Abstract
Interactions between injected CO2, brine and rock during CO2 sequestration in deep saline aquifers alter their natural hydro-mechanical properties, affecting the safety and efficiency of the sequestration process. This study aims to identify such interaction-induced mineralogical changes in aquifers and in particular their impact on the reservoir rock’s flow characteristics. Sandstone samples were first exposed for 1.5 years to a mixture of brine and super-critical CO2 (scCO2), then tested to determine their altered geochemical and mineralogical properties. Changes caused uniquely by CO2 were identified by comparison with samples exposed over a similar period to either plain brine or brine saturated with N2. The results show that long-term reaction with CO2 causes a significant pH drop in the saline pore fluid, clearly due to carbonic acid (as dissolved CO2) in the brine. Free H+ ions released into the pore fluid alter the mineralogical structure of the rock formation, through the dissolution of minerals such as calcite, siderite, barite and quartz. Long-term CO2 injection also creates a significant CO2 drying-out effect and crystals of salt (NaCl) precipitate in the system, further changing the pore structure. Such mineralogical alterations significantly affect the saline aquifer’s permeability, with important practical consequences for the sequestration process.
Similar content being viewed by others
Introduction
Due to their wide availability, deep saline aquifers show great promise for the sequestration of carbon dioxide (CO2) and therefore the effective mitigation of anthropogenic emission of CO2 into the atmosphere1,2. However, this sequestration brings long-term interactions between CO2, rock and pore fluid (brine), causing unpredictable hydro-mechanical behaviour in the reservoir rock and uncertain long-term stability. In particular, changes in rock permeability critically affect the safety and efficiency of the sequestration process. Unexpected increases in reservoir permeability may cause sudden leakages, harmful to the environment and to human communities; they may also strengthen gradual migration of the CO2 plume, allowing it to reach poorly characterized areas that are at even greater risk of leakage. Such considerations have made the effects on reservoir rock permeability a topic of great research interest3,4,5,6,7, but most studies have focused only on the mineralogical and geochemical consequences8,9,10, with little attention to how reservoir flow is influenced. The impact on flow in deep saline aquifers can only be appreciated by the combined investigation of geochemical and permeability changes with CO2 sequestration.
A small number of experimental studies have set out to identify the combined geochemical and permeability effects11,12, but their reach is limited. For example, Shiraki and Dunn11 conducted a hydro-chemical laboratory experiment on dolomite- and anhydrite-cemented Tensleep sandstone saturated with a mixture of CO2 and brine for nearly one week, to understand the influence on permeability. They found a reduction in permeability after one week of saturation, arising from the formation of kaolinite in pore throats and other mineral reactions such as the dissolution of dolomite and K-feldspar. Muller et al.6 performed a combined hydro-chemical experiment on Berea sandstone by flushing NaCl saturated cores with dry CO2 and found a reduction in permeability due to the precipitation of halite minerals. Experiments on calcite- and dolomite-cemented sandstone by Ross et al.12 showed a permeability increase in the reservoir formation from the dissolution of carbonate minerals; they report this increase as being due to enhanced pore space modification, associated with the dissolution of carbonate cement bonds. However, all these experiments were limited to short-term effects (2–4 weeks), revealing nothing about flow under long-term CO2 exposure. This is a serious gap in our knowledge. In the long term, carbonic acid from CO2 in brine reacts with many minerals in the formation, such as calcite, siderite, dolomite, quartz, barite, muscovite, feldspar and clay minerals3,6,7,8,12,13,14,15. These reactions dissolve rock cementation, weakening grain bonds and altering flow characteristics in ways that compromise the efficiency and safety of the whole sequestration exercise. In fact, any correct identification of alterations from injected CO2 demands a rigorous and systematic comparison, with a bench study of inert gas injected into reservoir rock under the same conditions. That comparison would eliminate the effects on rock pore structure from gas injection generally, such as pore expansion and shrinkage. However, no such comparison has been undertaken in previous combined geochemical and permeability studies.
The present investigation is driven by that demand. It is a comprehensive, combined experimental study of chemical, mineralogical and permeability alterations in saline aquifers from reactions in long-term CO2 sequestration, over a realistic duration of 1.5 years. The study aims especially to provide the parameters needed for reservoir modelling of CO2 sequestration. By allowing sufficient time for interactions to run their course, mineral reactions can emerge that are never seen in short-term saturation. The investigation will therefore be highly relevant to our understanding of actual flow properties in reservoir rock under sequestration conditions. The selected conditions for CO2 reactions, i.e. a pressure of 10 MPa and a temperature of 40 °C, represent real field behaviour with CO2 in its super-critical state. Permeability tests were included for a wide range of injection pressures (2–6 MPa) and confining pressures (10–30 MPa) to simulate field conditions accurately. This innovation enables the first-ever evaluation of the effective stress fields induced by rock mineral alterations. Care has been taken to isolate the pure CO2 reaction effects on chemical, mineralogical and flow behaviour in reservoir rock, by comparing them with alterations brought on by nitrogen (N2), which is chemically inert for the present purposes.
Methods
Sample description
Brine-saturated Hawkesbury sandstone samples were used to represent the saline aquifer formation and their possible geochemical and mineralogical alterations upon CO2 injection were investigated. The sandstone samples were collected from the potential Gosford site for carbon capture and storage (CCS) in the Sydney basin, a formation belonging to the early Triassic period. Their composition is mainly quartz, calcite and kaolinite, according to X-ray diffraction (XRD) analysis (by weight, around 60% quartz, 26% calcite, 6% kaolinite, 5% barite, 1% siderite, 1% muscovite and 1% other clay minerals such as illite and smectite). It may be considered a carbonate-cemented sandstone formation, as it contains high percentages of calcite minerals in the pore structure.
Most current field-scale CO2 sequestration projects have used saline aquifers with sandstone host rocks, because preferable saline aquifers for CO2 sequestration require adequate permeability and porosity values for successful CO2 injection and storage. Among the possible sandstone formations, carbonate-cemented sandstone formations with more than 20% carbonate minerals have more preferable characteristics for CO2 sequestration, due to their potential to trap greater amounts of CO2 through carbonate reactions7. This was the main reason for using Hawkesbury sandstone in this study, since Hawkesbury sandstone has a high percentage of calcite minerals (26%) and many favourable characteristics for CO2 storage process in terms of its hydro-mechanical and mineralogical properties. A comprehensive description of the mineralogical, chemical and geo-mechanical information of Hawkesbury sandstone is given in Table 1.
The Hawkesbury formation consists of an un-layered, white-grey coloured and medium-to fine-grained rock mass that exhibits a mostly inhomogeneous structure. Therefore, great care was taken to obtain homogeneous samples from this formation and only core specimens without visible discontinuities were selected for the experiment.
Sample preparation and reaction process
Hawkesbury sandstone blocks were collected and cored according to the ISRM standards in the Deep Earth Energy Laboratory (DEEL), at Monash University. The sample diameter was selected to be 38 mm and the cored samples were cut into 76 mm-long cylinders. The two ends of the samples were carefully ground to create smooth parallel faces and the prepared specimens were oven-dried for 24 hours under 40 °C (a low temperature was selected to avoid possible thermal cracking) before beginning the reaction process.
Three different reaction conditions were selected: pure-brine-reacted (without any gas injection), brine+CO2-reacted and brine+N2-reacted. Samples were first saturated with brine at a 20% NaCl concentration (% by weight) in desiccators under vacuum (0.2 MPa suction pressure). The samples were weighed at regular intervals during the saturation process and when full saturation was reached, 15 samples were left inside the desiccator without applying vacuum for 1.5 years, to allow time for interaction. Later, this set was used as pure brine-reacted samples for both chemical and permeability measurements. The remaining 15 samples were removed from the desiccators and kept in reaction chambers to achieve CO2- and N2-reacted conditions. Before brine-reacted samples were placed in them the reaction chambers were filled with brine of the same concentration (20%) and samples were then inserted to facilitate the CO2 and N2 reactions. Two separate chambers were used for CO2 and N2 injection and both gases were injected at 10 MPa pressure at 40 °C for 1.5 years to obtain CO2+brine- and N2+brine-reacted sandstone samples.
In the present study, a reaction period of 1.5 years was investigated, based on the time required to complete the reaction of existing major rock minerals (quartz, calcite and kaolinite) with CO2 and brine to identify the ultimate alterations of these major rock minerals upon CO2 interaction and the corresponding aquifer flow response. It is known that different rock minerals need different timescales and degrees of disequilibrium to complete their reaction with CO2 and brine16. The time required to create the equilibrium of the resulting buffer solution (due to initial dissolution of CO2 in brine) is within 1 to 2 years of interaction under reservoir conditions17. If the time required for mineral dissolution is considered, according to Knauss and Wolery18 and Davis et al.19, the initiation of the quartz reaction with CO2 and brine requires a considerable geological time, which is certainly more than 1 year and kaolinite mineral also requires a considerable geological time-frame to initiate the early reaction. Therefore, conducting short-term experiments fails to identify such reaction-creating influence. As a result, considering the time-frame available for the study, 1.5 years was selected as being a reasonable time for the CO2/brine/rock mineral interaction in this study. However, even using a 1.5-year time period, it is not possible to capture all the possible rock minerals alterations that occur with CO2 and brine interaction (e.g. precipitation of feldspar and secondary precipitation of calcite and quartz). Therefore, only the dominant reactions, such as the initial dissolution of quartz, calcite, kaolinite, barite and siderite and the salt drying-out effect were considered in this study. However, it should be noted that the reaction of some rock minerals with CO2 and brine can occur within a very short time period. For example, carbonate mineral reactions, including calcite, magnesite and siderite, may occur within 2–4 weeks of interaction with CO2 and brine6,7,8 and therefore can be captured in even short-term experiments.
Permeability tests
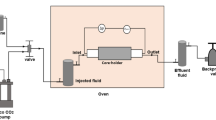
A series of high-pressure tri-axial permeability tests was conducted on the prepared variously- reacted sandstone samples (pure brine, brine+CO2, brine+N2), under undrained conditions. For the present study, three replicates were used in each test condition and permeability evaluation was performed taking its mean value (with standard deviation 1–3%). Details of the high-pressure tri-axial set-up and the sample assembly procedure can be found in Rathnaweera et al.20. The permeability tests were performed by injecting CO2 into the various prepared sandstone samples and the corresponding downstream pressure developments were recorded to find the CO2 permeability under each test condition using the pressure decay approach. Each sample was first kept inside the high-pressure cell; after assembly, the required confinement was applied under constant temperature and gas injection was initiated while recording the downstream pressure development. A high-precision syringe pump was used to inject CO2 into the sample at constant injection pressure (2–6 MPa) under the required confinement (between 10 and 30 confining pressures were considered, to simulate the reservoir depth effect).
Just before the permeability tests, the reacted sample was removed from the reaction chamber and inserted into the tri-axial cell. The brine inside the samples was removed by injecting CO2 at 1 MPa injection pressure under 10 MPa confining pressure and the corresponding flush-out brine weight was measured over time using an accurate balance. This process was performed until brine removal ceased (at which time the measured weight value becomes constant). After confirming that there was no mobile brine inside the sample (there would be brine held in place by capillary forces), the normal permeability tests were initiated for single-phase CO2 flow behaviour inside the sample. Here, the purpose of continuing the three kinds of reactions for 1.5 years was to provide sufficient time to initiate CO2/brine/rock mineral interactions and for the sample pore structure to be changed accordingly. Permeability tests on these altered samples gave an opportunity to see how the sample flow characteristics had changed following these interactions.
Permeability tests were initiated using brine-reacted samples after removing their mobile brine. CO2 was injected at 2 MPa injection pressure under 10 MPa confining pressure and the downstream pressure development was recorded. Once it became constant, the developed pressure was released by opening the downstream valve at the extremely slow rate of 0.02 MPa/s to avoid any damage to the sample’s pore structure. After this release, the downstream valve was closed and the experiment proceeded to the second stage of CO2 injection (at 3 MPa) under the same confining pressure and similar permeability tests were performed for a series of injection pressures (3, 4, 5 and 6 MPa). Once the permeability tests were completed with confining pressure set to 10 MPa, it was increased to the next level (first to 15 MPa confining pressure; then to 20, 25 and 30 MPa) and the permeability tests were conducted for the same injection pressures. All the brine-reacted, brine+CO2- and brine+N2-reacted samples were similarly tested, having first removed the mobile brine, for the same series of injection and confining pressure conditions.
Chemical and mineralogical analysis
Inductively-coupled plasma mass spectroscopy (ICP-MS) and inductively-coupled plasma atomic emission spectroscopy (ICP-AES)
ICP-MS and ICP-AES, two advanced analytical techniques for elemental determinations, were used in this study to examine the trace and ultra-trace elements21 of the brine samples taken from the reaction chambers and desiccators after the 1.5-year reaction period. The main purpose of these chemical analyses was to identify changes in pore fluid properties due to CO2/brine/rock mineral interactions. Table 2 shows the operating conditions for the ICP-MS and ICP-AES tests. A model SPQ 8000A instrument coupled with a quadrupole-type spectrometer was used for the ICP-MS tests and a model plasma atom comp MK11instrument was used for the ICP-AES tests.
Scanning electron microscopy (SEM) analysis
A detailed SEM analysis was also conducted to identify mineralogical changes in reservoir rock pore structures after three kinds of interaction. Samples reacted with brine+CO2 and brine+N2 were collected from the reaction chambers and pure-brine-reacted samples were taken from the desiccator. SEM analysis was also performed on natural samples to identify the natural condition of the rock microstructure and compare it with the saturated samples’ microstructures. A rock slice around 1 mm thick was prepared for each condition and a 3 μm titanium coating was applied before SEM testing, to avoid a charging effect during the image-scanning process. Tests were carried out under wet conditions for brine+CO2-, brine+N2- and pure-brine-reacted samples and dry conditions for natural samples. An FEI Nova Nano SEM machine coupled with two Brucker EDS and in-lens detectors was used in low-vacuum mode to capture changes in the sandstone microstructure. Furthermore, a spot size of 3.5 and a magnification of 10,000× were used to analyse the microstructure of the sandstone specimens under each reaction condition.
Results
Interacting brine, CO2 and rock produced mineralogical and geochemical alterations
Three differently-reacted pore fluid conditions brine/rock (plain brine, without gas), brine/CO2/rock and brine/N2/rock were analysed using ICP-MS, ICP-AES and SEM techniques and a plain unreacted brine sample was also tested as a control. The brine solution under each condition represented actual pore fluid in a saline aquifer. Dissolved  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  and
and  were determined using the ICP-AES method and
were determined using the ICP-AES method and  ,
,  and
and  using the ICP-MS method. Results of the desiccator and the chamber analyses are reported in Table 3. As the table shows, there is a significant pH drop in the brine+CO2-reacted solution of around 49% (7.41 to 4.81) after long-term CO2 injection, but no such drop in the brine+N2-reacted solution. The observed pH reduction is therefore believed to be from the dissolution of injected scCO2 in brine, resulting in the creation of an acidic medium: carbonic acid in the pore fluid, as shown in Eq. [1].
using the ICP-MS method. Results of the desiccator and the chamber analyses are reported in Table 3. As the table shows, there is a significant pH drop in the brine+CO2-reacted solution of around 49% (7.41 to 4.81) after long-term CO2 injection, but no such drop in the brine+N2-reacted solution. The observed pH reduction is therefore believed to be from the dissolution of injected scCO2 in brine, resulting in the creation of an acidic medium: carbonic acid in the pore fluid, as shown in Eq. [1].

As expected, this pH reduction occurred only in the brine+CO2-reacted solution, confirming the comparatively reactive nature of injected CO2. Past studies have emphasized the relevance of this CO2 dissolution in brine for the storage of injected CO2 to solubility trapping in deep saline aquifers22. However, this dissolution process is not only important for solubility trapping; it also greatly affects the mineral-trapping process, because free  ions released into the pore fluid may react with minerals in the formation, dissolving them and eventually changing the composition and structure of the reservoir rock. Since such alterations mainly occur in silicate- and carbonate-cemented grain-to-grain contacts, they are more evident in formations where these minerals predominate. Studies have shown that carbonate-cemented sandstone in deep saline reservoirs yields the required geological conditions for CO2 sequestration and more than 60% of petroleum reservoirs are carbonate reservoirs23.
ions released into the pore fluid may react with minerals in the formation, dissolving them and eventually changing the composition and structure of the reservoir rock. Since such alterations mainly occur in silicate- and carbonate-cemented grain-to-grain contacts, they are more evident in formations where these minerals predominate. Studies have shown that carbonate-cemented sandstone in deep saline reservoirs yields the required geological conditions for CO2 sequestration and more than 60% of petroleum reservoirs are carbonate reservoirs23.
ICP-AES and ICP-MS chemical analyses revealed the leachability of some minerals, when ions move from the sample into aquifer pore fluid as a result of these dissolution reactions. As mentioned previously, calcite dissolution is one of the most important dissolution reactions that occur during sequestration. Generally, the increment of  ion concentration in the pore fluid (compared to its initial stage) provides basic identifying evidence for the calcite mineral dissolution process, which can be further investigated by microstructural analysis using SEM.
ion concentration in the pore fluid (compared to its initial stage) provides basic identifying evidence for the calcite mineral dissolution process, which can be further investigated by microstructural analysis using SEM.
According to the ICP-AES analysis, significant quantities of  ions leached into the pore fluid from the samples reacted with brine+CO2, compared to either pure brine or brine+N2. The initial concentration of
ions leached into the pore fluid from the samples reacted with brine+CO2, compared to either pure brine or brine+N2. The initial concentration of  ions in pure brine was 100.1 mg/l. This increased to around 482.7 mg/l after introducing the sandstone sample into the brine, up to around 440.3 mg/l after introducing N2+sandstone and up to around 3237 mg/l after introducing CO2+sandstone. The presence of CO2-releasing
ions in pure brine was 100.1 mg/l. This increased to around 482.7 mg/l after introducing the sandstone sample into the brine, up to around 440.3 mg/l after introducing N2+sandstone and up to around 3237 mg/l after introducing CO2+sandstone. The presence of CO2-releasing  clearly accelerates calcite dissolution. SEM analysis confirmed this much greater effect, showing a significant calcite dissolution texture in brine+CO2-reacted samples compared to the other samples. Figure 1(a,b) show the mineral structure of a natural sample and Fig. 1(g–j) show the mineral structure of brine+CO2-reacted samples. The SEM image of a natural sample (Fig. 1(a,b)) shows the initial calcite mineral texture in the reservoir rock mass pore structure before significant changes caused by the CO2 interaction (Fig. 1(g–j)). The SEM images of brine+CO2-reacted samples (Fig. 1(g–j)) exhibit the dissolution textures of calcite minerals, with a relatively rough surface after CO2-brine-rock interaction compared to a smooth surface in the natural sample. According to Marbler et al.7 and Gledhill and Morse23, the dissolution of calcite in carbonate-cemented reservoir rocks significantly alters the arrangement of pores. It also helps create secondary pores by changing the effective stress and flow characteristics of the formation and the combined effect is permeability enhancement in the reservoir and caprock. Although this heightened permeability increases CO2 injectability into the reservoir and improves the aquifer’s storage capacity, the increase in reservoir permeability brings a greater risk of CO2 back-migration into the atmosphere.
clearly accelerates calcite dissolution. SEM analysis confirmed this much greater effect, showing a significant calcite dissolution texture in brine+CO2-reacted samples compared to the other samples. Figure 1(a,b) show the mineral structure of a natural sample and Fig. 1(g–j) show the mineral structure of brine+CO2-reacted samples. The SEM image of a natural sample (Fig. 1(a,b)) shows the initial calcite mineral texture in the reservoir rock mass pore structure before significant changes caused by the CO2 interaction (Fig. 1(g–j)). The SEM images of brine+CO2-reacted samples (Fig. 1(g–j)) exhibit the dissolution textures of calcite minerals, with a relatively rough surface after CO2-brine-rock interaction compared to a smooth surface in the natural sample. According to Marbler et al.7 and Gledhill and Morse23, the dissolution of calcite in carbonate-cemented reservoir rocks significantly alters the arrangement of pores. It also helps create secondary pores by changing the effective stress and flow characteristics of the formation and the combined effect is permeability enhancement in the reservoir and caprock. Although this heightened permeability increases CO2 injectability into the reservoir and improves the aquifer’s storage capacity, the increase in reservoir permeability brings a greater risk of CO2 back-migration into the atmosphere.
Moreover, the ICP-MS results for brine+CO2-saturated samples also showed some enrichment patterns in  ,
,  and
and  ions (see Table 3) compared to those in pure brine; and these patterns were not observed in samples reacted with pure brine or brine+N2. The accumulated ions in brine+CO2-reacted pore fluids are due solely to CO2. The increased
ions (see Table 3) compared to those in pure brine; and these patterns were not observed in samples reacted with pure brine or brine+N2. The accumulated ions in brine+CO2-reacted pore fluids are due solely to CO2. The increased  concentration is believed to be related to the dissolution of siderite minerals from the rock mass and the increase in
concentration is believed to be related to the dissolution of siderite minerals from the rock mass and the increase in  and
and  ions is probably from dissolution of clay minerals such as smectite, illite, kaolinite and muscovite.
ions is probably from dissolution of clay minerals such as smectite, illite, kaolinite and muscovite.
Apart from these mineral dissolutions, topographic SEM images of brine+CO2-reacted samples (Fig. 1(h)) display tiny rectangular etching caverns and pits inside the pore structure, which do not appear in the other samples. These are thought to come from barite minerals dissolved from the rock mass due to CO2 exposure, again confirming the effect of long-term injection of CO2 on reservoir mineral structure.
Significant quartz mineral corrosion was also found, in the chemical analyses.  concentration in the pore fluid was considerably increased after the introduction of CO2, most likely from dissolution of quartz in the rock mass. According to the ICP-AES analysis, the
concentration in the pore fluid was considerably increased after the introduction of CO2, most likely from dissolution of quartz in the rock mass. According to the ICP-AES analysis, the  ion concentration increased from 0.11 to 4118 mg/l with CO2 reaction and to 815.31 mg/l with N2 reaction. This quartz corrosion in the vicinity of CO2 is also confirmed by the SEM images of the brine+CO2-reacted samples (see Fig. 1(i,j)), consistent with the findings of Kaszuba et al.22, who found similar patterns in quartz minerals with CO2 introduced into brine-saturated sandstone. Marbler et al.7 provide valuable explanations of how quartz corrosion affects the structure of rock pores: the dissolution of primary and secondary silicate mineral rims around the quartz-cemented grains reduces the strength of quartz grain-grain contacts, significantly changing the rock mass pore structure. The small quartz mineral dissolution observed in brine+N2- and pure-brine-reacted samples (both negligible compared to the brine+CO2-reacted sample) is attributed to a slight corrosive effect of brine solution itself.
ion concentration increased from 0.11 to 4118 mg/l with CO2 reaction and to 815.31 mg/l with N2 reaction. This quartz corrosion in the vicinity of CO2 is also confirmed by the SEM images of the brine+CO2-reacted samples (see Fig. 1(i,j)), consistent with the findings of Kaszuba et al.22, who found similar patterns in quartz minerals with CO2 introduced into brine-saturated sandstone. Marbler et al.7 provide valuable explanations of how quartz corrosion affects the structure of rock pores: the dissolution of primary and secondary silicate mineral rims around the quartz-cemented grains reduces the strength of quartz grain-grain contacts, significantly changing the rock mass pore structure. The small quartz mineral dissolution observed in brine+N2- and pure-brine-reacted samples (both negligible compared to the brine+CO2-reacted sample) is attributed to a slight corrosive effect of brine solution itself.
Apart from mineral dissolution, chemical analyses uncovered a significant CO2 dry-out effect and NaCl crystal precipitation in the rock mass pore system in brine+CO2-reacted samples. This is consistent with the findings of Pruess and Muller24, who explained that the injection of CO2 into saline aquifers causes formation dry-out and salt precipitation near the injection well, reducing porosity, permeability and injectivity in the formation. When scCO2 is injected into a saline aquifer, water evaporates into the scCO2-phase, leaving the remaining brine with a higher salt concentration. If this concentration is greater than the solubility of salt under the prevailing pressure and temperature conditions, salt will precipitate; so salt precipitation is clearly associated an increase in salinity (Na+ concentration). According to the ICP-AES results,  in the initial brine sample increases from 191,433 to 277,863 mg/l with CO2 injection, thought to be related to salt precipitation. This was confirmed by SEM analysis, according to which the brine+CO2-reacted samples display some NaCl crystal depositions inside the rock pore structure. Visual inspection of the brine+CO2-reacted samples confirmed displacement of brine from the sample by injected CO2 and there were consequent deposits of NaCl crystals at the outer surface of the samples (see Fig. 2). Interestingly, the SEM results for samples reacted with either plain brine or brine+N2 also showed a small amount of NaCl deposition inside the pore structure (see Fig. 1(e)). According to the ICP-AES analysis,
in the initial brine sample increases from 191,433 to 277,863 mg/l with CO2 injection, thought to be related to salt precipitation. This was confirmed by SEM analysis, according to which the brine+CO2-reacted samples display some NaCl crystal depositions inside the rock pore structure. Visual inspection of the brine+CO2-reacted samples confirmed displacement of brine from the sample by injected CO2 and there were consequent deposits of NaCl crystals at the outer surface of the samples (see Fig. 2). Interestingly, the SEM results for samples reacted with either plain brine or brine+N2 also showed a small amount of NaCl deposition inside the pore structure (see Fig. 1(e)). According to the ICP-AES analysis,  concentration from 191,433 to 198,304 mg/l for plain brine samples and from 191,433 to 195,917 mg/l for brine+N2 reacted samples. The
concentration from 191,433 to 198,304 mg/l for plain brine samples and from 191,433 to 195,917 mg/l for brine+N2 reacted samples. The  concentration increase in plain-brine-reacted samples is therefore greater than in brine+N2-reacted samples and considerably less than in brine+CO2-reacted samples. In addition to
concentration increase in plain-brine-reacted samples is therefore greater than in brine+N2-reacted samples and considerably less than in brine+CO2-reacted samples. In addition to  , slight increments in
, slight increments in  and
and  content (compared to the initial brine solution) were observed in all the reaction conditions, presumably related to salt precipitation from the pore fluid. The
content (compared to the initial brine solution) were observed in all the reaction conditions, presumably related to salt precipitation from the pore fluid. The  and
and  content increments are more significant in brine+CO2-reacted samples than in all other samples (Table 3), probably due to the greater salt precipitation from the CO2 dry-out effect.
content increments are more significant in brine+CO2-reacted samples than in all other samples (Table 3), probably due to the greater salt precipitation from the CO2 dry-out effect.
According to Table 3, it is clear that significant rock mineral alterations are caused by CO2 injection, leading to changes in the chemical and mineral structure of the rock mass. To facilitate further understanding of this CO2-related dissolution process, the amounts of dissolved rock minerals were calculated as percentages of the initial compositions of each rock mineral in natural samples. Table 4 displays the calculated percentage values of each dissolved rock compound. According to Table 4%, 1.11% and 1.21% of calcite was dissolved in brine+CO2, brine+N2 and plain-brine-reacted samples respectively, compared to an initial measure of 39.73 g (26% of total rock mass). Moreover, 4.49%, 0.88% and 0.95% of quartz was dissolved in brine+CO2, brine+N2 and brine-reacted samples respectively, compared to an initial measure of 91.68 g (60% of total rock mass). As Table 4 shows, significant dissolution of barite mineral occurred in brine+CO2-reacted samples (10.79%) compared to the other two saturation conditions, with 0.16% and 0.17% of its initial barite mineral composition being dissolved in brine+N2 and brine-reacted samples respectively. The calculated dissolved mineral percentage values based on the initial composition indicate significant effects of CO2 saturation on the structure of reservoir rock minerals compared to the other two tested conditions. Table 4 also reveals that this CO2 injection-induced mineral alteration process (mineral trapping) is truly a long-term phenomenon; it dissolved only between 4% and 10% of the total mineral composition over 1.5 years of reaction in the present study. Significantly more dissolution can be expected over the large time-scales (more than 100 years) that are relevant in practice. This argument is consistent with previous research by Ranganathan et al.25.
Flow characteristics affected by rock alteration in reservoir formations
Variation of flow characteristics in reservoir formations during and after CO2 injection is one of the critical challenges for CO2 sequestration in saline aquifers. Alterations in the permeability and porosity of formations during long-term injection consequent upon interactions of minerals, brine and CO2 have been widely reported in field-scale studies13,14. The corresponding permeability of the sample under each injection condition was determined using the pressure decay approach26,27. According to Pan et al.27, pressure decay curves can be modelled as Eq. [2]:

where  is the pressure difference between the gas inlet and outlet measured by a differential pressure transducer,
is the pressure difference between the gas inlet and outlet measured by a differential pressure transducer,  is the initial pressure difference between gas inlet and outlet, t is the time and α is as given in Eq. [3]:
is the initial pressure difference between gas inlet and outlet, t is the time and α is as given in Eq. [3]:

where k is the permeability, μ is the flowing gas viscosity, β is the gas compressibility, L is the sample length,  is the sample volume and
is the sample volume and  and
and  are the volume of the gas inlet and outlet plumbing systems. The effect on CO2 permeability of pore structure modifications created by brine–CO2 interactions in the tested sandstone was investigated for five injection pressures (from 2 to 6 MPa) under five confining pressures (from 10 to 30 MPa), at 40 °C. Figure 3 shows the calculated CO2 permeability values; the CO2 injection pressure’s influence on permeability is shown in Fig. 3(a) and the confining pressure’s influence on permeability is shown in Fig. 3(b).
are the volume of the gas inlet and outlet plumbing systems. The effect on CO2 permeability of pore structure modifications created by brine–CO2 interactions in the tested sandstone was investigated for five injection pressures (from 2 to 6 MPa) under five confining pressures (from 10 to 30 MPa), at 40 °C. Figure 3 shows the calculated CO2 permeability values; the CO2 injection pressure’s influence on permeability is shown in Fig. 3(a) and the confining pressure’s influence on permeability is shown in Fig. 3(b).
As expected, sample permeability increases with increased injection pressure and reduced confining pressure, both of which are mainly related to the effective stress, which is lowered by increasing the injection pressure or by reducing the confining pressure applied to the sample2,20. For instance, increasing injection pressure from 2 to 6 MPa under 20 MPa confining pressure raises the sample permeability by around 67% and increasing the confining pressure from 10 to 30 MPa lowers it by around 43%.
The main purpose of this study, however, was to identify the effect on sample permeability of interactions between minerals, brine and CO2 in rock. Therefore, CO2 permeability values for brine+CO2-reacted samples were compared with CO2 permeability through brine+N2 and brine-only samples. Figure 4 shows the calculated CO2 permeability values under 20 MPa confining pressure for each saturation condition. According to the figure, rock minerals-brine-CO2 interaction has a significant effect on CO2 permeability in the reservoir rock samples and CO2 permeability through brine+CO2-reacted samples is clearly higher than through brine+N2 and brine-only samples. For example, at 4 MPa injection pressure and 20 MPa confining pressure, CO2 permeability through brine+CO2-reacted samples is around 17% and 19% higher than through brine-only and brine+N2 samples, respectively. The permeability enhancement in the brine+CO2 samples clearly reveals the effect of injected CO2/brine/rock interactions on the flow characteristics of deep saline aquifers and its influence on rock mineralogical alterations.
Permeability was found to be essentially the same through the brine+N2-reacted samples and the plain-brine-reacted samples, confirming the negligible influence of N2 saturation on sample flow characteristics. This is because, since non-reactive N2 causes no mineralogical or chemical reaction in the sample during saturation, its pore structure is not noticeably altered, even over 1.5 years. This is further confirmed by the results of the SEM and ICP analyses. Sandstone permeability enhancement with CO2 injection must therefore be seen as related to the reactions between rock minerals and carbonic acid produced by the interaction of CO2 and brine. This conclusion is reinforced by the results of ICP-AES analysis, where the collected chamber pore fluid showed significant silicate and calcite mineral dissolution from the sandstone sample and also by the SEM images, which clearly exhibit alterations in rock cement from CO2 reactions. The dissolution of pore-filling calcite and calcite coatings of detrital minerals changes the pore structure and consequently the porosity of the reservoir rock, eventually creating new pathways for CO2 migration and enhancing the permeability characteristics of the reservoir formation.
Weakening of the rock mass mineral grains with CO2 injection-induced chemical and mineralogical changes also affects the effective stress patterns acting on the pore space system. It was therefore important to assess the impact of the effective stress field on permeability during brine/rock/CO2 interaction and the variation of the effective stress coefficient for permeability under various conditions. The effective stress coefficient for the variation of permeability can be obtained from iso-permeability lines drawn as a function of pore pressure and confining pressure and the slope of each curve gives the effective stress coefficient28. Figure 5 shows the iso-permeability lines for the brine+CO2 and brine-only samples, which give respectively 3.5 and 0.95 effective stress coefficients. The introduction of CO2 into brine-saturated rock samples has therefore significantly raised the effective stress coefficient. Such observations indicate the importance of precisely understanding the possible consequences of rock hydro-mechanical changes as a result of mineralogical changes during the CO2 injection process for any CO2 sequestration field project.
Discussion
Although the laboratory experimental results provide crucial evidence related to mineralogical rock alterations in deep saline aquifers, modelling of reservoir simulations, coupled with both geochemical and geophysical behaviours, are also important to understand the reaction mechanisms of rock minerals upon exposure to CO2/brine during real field time-frames due to the practical difficulties of laboratory conditions, particularly time limitations. However, the precise simulation of such mechanisms requires correct identification of potential reaction mechanisms and their behaviour over the time of CO2/brine interaction and their variation with surrounding factors (CO2 partial pressure, reservoir temperature and pH). To date, most related laboratory experiments have been conducted over short-term time durations and they have therefore failed to capture long-term reaction mechanisms.
Among the various possible reaction mechanisms, if the possible short-term mechanisms are first considered, calcite dissolution is predominant. According to the existing findings, the calcite dissolution process mainly involves three simultaneous reaction mechanisms, as given below29;



These reactions are dependent on the CO2 partial pressure, reservoir temperature and pH and the calcite dissolution rate increases with increasing CO2 partial pressure and decreasing pore fluid pH29 and varies with reservoir temperature30 and foreign ions (e.g. orthophosphate)31. The short-term experimental study conducted by Wigand et al.17 found an enhancement of calcite dissolution over time (after five days of CO2 interaction) with associated reduction of pH and the reduction of the calcite reaction rate after a certain time (two months) with the initiated pH increment. In this present study it was not possible to calculate the calcite dissolution rate over time. However, the long-term interaction of CO2/brine/ rock caused a significant calcite dissolution, as evidenced by the observed 100.1 mg/l to 3237 mg/l Ca2+ concentration increment in the pore fluid. Since CO2 geological sequestration is conducted over long time-scales in the field, according to this study, there is a greater possibility of enhancing the calcite dissolution rate with the CO2/brine interaction.
In relation to possible long-term reactions, the quartz mineral reaction dominates the mineral trapping process of CO2 in deep saline aquifers, as the reservoir rocks are generally abundant in quartz minerals. The general reaction mechanism of quartz is given bellow:

According to existing studies, the quartz dissolution rate reduces with increasing pore fluid pH and increases with the presence of cations in the pore fluid (e.g. sodium, calcium and magnesium)18,32,33. However, since this quartz dissolution process takes extensive time to initiate, this cannot be identified in short-term laboratory experiments. For example, the short-term experimental work conducted by Wigand et al.17 did not find any quartz dissolution in their experiments. However, the long-term experiments conducted in the present study revealed that the interaction of quartz with CO2 and brine causes significant quartz dissolution, as evidenced by the observed 0.11 to 4118 mg/l  ion concentration in the pore fluid after 1.5 years of interaction.
ion concentration in the pore fluid after 1.5 years of interaction.
Apart from the quartz dissolution, kaolinite dissolution is also a dominant long-term reaction mechanism that occur during CO2 sequestration in saline aquifers, which mainly affects the reservoir rock mass pore structure by altering grain-to-grain contacts. The basic kaolinite dissolution reaction mechanism in acidic systems is given below:

According to the research, the rate of kaolinite dissolution increases with increasing reservoir temperature and pH of the aquifer pore fluid34 and varies with the available  and
and  complexes34. According to the study conducted by Gunter et al.35, the final influence of CO2/brine interaction on this kaolinite reaction is hard to determine, as the dissolution of Ca− feldspar can re-precipitate kaolinite minerals during the sequestration process. However, 1.5 years of long-term interaction of kaolinite /brine/CO2 in this study clearly caused a significant dissolution of kaolinite mineral, as evidenced by the observed increase of
complexes34. According to the study conducted by Gunter et al.35, the final influence of CO2/brine interaction on this kaolinite reaction is hard to determine, as the dissolution of Ca− feldspar can re-precipitate kaolinite minerals during the sequestration process. However, 1.5 years of long-term interaction of kaolinite /brine/CO2 in this study clearly caused a significant dissolution of kaolinite mineral, as evidenced by the observed increase of  ion concentration in the pore fluid after 1.5 years of interaction. This may have been influenced by the fact that the sandstone samples in this study do not contain feldspar minerals.
ion concentration in the pore fluid after 1.5 years of interaction. This may have been influenced by the fact that the sandstone samples in this study do not contain feldspar minerals.
In general, the incorporation of laboratory data with field simulations has become one of the major challenges in predicting the long-term fate of mineral reactivity in reservoirs. This is because, reservoir rock mineral dissolution and precipitation may alter the reaction mechanisms over time, which progressively modifies the existing flow pathways for CO2 movement. Therefore, it is important to consider these processes, including the coupling between reaction kinetics and mass transport processes when modelling reservoir-scale simulations. Long-term laboratory experiments therefore offer more promising data for the numerical simulation of CO2 sequestration.
The effective implementation of CO2 sequestration in deep saline aquifers requires significant development in the scientific understanding of mineral reaction-induced reservoir flow property alterations and the corresponding influence on the safety of the process in terms of the effect on caprock integrity and the corresponding possibility of CO2 leakage. According to the findings of this study, the significant rock mass mineralogical and pore structures alterations caused by around 1.5 years of long-term CO2 interaction with the reservoir may cause its permeability to be enhanced by around 10%. According to Rochelle et al.36, even such a small alteration in reservoir rock permeability may have a significant effect on the effectiveness of the sequestration process. According to the field-scale observations of Arsyad et al.37, CO2 sequestration created permeability enhancements in Ainoura and Berea sandstone formations, leading to easy movement for CO2 plumes to migrate into upper cap rock layers, generating high risk of CO2 leakage from the aquifer to surrounding groundwater zones. According to these researchers, enhanced reservoir permeability during the CO2 sequestration process in saline aquifers may cause pore pressure enhancement in the aquifer and consequently affect the caprock, creating a negative impact on caprock stability. This is because, if pressure reaches the overburden pressure of the caprock, it may fail, creating hydraulic fractures that will cause CO2 leakage into the surrounding aquifers and the atmosphere.
Apart from this, the moving CO2 from the aquifer to caprock start to dissolute rock minerals in the caprock17. Generally, in deep saline sequestration, mudstone is widely found as a caprock sealing38 and contains considerable amounts of clay minerals, quartz and feldspar39. Therefore, the presence of these rock minerals has the potential to cause reactions with the dissolved CO2 in brine, creating major changes in the caprock structure. Rutqvist and Tasang39 confirmed the possibility of reacting these caprock minerals with dissolved CO2 in brine and showed that supercritical CO2 can react with the organic contents of the caprock including clay minerals and cause considerable changes in the permeability and porosity of the caprock. Moreover, Rochelle et al.40 also stated that dissolved CO2 in brine can react with the overlying caprock, thus reducing the caprock’s sealing properties. Such caprock mineral alterations lead to the formation of new flow pathways along the caprock, creating a leakage risk from the aquifer to surrounding fresh water aquifers and ultimately back-migration into the atmosphere. This is a serious issue and negatively affects the potential implementation of CO2 sequestration in deep saline aquifers.
Conclusions
CO2 injection into a deep saline aquifer during the sequestration process causes its hydro-mechanical properties to be significantly altered and the purpose of this study is to identify how CO2 sequestration affects the mineralogical structure and alters the aquifer’s flow response. According to the results of permeability tests on brine-saturated sandstone samples obtained from the Sydney basin, the following conclusions can be drawn:
-
➢ Long-term CO2 reaction causes a carbonic acid to form in the aquifer, which causes a significant pH drop in the pore fluid, the observed drop in this study being around 49% after a 1.5 years.
-
➢ Importantly, a huge free
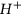 ions release occurs during this acid formation process, which has a significant influence on the aquifer’s mineralogical structure. The 1.5 years of CO2 + brine reaction in this study caused a significant dissolution of some rock minerals, including
ions release occurs during this acid formation process, which has a significant influence on the aquifer’s mineralogical structure. The 1.5 years of CO2 + brine reaction in this study caused a significant dissolution of some rock minerals, including 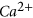 ,
,  ,
, 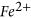 , quartz and barite minerals. Of the,
, quartz and barite minerals. Of the, 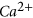 and quartz dissolutions were prominent and caused the pore fluid
and quartz dissolutions were prominent and caused the pore fluid 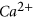 concentration to increase from 100.1 mg/l to 3237 mg/l and the pore fluid
concentration to increase from 100.1 mg/l to 3237 mg/l and the pore fluid 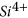 ion concentration to increase from 0.11 to 4118 mg/l.
ion concentration to increase from 0.11 to 4118 mg/l. -
➢ Long-term CO2 reaction also creates a significant CO2 drying-out effect and NaCl crystallization (salt) in the aquifer’s rock pore space by altering the pore structure. The tests showed a 191,433 to 277,863 mg/l increment in
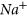 concentration and also considerable enhancements of
concentration and also considerable enhancements of  and
and 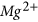 in the pore fluid after the CO2 reaction.
in the pore fluid after the CO2 reaction. -
➢ Such significant rock mass mineralogical structure alterations certainly affect the aquifer’s flow characteristics and aquifer permeability is enhanced by the long-term CO2 reaction in this study. For example, CO2 permeability at 4 MPa injection pressure and 20 MPa confining pressure increased by around 17% with CO2 saturation.
-
➢ The pore structure changes caused by the CO2 reaction also affect the effective stress response of the aquifer rock mass and a significant rise in effective stress coefficient from around 0.95 to 3.5 was observed in this study.
Additional Information
How to cite this article: Rathnaweera, T. D. et al. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 6, 19362; doi: 10.1038/srep19362 (2016).
References
Holloway, S., Rochelle, C. A. & Pearce, J. M. Geological sequestration of carbon dioxide: implications for the coal industry. T. I. Min. Metall. 108, 19–28 (1999).
Bachu, S. Sequestration of CO2 in geological media: criteria and approach for site selection in response to climate change. Energy Convers. Manage. 41, 953–970 (2000).
Moore, J., Adams, M., Allis, R., Lutz, S. & Rauzi, S. Mineralogical and geochemical consequences of the long-term presence of CO2 in natural reservoirs: an example from the Springerville-St. Johns field, Arizona and New Mexico, USA. Chem. Geol. 217, 183–186 (2005).
White, S. P. et al. Simulation of reactive transport of injected CO2 on the Colorado Plateau, Utah, USA. Chem. Geol. 217, 387–405 (2005).
Xu, T., Apps, J. & Pruess, K. Mineral sequestration of a sandstone-shale system. Chem. Geol. 217, 295–318 (2005).
Muller, N., Qi, R., Mackie, E., Pruess, K. & Blunt, M. CO2 injection impairment due to halite precipitation. Energy 112, 3507–3514 (2009).
Marbler, H., Erickson, K. P., Schmidt, M., Lempp, C. & Pollmann, H. Geomechanical and geochemical effects on sandstones caused by the reaction with supercritical CO2: an experimental approach to in situ conditions in deep geological reservoirs. Environ. Earth Sci. 69, 1981–1998 (2013).
Chou, L., Garrels, R. M. & Wollast, R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 78, 269–282 (1989).
Palandri, J. L. & Kharaka, Y. K. Ferric iron-bearing sediments as a mineral trap for CO2 sequestration: iron reduction using sulfur-bearing waste gas. Chem. Geol. 217, 351–364 (2005).
Pokrovsky, O. S., Golubev, S. V. & Schott, J. Dissolution kinetics of calcite, dolomite and magnesite at 25 8 C and 0 to 50 atm pCO2 . Chem. Geol. 217, 239–255 (2005).
Shiraki, R. & Dunn, T. L. Experimental study on water-rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl. Geochem. 15, 265–279 (2000).
Ross, G. D., Todd, A. C. & Tweedie, J. A. The effect of CO2, flooding on the permeability of reservoir rocks. Dev. Petrol. Sci. 13, 351–366 (1981).
Gaus, I. et al. Geochemical modelling and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenhouse Gas Cont. 2, 605–625 (2008).
Gilfillan, S. M. V. et al. Solubility trapping in formation water as dominant CO2 sink in natural gas fields. Nature 458, 614–618 (2009).
Gautelier, M., Oelkers, E. H. & Schott, J. An experimental study of dolomite dissolution rates as a function of pH from −0.5 to 5 and temperatures from 25 to 80 °C. Chem. Geol. 157, 13–26 (1999).
Riaz, A., Hesse, M., Tchelepi, H. A. & Orr, F. M. Onset of convection in a gravitationally unstable diffusive boundary layer in porous media. J. Fluid Mech. 111, 548–587 (2006).
Wigand, M., Carey, J. W., Schütt, H., Spangenberg, E. & Erzinger, J. Geochemical effects of CO2 sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Appl. Geochem. 23(9), 2735–2745 (2008).
Knauss, K. G. & Wolery, T. J. The dissolution kinetics of quartz as a function of pH and time at 70 °C. Geochim. Cosmochim. Ac. 52(1), 43–53 (1988).
Davis, M. C., Wesolowski, D. J., Rosenqvist, J., Brantley, S. L. & Mueller, K. T. Solubility and near-equilibrium dissolution rates of quartz in dilute NaCl solutions at 398–473 K under alkaline conditions. Geochim. Cosmochim. Ac. 75(2), 401–415 (2011).
Rathnaweera, T. D., Ranjith, P. G. & Perera, M. S. A. Effect of salinity on effective CO2 permeability in reservoir rock determined by pressure transient methods: An experimental study on Hawkesbury sandstone. Rock Mech. Rock Eng. 48, 2093–2110 (2015).
Seyfried, W. E., Janecky, D. R. & Berndt, M. E. Rocking Autoclaves For Hydrothermal Experiments, II. The Flexible Reaction-Cell System. (eds. Ulmer, G. C. & Barnes, H. L. ), 216–239 (John Wiley & Sons, 1987).
Kaszuba, J. P., Janecky, D. R. & Snow, M. G. Carbon dioxide reaction processes in a model brine aquifer at 200 °C and 200 bars: implications for geologic sequestration of carbon. Appl. Geochem. 18(7), 1065–1080 (2003).
Gledhill, D. K. & Morse, J. W. Calcite dissolution kinetics in Na-Ca-Mg-Cl brines. Geochim. Cosmochim. Ac. 70, 5802–5813 (2006).
Pruess, K. & Muller, N. Formation dry-out from CO2 injection into saline aquifers: Effects of solids precipitation and their mitigation. Water Resour. Res. 45, 3402–3412 (2009).
Ranganathan, P., Van, H. P., Rudolph, E., Susanne, J. & Zitha, P. Z. J. Numerical modeling of CO2 mineralisation during storage in deep saline aquifers. Energy 4, 4538–4545 (2011).
Brace, W. F., Walsh, J. B. & Frangos, W. T. Permeability of granite under high pressure. J. Geophys. Res. 73(6), 2225–2236 (1968).
Pan, Z., Connell, L. D. & Camilleri, M. Laboratory characterisation of coal reservoir permeability for primary and enhanced coalbed methane recovery. Int. J. Coal Geol. 82, 252–261 (2010).
Rathnaweera, T. D., Ranjith, P. G., Perera, M. S. A. & Yang, S. Q. Determination of effective stress parameters for effective CO2 permeability in deep saline aquifers: An experimental study. J. Natural Gas Sci. Eng. 24, 64–79 (2015).
Plummer, L. N., Wigley, T. M. L. & Parkhurst, D. L. The kinetics of calcite dissolution in CO2-water systems at 5 degrees to 60 degrees C and 0.0 to 1.0 atm CO2 . American J. Sci. 278(2), 179–216 (1978).
Pokrovsky, O. S., Golubev, S. V. & Jordan, G. Effect of organic and inorganic ligands on calcite and magnesite dissolution rates at 60 C and 30 atm pCO2 . Chem. Geol. 265(1), 33–43 (2008).
Berner, R. A. & Morse, J. W. Dissolution kinetics of calcium carbonate in sea water; IV, Theory of calcite dissolution. American J. Sci. 274(2), 108–134 (1974).
Sardini, P., Ledesert, B. & Touchard, G. Quantification of microscopic porous networks by image analysis and measurements of permeability in the Soultz-sous-Forêts granite (Alsace, France). Fluid Flow Transport Rocks, 24, 171–189 (1995).
Dove, P. M. & Crerar, D. A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Cosmochim. Ac. 54(4), 955–969 (1990).
Huertas, F. J., Chou, L. & Wollast, R. Mechanism of kaolinite dissolution at room temperature and pressure Part II: Kinetic study. Geochim. Cosmochim. Ac. 63(19), 3261–3275 (1999).
Gunter, W. D., Perkins, E. H. & McCann, T. J. Aquifer disposal of CO2-rich gases: reaction design for added capacity. Energy Convers. Mgmt. 34(9), 941–948 (1993).
Rochelle, C. A., Czernichowski-Lauriol, I. & Milodowski, A. E. The impact of chemical reactions on CO2 storage in geological formations: a brief review. Geological Society, London, Special Publications. 233(1), 87–106 (2004).
Arsyad, A., Mitani, Y. & Babadagli, T. Comparative assessment of potential ground uplift induced by injection of CO2 into Ainoura and Berea sandstone formations. Earth Planet. Sci. 6, 278–286 (2013).
Kharaka, Y. K. et al. Potential environmental issues of CO2 storage in deep saline aquifers: geochemical results from the Frio-I Brine Pilot test, Texas, USA. Appl. Geochem. 24(6), 1106–1112 (2009).
Rutqvist, J. & Tsang, C. F. A. study of caprock hydromechanical changes associated with CO2-injection into a brine formation. Enviro. Geol. 42, 296–305 (2002).
Rochelle, C., Pearce, J. & Holloway, S. The under-ground sequestration of carbon dioxide: containment by chemical reactions in the deep geosphere. In: Metcalfe, R., Rochelle, C. (Eds.), Chemical Containment of Waste in the Geosphere, special publication, The Geological Society of London. 157, 117–129 (1999).
Acknowledgements
This research was supported under Australian Research Council’s Discovery Projects funding scheme (project number DP120103003). The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Saudi Arabia, for funding the work through the international research group project IRG14-36.
Author information
Authors and Affiliations
Contributions
The studies were designed and analysed by all authors. R.P.G. planned the experiments and wrote the introduction section. T.D.R. carried out the chemical and microstructural analyses and wrote the chemical and microstructural part of the paper. M.S.A.P. provided permeability measurements and wrote the flow behaviour section.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rathnaweera, T., Ranjith, P. & Perera, M. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci Rep 6, 19362 (2016). https://doi.org/10.1038/srep19362
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19362
This article is cited by
-
The permeability of shale exposed to supercritical carbon dioxide
Scientific Reports (2023)
-
The effect of different synthetic methods of silica-based matrices compounds on the CO2 sequestration
Reaction Kinetics, Mechanisms and Catalysis (2023)
-
Influence of Brine–Rock Parameters on Rock Physical Changes During CO2 Sequestration in Saline Aquifer
Arabian Journal for Science and Engineering (2022)
-
Variation of failure properties, creep response and ultrasonic velocities of sandstone upon injecting CO2-enriched brine
Geomechanics and Geophysics for Geo-Energy and Geo-Resources (2021)
-
Temperature Dependence of the Permeability of Sandstone Under Loading and Unloading Conditions of Confining Pressure
Mathematical Geosciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.






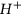 ions release occurs during this acid formation process, which has a significant influence on the aquifer’s mineralogical structure. The 1.5 years of CO2 + brine reaction in this study caused a significant dissolution of some rock minerals, including
ions release occurs during this acid formation process, which has a significant influence on the aquifer’s mineralogical structure. The 1.5 years of CO2 + brine reaction in this study caused a significant dissolution of some rock minerals, including 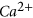 ,
,  ,
, 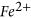 , quartz and barite minerals. Of the,
, quartz and barite minerals. Of the, 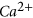 and quartz dissolutions were prominent and caused the pore fluid
and quartz dissolutions were prominent and caused the pore fluid 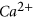 concentration to increase from 100.1 mg/l to 3237 mg/l and the pore fluid
concentration to increase from 100.1 mg/l to 3237 mg/l and the pore fluid 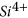 ion concentration to increase from 0.11 to 4118 mg/l.
ion concentration to increase from 0.11 to 4118 mg/l.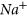 concentration and also considerable enhancements of
concentration and also considerable enhancements of  and
and 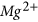 in the pore fluid after the CO2 reaction.
in the pore fluid after the CO2 reaction.