the involution coefficient of (a) the adhesion rate as determined by equations 1, 2, 3, 4, and (b) the survival rate as determined by equations 5, 6, 7, 8.

Combining equations (1, 2, 3, 4) with equation (9), the zoospore adhesion rate (%), Ar, can be expressed by the mean particle diameter D (μm) and the total quantity Q (mg cm−2) of sediment:

The E. bicyclis zoospore adhesion rate can thus be calculated when the mean particle diameter and quantity of sediment are known.
Similarly, the effect of sediment particles on the E. bicyclis gametophyte survival rate can be calculated using the inverse of particle diameter (x = 1/D) and the coefficients (y) from equations (5, 6, 7, 8) (Fig. 3b):

Combining equations (5, 6, 7, 8) with equation (11), the gametophyte survival rate (%), Sr, can also be expressed as a function of D (μm) and Q (mg cm−2), which allows its calculation when D and Q are known:

Consequently, the overall effect of sediment particles on the initial E. bicyclis depletion rate (%) (zoospores and gametophyte survival) can be derived from the mathematical product of equations (10) and (12):

where, Lr, Ar and Sr denote the initial depletion rate, the zoospore adhesion rate and the gametophyte survival rate, respectively. Lr represents the rates of detachment and death of zoospores and gametophytes caused by sediment particles. By inserting equations (10) and (12) into equation (13), an equation can be derived that expresses the effect of the quantity (Q) and particle diameter (D) of sediments on the early stages of E. bicyclis (Lr):

Because sediments on the seabed have a wide range of diameters and the use of mean particle diameter underestimates the effects of sediment30, we applied the technique of Arakawa et al.30 to solve this problem. When the range of particle diameters in equation (14) are divided into n groupings, the initial E. bicyclis depletion rate (Lr) can be expressed as the mathematical product of the effects of particles in each diameter grouping:


Thus, initial E. bicyclis depletion can be estimated from the quantity and size distribution of sediment particles on the substrate.
Discussion
Some of the factors that affect kelp forest dynamics include temperature and nutrient stress, strong surge and flow during storms, sand and sediment scour, grazing and competition from other algae2. Canopy-forming communities are depleted by unusually high water temperatures and low nutrient concentrations8. Dayton et al. also reported the destruction of a Macrocystis kelp community by strong winter storms2. There are many reports of biological disturbances like grazing and competition in kelp communities7,8,9. That is, these physical and biological factors cause the depletion and expansion of canopy-forming communities, interfering with the very complex dynamics of the communities. Fluctuations in suspended seawater particles and seabed sediment volumes also affect kelp communities31. In coastal rocky areas, natural phenomena such as river floods11,12, accumulation after storms14,15 and sediment scour by strong water currents create sediment loads32,33,34.
The sediment loads from natural phenomena on rocky reefs have a considerable influence on the early life-stages of kelp forest species. In this paper we focused on the effects of sediments on the early life-stages of canopy-forming algae. Our study found that increasing sediment deposition on the substrate inhibited E. bicyclis zoospore adhesion and the inhibitory effect was more prominent as particle diameter decreased. Larger sediment quantities also reduced E. bicyclis gametophyte growth and survival.
Inhibition of the larval and juvenile stages by sediments has been reported in many algal species21,22,23,27,28,35,36,37,38,39,40,41,42,43,44. However, there are few reports on the differences in negative effects on canopy-forming algae by different size sediment particles. According to reports, smaller sediment particles have larger negative effects on early stage canopy-forming alga30,45,46.
Arakawa et al.30 also investigated the adhesion of G. elegans spores on substrates upon which sediment particles of varying sizes were deposited in different quantities. Similar to the current study, they showed that less spores adhered as the quantity of particles of any size increased. Additionally, the inhibitory effect on adhesion increased with decreasing particle size. However, the effects were stronger on E. bicyclis than on G. elegans for a given particle size. Arakawa et al.30 also found that kaolinite particles inhibited G. elegans spore adhesion at loads of 30 mg cm−2. This result, combined with those of previous studies28,29, indicates that a given quantity of sediment particles also inhibits substrate adhesion in E. bicyclis, E. cava and U. pinnatifida zoospores, but is less inhibitory to G. elegans. This effect is likely attributable to size differences, where E. bicyclis, E. cava and U. pinnatifida zoospores are ~5 μm in diameter27, those of G. elegans are 30 μm47. Thus, comparison between our results on E. bicyclis and those on G. elegans by Arakawa et al.30 suggest that the effect of sediment depends on spore size. Because spore size is highly variable among algal species, further studies are needed to understand the effects of sediment on different algal species.
A study on substrate adhesion in Ulva prolifera spores under sediment deposition showed that particles with a comparable or smaller diameter than the spores do not leave sufficient inter-particle space for spores to reach and adhere to the substrate46. A similar mechanism may be at play in E. bicyclis. That is, when small sediment particles were deposited on the substrate, the amount of free space on was reduced and spore adhesion decreased dramatically.
For a given quantity of sediment, smaller particles had greater inhibitory effects on gametophyte growth and survival. In this experiment, we observed that many of the 15.0- and 48.2-μm particles adhered to the surface of the gametophytes. Larger numbers of fine particles on the gametophyte surface reduced the area in contact between the seawater and the gametophyte cell, obstructing nutrient uptake and gas exchange, leading to slow growth and mortality.
Gorgula & Connell24 suggested that although increasing sediments on rocky reefs inhibits canopy-forming macrophytes, turf algae benefit. Other studies on turf algae23,25 on rocky reefs have shown that the species are tolerant of sediment deposition and exhibit a strong negative influence of large sediment particles on turf algae. In this study smaller sediment particles had larger negative effects on early stage canopy-forming algae. That is, sediments loads on reefs as a result of storms or floods, inhibited canopy-forming macrophyte adhesion to the substrate and slowed growth. The effects are worst from fine particle sediments, which causes the complete destruction the macrophyte community. When the canopy-forming macrophytes die, sufficient light reaches the turf-forming algae providing them with an advantageous environment. Both communities are affected by sediment in their environment.
Airoldi31 reviewed the influence of sediment loads on rocky reef seaweed communities in detail. Sudden or continuous sediment influx into coastal ecosystems occurring by either natural (e.g., flooding or storms) or human causes increases sediment accumulation, which has negative effects on the kelp community. There are many studies on the negative effects on the kelp communities; however, a few examples were reported in a study on the mathematical estimation of the effects of sediment22,23,28,32,48,49. Furthermore, we do not know if they estimated the effect of particle size for the influence of the sediments.
We developed an equation to estimate the early depletion of the canopy-forming macrophyte E. bicyclis from the particle size and quantity of sediment settling on the substrate. The equation was parameterized by exponential approximation of the experimental results performed using 15–600-μm diameter particles. The resulting equation provides a good estimate of the influence of sediment accumulation of a given particle size on the existing seaweed community. It is possible, however, that this equation may overestimate the influence of particles <15 μm. The influence of particles <15 μm requires further research.
We can evaluate the influence of sediments using equation (15) and the quantity and particle size distribution of sediments on in situ rocky reefs. Sudden sediment loads occur on rocky reefs during strong storms and floods. Arakawa et al.50 investigated the amount of sediments on a rocky reef seaweed forest after a river flood in Wakayama Prefecture, Japan. Using the sediment data (sediment quantity and particle size) from equation (15), the initial depletion of E. bicyclis in this area can be estimated.
However, we can examine the model predictions using a case study on the effects of particle transport on the coastal seaweed community off east Japan after the tsunami in the spring of 2011. It has been reported that the kelp forest in this area did not suffer significant impacts from the strong flow of the tsunami51. A large quantity of sediment particles rolled up by the tsunami were later gradually deposited on surviving kelp forest substrates52.
The tsunami that occurred in Japan in the spring of 2011 suspended large quantities of sediment particles in coastal waters that were eventually deposited on the substrate in kelp forests, among other places. E. bicyclis zoospores are released in autumn; thus, the tsunami occurred during the sporophyte growing season. Although the influx of particulate matter is likely to have affected sporophyte growth and survival, this effect cannot be determined from the results of the present study. Sediment influx to the rocky reef at Shizugawa in Miyagi Prefecture remained on the reef until the following year (Agatsuma et al., unpublished data). Because the sediment load was up to several cm thick, zoospore adhesion is unlikely. Future monitoring of sediment loads and particle size distributions in this area will allow us to estimate the initial depletion of zoospores and gametophytes and the regrowth potential of the kelp forests.
Kelp forest destruction by coastal development, dredging and river inflow has been documented in numerous coastal areas53,54. The equations derived in this study to estimate initial kelp depletion rates could be used to evaluate the resulting negative effects on canopy-forming macrophytes in coastal ecosystems. However, we cannot understand how initial kelp depletion affects the development of future seaweed communities. We have to clarify the threshold of the initial depletion for natural kelp community formation in future study.
Materials and Methods
The effects of sediment particles on zoospore substrate adhesion and on the growth and survival of the canopy-forming kelp E. bicyclis gametophytes were examined using laboratory experiments. Our experiments utilized various sediment particle sizes to the maximize relevance to actual kelp forest ecosystems. Effects on both male and female mature gametophytes were analysed. A model estimating the depletion of early stage E. bicyclis under deposition of sediment particles in the field was formulated using the laboratory results.
Seaweed samples and sediment particles
Mature E. bicyclis sporophytes were collected from a subtidal reef in Shirahama, Minamiboso city, Chiba Prefecture, Japan. Sori were immediately cut from the thalli, washed, wiped dry and left in the shade for 1–2 h. A zoospore suspension was prepared by soaking the sori in sterile filtered seawater (Millipore Filter HA, 0.45 μm pore size; sterilized at 120 °C for 35 min) for 20 min to release the zoospores and then allowing the mixture to stand in the dark for 1 h.
Particles of the clay mineral kaolinite (mean diameter: 15.0 μm) and glass beads of three different diameters (mean diameters: 48.2, 164 and 599 μm) were used as the four sediment treatments (Fig. 4a–d). Particle size distributions were measured using a LS-200 instrument (Beckman-Coulter Inc., California).
Effect of sediment particles on zoospore substrate adhesion
To determine the effect of sediment loads on zoospore adhesion to the substrate, cylindrical tanks (34 cm × 30 cm, diameter × height) were filled to a 28-cm depth with filtered seawater and glass slides with a covering of sediment particles were placed on the bottom of the tank. Sediment treatments applied to the glass slides included: 0.5, 1, 2, 3 and 5 mg cm−2 of 15.0-μm particles; 3, 5, 10, 25 and 50 mg cm−2 of 48.2-μm particles; 5, 10, 25, 50 and 100 mg cm−2 of 164-μm particles; and 10, 25, 50, 100 and 150 mg cm−2 of 599-μm particles. Control slides had no sediment. We added 75-mL of zoospore suspension (400,000 inds. mL−1) to the centre of the tank and allowed it to stand for 12 h. The slides were then taken from the tank and rinsed gently with filtered seawater to remove sediment. The rate of zoospore adhesion to the slides was determined using a microscope. Five replicate samples were used for the 15.0-μm particle treatments and 10 replicates were used for the other particle treatments. The adhesion rate was calculated from the number of adhering zoospores according to the following equation:

where, Ar, Ns and Nno denote the adhesion rate and the adhesion densities in the presence and absence of sediment particles, respectively.
Effects of sediment particles on gametophyte growth and survival
Zoospore adhesion to glass slides was accomplished by allowing a zoospore suspension of 500,000 zoospores to stand for 1 h in a tall Petri dish (15 cm × 9 cm, diameter × height) containing glass slides and 1 L sterile filtered seawater. Sediment particles were then deposited on the slides according to the following treatments: 1, 5, 10, 15 and 25 mg cm−2 of 15.0-μm particles; 10, 25, 50 and 100 mg cm−2 of 48.2-μm particles; 10, 25, 50, 100 and 150 mg cm−2 of 164-μm particles; and 25, 50, 150 and 200 mg cm−2 of 599-μm particles. Three replicates were used for each combination of particle diameter and quantity.
Seeded glass slides with sediment particles were placed two each in Petri dishes filled with PESI culture medium55. Petri dishes were kept in a 20 °C incubator for 12 days under neutral white fluorescent light with an intensity of 71 μmol photons m−2 s−1 and a 12: 12-h light: dark photoperiod. Six and twelve days after the start of the experiment, one slide was removed from each Petri dish. Using a microscope, the total lengths of 30–40 randomly selected gametophytes were measured and the surviving gametophytes were counted. When the surviving gametophytes numbered less than 30–40, all live gametophytes were measured. On day 12, the body lengths of male and female gametophytes were measured separately. Discoloured gametophytes or those that had fallen off the slide were considered dead. The rate of gametophyte survival was calculated as:

where, Sr, Ns and Nno denote the survival rate and the densities of gametophytes after 12 days on slides with or without sediment particles, respectively.
The influence of sediment quantity and particle size on the growth of E. bicyclis gametophytes was analysed using ANCOVA. The terms of the model were gametophyte sex (categorical factor), quantity and size of sediment particles (covariates) and their interactions.
Additional Information
How to cite this article: Watanabe, H. et al. Effects of sediment influx on the settlement and survival of canopy-forming macrophytes. Sci. Rep. 6, 18677; doi: 10.1038/srep18677 (2016).
References
Ayling, A. M. The role of biological disturbance in temperate subtidal encrusting communities. Ecology 62, 830–847 (1981), doi: 10.2307/1937749.
Dayton, P. K. et al. Patch dynamics and stability of some California kelp communities. Ecol. Monographs 54, 253–289 (1984).
Jackson, G. A. Nutrients and production of giant kelp, Macrocystis pyrifera, off southern California. Limnol. Oceanogr. 22, 979–995 (1977).
Arakawa, H., Ido, M., Arimoto, M. & Agatsuma, Y. Combined effects of high water temperature and low flow velocity on survival of brown algae Eisenia bicyclis and Ecklonia cava. Can. J. Plant Protection 1, 125–128 (2013).
North, W. J. editor. The biology of giant kelp beds (Macrocystis) in California. Beih. Nova Hedwigia 32, 1–600 (1971).
Hurd, C. L., Harrison, P. J. & Druehl, L. D. Effect of seawater velocity on inorganic nitrogen uptake by morphologically distinct forms of Macrocystis integrifolia from wave-sheltered and exposed sites. Mar. Biol. 126, 205–214 (1996).
Lawrence, J. M. On the relationships between marine plants and sea urchins. Oceanogr. Mar. Biol. Ann. Rev. 13, 213–286 (1975).
Nelson, B. V. & Vance, R. R. Diel foraging patterns of the sea urchin Centrostephanus coronatus as a predator avoidance strategy. Mar. Biol. 51, 251–258 (1979).
Tegner, M. J. & Dayton, P. K. Population structure, recruitment and mortality of two sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in a kelp forest. Mar. Ecol. Prog. Ser. 5, 255–268 (1981).
McNaughton, S. J. Stability and diversity of ecological communities. Nature 274, 251–253 (1978).
Branch, G. M., Eekhout, S. & Bosman, A. L. Short-term effects of the 1988 Orange River floods on the intertidal rocky-shore communities of the open coast. Transact. Royal Society Africa 47, 331–354 (1990), doi: 10.1080/00359199009520246.
Gillanders, B. M. & Kingsford, M. J. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology, an Annual review 40, 233–309 (2002).
Kendrick, G. A. Recruitment of coralline crusts and filamentous turf algae in the Galapagos archipelago: effect of simulated scour, erosion and accretion. J. Exp. Mar. Biol. Ecol. 147, 47–63 (1991).
Storlazzi, C. D. & Field, M. E. Sediment distribution and transport along a rocky, embayed coast: Monterey Peninsula and Carmel Bay, California. Mar. Geol. 170, 289–316 (2000).
Moring, J. R. Short-term changes in tide pools following two hurricanes. Hydrobiologia 328, 155–160 (1996).
MacDonald, L. H., Anderson, D. M. & Dietrich, W. E. Paradise threatened: land use and erosion on St. John, US Virgin Islands. Environ. Manage. 21, 851–863 (1997).
Turner, S. J. et al. Changes in epifaunal assemblages in response to marina operations and boating activities. Mar. Environ. Res. 43, 181–199 (1997).
Eagle, R. A. et al. The field assessment of effects of dumping wastes at sea: 5. The disposal of solid wastes off the north-east coast of England. Lowestoft: MAFF Fisheries Research Technical Report, Report 51 (1997).
Longstaff, B. J. & Dennison, W. C. Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aqua. Bot. 65, 105–121 (1999), doi: 10.1016/S0304-3770(99)00035-2.
Thibaut, T., Pinedo, S., Torras, X. & Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes coast (France, North-western Mediterranean). Mar. Pollut. Bull. 50, 1472–1489 (2005), doi: 10.1016/j.marpolbul.2005.06.014.
Vogt, H. & Schramm, W. Conspicuous decline of Fucus in Kiel Bay (Western Balti): what are the causes? Mar. Ecol. Prog. Ser. 69, 189–194 (1991).
Devinny, J. S. & Volse, L. A. Effects of Sediments on the Development of Macrocystis Pyrifera Gametophytes. Mar. Biol. 48, 343–348 (1978), doi: 10.1007/BF00391638.
Airoldi, L. & Virgilio, M. Responses of turf-forming algae to spatial variations in the deposition of sediments. Mar. Ecol. Prog. Ser. 165, 271–282 (1998).
Gorgula, S. K. & Connell, S. D. Expansive covers of turf-forming algae on human-dominated coast: the relative effects of increasing nutrient and sediment loads. Mar. Biol. 145, 613–619 (2004), doi: 10.1007/s00227-004-1335-5.
Eriksson, B. K. & Johansson, G. Effects of sedimentation on macroalgae: species-specific responses are related to reproductive traits. Oecologia 143, 438–448 (2005), doi: 10.1007/s00442-004-1810-1.
Suzuki, Y., Maruyama, T., Takami, T. & Miura, A. Inhibition effects of suspended and accumulated particles on adhesion and development of Undaria pinnatifida zoospores. J. Jpn. Soc. Wat. Env. 21, 670–675 (1998).
Arakawa, H. & Matsuike, K. Influence on sedimentation velocity of brown algae zoospores and their base-plate insertion exerted by suspended matters (in Japanese with English abstract). Nip. Sui. Gak. 56, 1741–1748 (1990).
Arakawa, H. & Matsuike, K. Influence on insertion of zoospores, germination, survival and maturation of gametophytes of brown algae exerted by sediments (in Japanese with English abstract). Nip. Sui. Gak. 58, 619–625 (1992).
Arakawa, H. Lethal effects caused by suspended particles and sediment load on zoospores and gametophytes of the brown alga Eisenia bicyclis. Fish. Sci. 71, 133–140 (2005), doi: 10.1111/j.1444-2906.2005.00940.x.
Arakawa, H. et al. Influence of volcanic ash sediments on spore adhesion of the red alga Gelidium elegans from the coastal seabed around Miyakejima Island, Japan. Fish. Sci. 78, 1083–1090 (2012), doi: 10.1007/s12562-012-0521-5.
Airoldi, L. The effects of sedimentation on rocky coast assemblages. Oceanogr. Mar. Biol. 41, 161–236 (2003).
Airoldi, L. Roles of disturbance, sediment stress and substratum retention on spatial dominance in algal turf. Ecology 79, 2759–2770 (1998).
Airoldi, L. Effects of disturbance, life histories and overgrowth on coexistence of algal crusts and turf. Ecology 81, 798–814 (2000).
Arakawa, H., Arai, Y., Seto, M. & Morinaga, T. Influences on survival of brown algal zoospore exerted by drifting particles. Fisheries Sci. 68, Supplement II, 1893–1894 (2002).
Dayton, P. K. Experimental evaluation of ecological dominance in a rocky intertidal algal community. Ecological Monographs 45, 137–159 (1975).
Norton, T. A. The factors influencing the distribution of Saccorhiza polyschides in the region of Lough Ine. J. Mar. Biol. Ass. UK 58, 527–536 (1978).
Deysher, L. E. & Dean, T. A. In situ recruitment of sporophytes of the giant kelp Macrocystis pyrifera (L.) C.A. Agardh: effects of physical factors. J. Exp. Mar. Biol. Ecol. 103, 41–63 (1986).
Reed, D. C., Laur, D. R. & Ebeling, A. W. Variation in algal dispersal and recruitment: the importance of episodic events. Ecol. Monog. 58, 321–335 (1988).
Renaud, P. E. et al. Multi-level effects of severe storms on an offshore temperate reef system: benthic sediments, macroalgae and implications for fisheries. Mar. Ecol. 17, 383–398 (1996).
Steneck, R. S., Macintyre, I. G. & Reid, R. P. A unique algal ridge system in the Exuma Cays, Bahamas. Coral Reefs 16, 29–37 (1997).
Suzuki, Y., Maruyama, T., Miura, A. & Shin, J. Effects of suspended or accumulated kaolinite particles on adhesion and germination of Porphyra yezoensis conchospores. Proc. Jap. Soc. Civ. Eng. 559, 73–79 (1997).
Suzuki, Y., Maruyama, T. & Miura, A. Effect of suspended matters on the adhesion of Porphyra yezoensis conchospores. Proc. Jap. Soc. Civ. Eng. 580, 19–26 (1997).
Schiel, D. R., Wood, S. A., Dunmore, R. A. & Taylor, D. I. Sediment on rocky intertidal reefs: Effects on early post-settlement stages of habitat-forming seaweeds. J. Exp. Mar. Biol. Ecol. 331, 158–172 (2006).
Balata, D., Piazzi, L. & Cinelli, F. Increase of sedimentation in a subtidal system: Effects on the structure and diversity of macroalgal assemblages. J. Exp. Mar. Biol. Ecol. 351, 73–82 (2007).
Chapman, A. S. & Fletcher, R. L. Differential effects of sediments on survival and growth of Fucus serratus Embryos (Fucales, Phaeophyceae). J. Phycol. 38, 894–903 (2002).
Park, C. S. & Hwang, E. K. An investigation of the relationship between sediment particles size and development of green algal mats (Ulva prolifera) on the intertidal flats of Muan. Korea. J. Appl. Phys. 23, 515–522 (2010), doi: 10.1007/s10811-010-9620-9.
Ueta, S. & Katada, M. Study on the culture of Gelidium sp. II. Nip. Sui. Gak. 11, 175–178 (1943).
Airoldi, L., Fabiano, M. & Cinelli, F. Sediment deposition and movement over a turf assemblage in a shallow rocky coastal area on the Ligurian Sea. Mar. Ecol. Prog. Ser. 133, 241–251 (1996).
Arakawa, H. & Morinaga, T. Influence of suspended particles on dispersion of brown algal zoospores. Nip. Sui. Gak. 60, 61–64 (1994).
Arakawa, H. et al. Physical factors involved in the Isoyake (Seaweed forest depletion) at Mio, Pacific coast of central Japan. J. Mar. Biol. Oceanogr. 3, 4, doi: org/10.4172/2324-8661.1000135.
Komatsu, T. et al. Impact of the 2011 Tsunami on seagrass and seaweed beds in Otsuchi Bay, Sanriku coast, Japan. Ceccaldi, H. J. et al. (eds.), Marine productivity: Perturbations and resilience of socio-ecosystems 43–53 (2015), Springer International Publishing, Switzerland, doi: 10.1007/978-3-319-13878-7_5.
Razjigaeva, N. G. et al. Coastal sedimentation associated with the Tohoku Tsunami of 11 March 2011 in south Kuril Islands, NW Pacific Ocean. Pure Appl. Geophys. 170, 1081–1102 (2013), doi: 10.1007/s00024-012-0478-4.
Susana, M. C., Rijstenbil, J. W. & Brown, M. T. Impacts of anthropogenic stresses on the early development stages of seaweeds. J. Aqua. Ecos. Stress Rec. 7, 317–333 (2000), doi: 10.1023/A:1009916129009.
Schroeter, S. C. et al. Detecting the ecological effects of environmental impacts: a case study of kelp forest invertebrates. Ecol. Appl. 3, 331–350 (1993).
Tatewaki, M. Formation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6, 62–66 (1966), doi: 10.2216/i0031-8884-6-1-62.1.
Acknowledgements
We thank the technical staff of the Tateyama Marine Field Station of Tokyo University of Marine Science and Technology for their cooperation during this study. Part of this research was funded by the MEXT Revitalization Project for the creation of Fisheries Research and Education Center in Sanriku.
Author information
Authors and Affiliations
Contributions
H.W. and H.A. wrote the manuscript and created the figures; M.I. conducted the gametophyte culture experiment; A.M. performed statistical analyses. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Watanabe, H., Ito, M., Matsumoto, A. et al. Effects of sediment influx on the settlement and survival of canopy-forming macrophytes. Sci Rep 6, 18677 (2016). https://doi.org/10.1038/srep18677
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18677
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.