Abstract
The lung is an important organ for air breathing in tetrapods and originated well before the terrestrialization of vertebrates. Therefore, to better understand lung evolution, we investigated lung development in the extant basal actinopterygian fish Senegal bichir (Polypterus senegalus). First, we histologically confirmed that lung development in this species is very similar to that of tetrapods. We also found that the mesenchymal expression patterns of three genes that are known to play important roles in early lung development in tetrapods (Fgf10, Tbx4 and Tbx5) were quite similar to those of tetrapods. Moreover, we found a Tbx4 core lung mesenchyme-specific enhancer (C-LME) in the genomes of bichir and coelacanth (Latimeria chalumnae) and experimentally confirmed that these were functional in tetrapods. These findings provide the first molecular evidence that the developmental program for lung was already established in the common ancestor of actinopterygians and sarcopterygians.
Similar content being viewed by others
Introduction
Tetrapods use lungs for respiration, which deliver oxygen from the air into the body and remove carbon dioxide from the body. Lung development has been well studied in tetrapods1, where it has been shown that signals from the lateral plate mesoderm first stimulate the foregut endoderm to form a primary lung bud from the ventral part. This lung bud then elongates and branches into two buds to form the lobes of the lungs. Lungs are not only found in tetrapods but also in the sarcopterygian lungfish, which also use these for respiration2. Moreover, extant coelacanths (Latimeria), which belong to the sister group of lungfish and tetrapods, possess a tubular “fat-filled organ” arising from the ventral part of the esophagus. This organ is believed as a “gas bladder” that is used to control buoyancy3. It is thought that fossil coelacanths possessed lungs and therefore, it was long believed that coelacanths lost or transformed these lungs into this fat-filled organ4. However, it has recently been shown that at the embryo stage, coelacanths possess a single lung bud in addition to this fat-filled organ5. Furthermore, it has been found that a Tbx4 lung mesenchyme-specific enhancer (LME), which was first identified in the mouse (Mus musculus) genome and regulates the expression of the Tbx4 gene in the lung mesenchyme from early development6,7, is well-conserved in the coelacanth (Latimeria chalumnae) genome8,9. Taken together, these findings suggest that coelacanths possess the homologous lung similar to other members of the sarcopterygian crown group and that the Tbx4 lung enhancer (and lungs) may be present in the last common ancestor of crown sarcopterygians.
The lung developments of lobe-finned fish (lungfish and coelacanths) are ideal candidates for understanding vertebrate lung evolution. However, it is difficult to obtain embryos from these species and the whole genome sequence of the lungfish is currently unavailable due to its extremely large size10, which makes it difficult to read the sequence. Consequently, polypteriformes (bichirs and reedfish) may be better candidates for investigating the evolution of the vertebrate lung. Because the family Polypteridae (Actinopterygii) also possess lungs (gas-filled organs on the ventral side)2,11,12,13 for air breathing, it is believed that the common ancestor of sarcopterygians and actinopterygians already possessed lungs2,3,12,14,15. Polypterids are now recognized as the earliest diverging lineage of the Actinopterygii (ray-finned fish)16,17,18 and display many primitive characters that are not found in other living actinopterygians19,20,21,22,23. A histological analysis of lung development in Senegal bichir (Polypterus senegalus) indicated that this species has a similar developmental mechanism to tetrapods11. Although it is difficult to obtain bichir embryos, it is possible to breed these fish under laboratory conditions24,25. Moreover, several molecular biological studies using bichir embryos have been published in recent years25,26.
In this study, we investigated the lung development of P. senegalus in an attempt to further elucidate the evolution of vertebrate lungs. We histologically examined lung development in bichir and then investigated the expression patterns of four genes that are known to play an important role in early lung development in mouse and chicken (Gallus gallus), demonstrating strong similarities between the bichir and tetrapods. We also found a Tbx4 core lung mesenchyme-specific enhancer (C-LME) in the genome of P. senegalus as well as the coelacanth L. chalumnae and experimentally confirmed that these are functional in chicken (tetrapod) embryos. Thus, our findings indicate that the molecular mechanism for lung development in tetrapods is conserved in bichir and strongly suggest that lungs were already present in the common ancestor of actinopterygians and sarcopterygians.
Results
Lung development
To observe lung development in P. senegalus in more detail, we made paraffin sections of larvae at several stages. At 8 days post fertilization (dpf, Fig. 1a), the endodermal foregut was still a tubular structure that was surrounded by mesenchymal cells, which were denser in the ventral part of the foregut than in the dorsal part (Fig. 1b). At 9 dpf (Fig. 1c), a primary lung bud had arisen from the foregut tube (Fig. 1d), which closely resembled that observed during tetrapod lung development. At 13 dpf (Fig. 1e), the primary lung bud had already divided into the left and right buds (Fig. 1f,g). It is known that the lungs of Polypterus are asymmetrical13, with the right lung being longer than the left lung and caudal serial sections at 13 dpf supported this, showing that the right lung extended more posteriorly than the left lung (red arrows on Fig. 1h,i). These results demonstrate that the primary lung bud begins to develop after hatching.
Lung development in Polypterus senegalus.
Specimens at (a) 8 days post fertilization (dpf), (c) 9 dpf and (e) 13 dpf; and sections at (b) 8 dpf, (d) 9 dpf and (f–i) 13 dpf stained with hematoxylin and eosin. Red arrows indicate the right lung bud. Scale bars, 100 μm. fg, foregut; lb, lung bud; m, mesenchyme.
Gene expression patterns
To clarify the similarity in early lung development between bichir and tetrapods at the molecular level, we investigated the expression patterns of four genes (Nkx2.1, Fgf10, Tbx4 and Tbx5) that are known to play an important role in early lung development in mouse and chicken27,28,29,30,31,32 (Fig. 2a,f). We cloned the orthologs of these four genes and compared the amino acid sequences to confirm their similarities across several species (see Supplementary Fig. S1). We then used RNA probes constructed from the clones of the four genes and performed in situ hybridization to observe the expression patterns during lung development in bichir (Fig. 2b–e,g–j).
Gene expression patterns of Polypterus senegalus embryos.
Gene expression patterns of schematic images in tetrapods and of P. senegalus at (a–e) 8.5 days post fertilization (dpf) and (f–j) 12 dpf for the genes Fgf10 (b,g), Nkx2.1 (c,h), Tbx4 (d,i) and Tbx5 (e,j). Dotted lines in (b–e) indicate the foregut endoderm and lung bud. Scale bars, 100 μm. fg, foregut; lb, lung bud; m, mesenchyme.
At 8.5 dpf, no expression of Fgf10 and Nkx2.1 was observed (Fig. 2b,c); however, expression of Tbx4 and Tbx5 was detected at the mesenchyme of the developing lung bud, with Tbx4 being weakly expressed in the ventral part (Fig. 2d) and Tbx5 being strongly expressed in the mesenchyme (Fig. 2e). At 12 dpf, the expression of Fgf10 was also detected in the surrounding mesenchyme (Fig. 2g), while Nkx2.1 had very weak expression in the foregut and lung bud (Fig. 2h). At this time, Tbx4 expression was observed in the more ventral part of the mesenchyme, at the pointed tips of the left and right branched buds (Fig. 2i), whereas Tbx5 was strongly detected in the mesenchyme around the entire lung bud (Fig. 2j).
Core lung mesenchyme-specific enhancer in the P. senegalus genome
The patterns of gene expression present in P. senegalus were similar to those of tetrapods suggest that they may be driven by the same regulatory mechanism. Therefore, we investigated whether bichir has conserved regulatory elements for lung development in its genome. As the mouse lung mesenchyme-specific enhancer (LME) of Tbx4 has previously been identified in several species6,7, including coelacanths8,9, we focused on the regulatory elements of this gene.
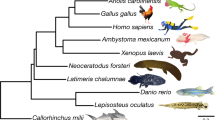
VISTA plots (Fig. 3a) showed that a conserved region corresponding to the LME was found in each of the genomes of the sarcopterygians, but not in zebrafish (Danio rerio) or medaka (Oryzias latipes), both of which are actinopterygians. However, interestingly, we found that P. senegalus also has the conserved region, despite this species also being included in the actinopterygians. The multiple sequence alignment showed that the 150-bp region was conserved in the genomes of P. senegalus and the sarcopterygians, consistent with the possession of lungs of these species (Fig. 3b). We named this conserved sequence a “core lung mesenchyme-specific enhancer” (C-LME).
The mouse lung mesenchyme-specific enhancer (LME) of the Tbx4 gene.
(a) VISTA plots comparing the mouse LME sequences of the Tbx4 gene in human (Homo sapiens), chicken (Gallus gallus), frog (Xenopus tropicalis), coelacanth (Latimeria chalumnae), bichir (Polypterus senegalus), zebrafish (Danio rerio) and medaka (Oryzias latipes) with that in mouse (Mus musculus). The green dashed box indicates the conserved region in the LME. (b) Multiple sequence alignment of the conserved region among human, mouse, chicken, frog, coelacanth and bichir. A core lung mesenchyme-specific enhancer (C-LME) of 150 bp was found in bichir as well as the sarcopterygians, as shown in orange. The predicted binding sites of the transcription factors are indicated by blue boxes.
Activity of the C-LME in chicken embryos
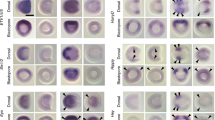
To verify whether the C-LME is functional, we tested its expression activity in chicken embryos (Fig. 4a–l). Four tandemly repeated C-LMEs of P. senegalus were cloned into the ptk-EGFP vector. The plasmids were then introduced into the prospective lung mesenchyme of chicken embryos [Hamburger–Hamilton (HH)33 stage 12]. The embryos were observed 48 h later, using Kusabira-orange to monitor the plasmid-positive area (Fig. 4b,f,j). No expression was observed in the control embryos (Fig. 4c; n = 5), but enhanced green fluorescent protein (EGFP) expression from psTbx4C-LME 4× was detected in the lung mesenchyme (Fig. 4g; n = 6), demonstrating that the C-LME of P. senegalus is functional for expressing the EGFP gene in the tetrapod.
Functional assay for the core lung mesenchyme-specific enhancer of the Tbx4 gene.
Various vectors were introduced into the right-hand side of the chicken lung mesenchyme: (a–d) the ptk-EGFP vector alone as a control, (e–h) psTbx4C-LME4× and (i–l) lcTbx4C-LME4×. pCAGGS-Kusabira-orange was co-introduced to monitor the plasmid-positive area (b,f,j). The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (a,e,i). No expression was observed in the control (c), whereas enhanced green fluorescent protein (EGFP) expression from psTbx4C-LME4× and lcTbx4C-LME4× was detected in the lung mesenchyme (g,k). The signals from Kusabira-orange were merged with the EGFP expression (d,h,l). Asterisks indicate the right lung bud. Scale bars, 200 μm.
We found C-LME sequences were highly conserved between bichir and coelacanth Latimeria chalumnae. Therefore, we also performed the same experiments using the C-LME from L. chalumnae genome (lcTbx4 C-LME 4× Fig. 4k; n = 7), which provided the same results as that of bichir.
Discussion
It is now believed that lungs were not acquired during the terrestrialization of vertebrates, but rather originated in a common ancestor of the actinopterygians and sarcopterygians2,3,12,14,15. Evidences from the fossil record and phylogenies based on morphological characteristics indicate that the last common ancestor of actinopterygians and sarcopterygians lived during the late Silurian34,35,36,37,38,39,40. Our molecular data from P. senegalus strongly supported the presence of lungs at the osteichthyan crown group node at least 423 million years ago.
Polypterids diverged from the common ancestor of the actinopterygians approximately 380 million years ago in the Devonian period16,17,18 and consequently, possess many primitive characters that are not observed in other actinopterygians19,22. One particular characteristic of this family is the presence of lungs, which developed from the ventral side of the foregut endoderm. Our histological observation of lung development in P. senegalus showed that the lungs of this species develop in a similar manner as those of tetrapods and compensated for that of Kerr11, who shows the development of this species based on the limited specimens.
Investigation of the expression patterns of four genes that are known to play an important role in early lung development in mouse and chicken showed that three of these (Fgf10, Tbx4 and Tbx5) had similar expression patterns in bichir. Tbx4 and Tbx5 are transcription factors that are involved in the induction and elongation of the tetrapod lung bud30 and were expressed in the mesenchyme of both the foregut and lung bud in bichir; Fgf signaling is known to be important for the elongation and growth of the tetrapod lung bud32,41, confirming that Fgf10 expression was observed in the mesenchyme of the elongating lung bud in bichir. However, the expression pattern of Nkx2.1 in bichir appeared to be different from that of mouse and chicken, where it is expressed during very early development27,28 and is involved in the specification of the foregut endoderm into the lungs. In contrast, no expression of this gene was detected in bichir at 8.5 dpf. Similarly in Xenopus, it appears that Nkx2.1 does not play this early developmental role, only being detectable from the start of lung bud development42. This result suggests that the timing of Nkx2.1 expression during early lung development is a specialization of amniotes with Xenopus and Polypterus both retaining the primitive osteichthyan condition. Thus, overall, the gene expression patterns during bichir lung development suggest that the molecular mechanism for lung development in bichir is similar to that of tetrapods.
To further investigate this idea, we examined whether polypterids possesses conserved regulatory elements for lung development in its genome. We found that the C-LME of the Tbx4 gene was conserved in the genomes of P. senegalus and the sarcopterygians (coelacanth and tetrapods). Furthermore, functional experiments showed that the C-LMEs of both bichir and coelacanth act as enhancers, driving the expression of EGFP in the tetrapod lung mesenchyme in our studies. Thus, these results suggest that the polypterids have regulatory mechanisms of lung development that has been conserved in the sarcopterygians. The sarcopterygii and actinopterygii diverged more than 425 million years ago36,38,39 and yet, bichir has conserved the C-LME. This suggests that there are some important regulatory elements in C-LME for lung development. The bichir C-LME sequence had 44% and 66% similarities with mouse and coelacanth sequences, respectively. Coelacanth C-LME was much more similar to that in tetrapods than that in P. senegalus, but some regions were well conserved through the sarcopterygians and bichir. We predicted that C-LME would contain several transcription factor binding sites and found well-conserved regions indicating such sites. In total, five transcription factor sites were found [HNF4, HNF3 (and related binding sites FOXF2, etc.), SOX, TCF1/LEF1 and GATA6], all of which are known to contribute to lung development in some way (blue boxes in Fig. 3b). Among these transcription factors, it has been reported that Foxf2 and Gata6 are expressed in the lung mesenchyme43,44,45,46, whereas the others are expressed in the endoderm1,47. These well-conserved sequences in C-LME indicate that transcription factor binding sites are candidate “trans-factors” that drive the expression of Tbx4 in the lung mesenchyme during lung development.
Recent studies have shown that the Tbx4 LME sequence is not present in the actinopterygians and is only found in the sarcopterygians8,9. Thus, the C-LME sequence has only been conserved in air-breathing vertebrates with lung. There are several air-breathing teleost fish, all of which except the polypterids use a gas bladder2. A comparison of the lung and gas bladder reveals that the lung developed from the ventral portion of the foregut endoderm, while the gas bladder developed from the dorsal side2,12. However, it is not quite this simple, as the gas bladder also expresses the same genes that are expressed during lung development48,49, including the Tbx4 genes (M. Okabe, unpublished data) and many fish breathe air using the gas bladder2. Therefore, it is difficult to define the lung and gas bladder based on the genes that are expressed and the air-breathing function. However, our findings indicate that it may be possible to separate these organs based on the possession of the C-LME sequence, although additional basal group sequences of Actinopterygii are required to prove this. Fortunately, the genome sequences of spotted gar (Lepisosteus oculatus) and Asian arowana (Scleropages formosus) have recently been reported50,51, both of which are air-breathing fish with a gas bladder2. Gars are the sister lineage of teleosts and diverged from them before teleost-specific whole genome duplication (three-round whole-genome duplication; 3R-WGD) occurred17,52,53,54. In contrast, arowanas belong to the Osteoglossomorpha, which are the most basal group of teleosts after the 3R-WGD17. VISTA plots showed that both species seemed to possess the Tbx4 C-LME, but multiple sequence alignment revealed that many substitutions and deletions/insertions in their C-LME were observed, although only TCF1/LEF1 and GATA6 binding sites were conserved. (see Supplementary Fig. S2a and S2b). Furthermore, the Tbx4 genomic sequences of both species were much closer to those of other teleosts than those of tetrapods and several conserved elements were found in gars and teleosts (see Supplementary Fig. S2c). These data imply that the loss of lung in actinopterygians might result from functional loss of C-LME, although the genomes of other basal actinopterygians such as sturgeons should be analyzed.
This is the first study to demonstrate that the molecular mechanism of lung development in the air-breathing fish bichir is similar to that observed in tetrapods. Our findings also indicate that the molecular mechanism of lung development using the Tbx4 lung enhancer may already have been present in the common ancestor of the actinopterygians and sarcopterygians (Fig. 5; based on Perry and Sander14; Zhu and Yu36). Our data show that bichir possesses Tbx4 C-LME, which is also present in extant sarcopterygians but has been lost in teleosts. This result indicates that unique sarcopterygian specializations are shared osteichthyan characteristics that have been lost in teleosts. Similar recent findings for gar also suggest that unique genome sequences that were present in the sarcopterygians were lost in the teleosts50,55. This finding is particularly important for improving our understanding of osteichthyan evolution.
Summary of the Tbx4 C-LME in osteichthyan evolution.
Possession of the Tbx4 core lung mesenchyme-specific enhancer (C-LME) is summarized on a simplified phylogenetic tree of osteichthyes. The blue line shows the possession of Tbx4 C-LME in sarcopterygians and actinopterygians, corresponding to the possession of lungs. Lungfish are assumed to possess the Tbx4 C-LME due to the existence in coelacanths. The blue squares indicate presumptive positions for functional loss of the Tbx4 C-LME during the evolution of actinopterygians. We indicate the point at which this loss may have occurred. The red arrow indicates the timing of 3R-WGD (teleost specific whole-genome duplication).
Further experiments with Polypterus will provide us with great insights into such ancestral osteichthyan characteristics.
Methods
Specimens
Male and female adult P. senegalus were purchased from a local pet shop (Meito Suien, Japan) and naturally mated. We maintained the fertilized eggs at 28 °C until they reached the required developmental stages25, following which we fixed them in 4% paraformaldehyde (PFA) at 4 °C for 2 h or overnight. We recorded the developmental stages in days post fertilization (dpf). All animal experiments were approved by the Animal Care and Experimentation Committee of the Jikei University School of Medicine and were performed in accordance with the approved guidelines.
Paraffin- and cryosectioning
For paraffin sectioning, we passed the fixed larvae through a series of 70%, 80%, 90% and 100% ethanol and 100% acetone. They were then embedded in paraffin. We made 4-μm serial sections using a Leica RM2235 microtome and then deparaffinised and stained them with hematoxylin and eosin.
For cryosectioning, we embedded the fixed larvae in optimal cutting temperature compound (Sakura Finetek, Japan) and made 10-μm serial sections using a Leica CM3050S cryostat.
Cloning and identification of the genes for lung development
To isolate total RNA, we harvested and homogenized 10-day-old larvae using TRIzol Reagent (Life Technologies, CA, USA). The total RNA was then reverse-transcribed using the PrimeScript II 1st strand cDNA Synthesis Kit (TaKaRa Bio, Japan). Primers for the Fgf10, Nkx2.1, Tbx4 and Tbx5 genes were designed based on the genomic sequence of P. senegalus, which had been sequenced by our consortium. We performed a polymerase chain reaction (PCR) using Q5 High-Fidelity DNA Polymerase (New England Biolabs, MA, USA) and the following primer sets: Fgf10 forward, 5′-ATGTGGAAATGGATACTGACA-3′; Fgf10 reverse, 5′-TCATGCATGGCCCAAAATG-3′; Nkx2.1 forward, 5′-ATGTCGATGAGCCCCAAGCA-3′; Nkx2.1 reverse, 5′-TCACCAGGTCCTACCATATAATAGTG-3′; Tbx4 forward, 5′-ATGCTGCAGGAAAAATCTCCAGCT-3′; Tbx4 reverse, 5′-TTATTTATCTTCCTTATACCCATCTATCC-3′; Tbx5 forward, 5′-ATGGCGGACACCGAGGAA-3′; Tbx5 reverse, 5′-TTAGCTGTTTTCTCCCCATTCCG-3′. Adenine was added to the 3′ ends of the PCR products using Blend Taq Plus (TOYOBO Life Science, Japan) and these were then cloned into the pGEM-T Easy Vector (Promega, WI, USA) and sequenced to identify the full-length sequences of Nkx2.1, Tbx4, Tbx5 and Fgf10 (DDBJ56 accession numbers LC031499, LC031500, LC031501 and LC031502, respectively).
In situ hybridization
Using the plasmids for Fgf10, Nkx2.1, Tbx4 and Tbx5, we amplified the inserted DNA using Blend Taq Plus with the M13 primers. The amplified PCR products were then used as templates to synthesize probes for the four genes using Sp6 RNA polymerase. We performed in situ hybridization on the sections as previously described57.
Identification of the lung mesenchyme-specific enhancer for the Tbx4 gene
The LME of Tbx4 was previously identified by Menke et al.6. To construct VISTA plots58 based on mouse LME, we obtained the genomic sequences for mouse, human, chicken, frog (X. tropicalis), coelacanth, zebrafish and medaka from Ensembl and used these alongside the genomic sequence of P. senegalus (DDBJ56 accession number: LC031503), which had been sequenced by our consortium. The binding sites of the transcription factors were then predicted using the rVISTA program59 with the TRANSFAC library.
Functional assay for the core lung mesenchyme-specific enhancer
The P. senegalus genome was isolated from a caudal fin clip using the DNeasy Blood & Tissue Kit (Qiagen, Germany), whereas coelacanth L. chalumnae genome was obtained from Nikaido et al.8. We amplified the conserved enhancer region using PrimeSTAR HS DNA Polymerase (TaKaRa Bio, Japan) with two sets of primers for each species: P. senegalus (ps) Tbx4C-LME-1 Fwd, 5′-ACGCGTCGACTGACAAATGAACTTCTGAGGAGAACTC-3′; psTbx4C-LME-1 Rev, 5′-GGGGTACCCTTATCACCAACGCCAGCCC-3′; psTbx4C-LME-2 Fwd, 5′-CCGCCTCGAGCTTATCACCAACGCCAGCCC-3′; psTbx4C-LME-2 Rev, 5′-ACGCGTCGACTGAAAAATGAACTACTGATGAGAACTCCT-3′; L. chalumnae (lc) Tbx4C-LME-1 Fwd, 5′-ACGCGTCGACTGAAAAATGAACTACTGAGGAGAACTC-3′; lcTbx4C-LME-1 Rev, 5′-GGGGTACCCTTATCACCCACGCTACTCTGC-3′; lcTbx4C-LME-2 Fwd, 5′-GGGGTACCTGAAAAATGAACTACTGAGGAGAACTC-3′; lcTbx4C-LME-2 Rev, 5′-CCGCCTCGAGCTTATCACCCACGCTACTCTGC-3′.
The PCR products with the first primer set were cut using SalI and KpnI, whereas those with the second primer set were cut using KpnI and XhoI. We then ligated both products into a ptk-EGFP vector to make constructs with 2× enhancers (psTbx4C-LME2× and lcTbx4C-LME2×). Each plasmid of the psTbx4C-LME2× and lcTbx4C-LME2× constructs was cut using SalI and SphI and the shorter fragment was separated with gel electrophoresis, whereas each of the psTbx4C-LME2× and lcTbx4C-LME2× plasmids was cut using XhoI and SphI and the longer fragment were separated. The shorter and longer fragments were then ligated to each other to make constructs with 4× enhancers (psTbx4C-LME4× and lcTbx4C-LME4×).
To evaluate the activity of the C-LMEs, the plasmid DNAs were introduced into the lung mesenchyme on the right-hand side of 2-day-old (HH33 stage 12) chicken embryos using a CUY21 electroporator (Tokiwa Science, Japan), as previously described27. Chicken eggs were purchased from a local supplier (Shiroyama Keien, Japan). The concentration of the plasmid DNA was adjusted to 10–15 μg/μl. The expression vector pCAGGS-Kusabira-orange (1 μg/μl) was co-introduced to monitor the electroporation efficiency. After 45–48 h in culture, we harvested the embryos and fixed them in 4% PFA at 4 °C for 2 h or overnight.
To observe the expression of the transgenes, we made cryosections and immunofluorescently stained them using 1:500 rabbit anti-GFP antibody (MBL, Japan) and 1:500 mouse anti-Kusabira antibody (MBL) as primary antibodies; and 1:500 anti-mouse IgG DyLight 549 (RockLand, PA, USA) and 1:500 anti-rabbit IgG Alexa fluor 488 (Life Technologies) as secondary antibodies. We did not detect any significant difference in the EGFP fluorescent intensity between psTbx4C-LME 2× and 4× and between lcTbx4C-LME 2× and 4× and therefore, only present the results for the 4× enhancers.
Additional Information
How to cite this article: Tatsumi, N. et al. Molecular developmental mechanism in polypterid fish provides insight into the origin of vertebrate lungs. Sci. Rep. 6, 30580; doi: 10.1038/srep30580 (2016).
References
Cardoso, W. V. & Lu, J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611–1624 (2006).
Graham, J. B. Air‐Breathing Fishes: Evolution, Diversity and Adaptation. (Academic Press, 1997).
Kardong, K. V. Vertebrates: comparative anatomy, function, evolution. 6th edn, (McGraw-Hill Higher Education, 2011).
Brito, P. M., Meunier, F. J., Clément, G. & Geffard-Kuriyama, D. The histological structure of the calcified lung of the fossil coelacanth Axelrodichthys araripensis (Actinistia: Mawsoniidae). Palaeontology 53, 1281–1290 (2010).
Cupello, C. et al. Allometric growth in the extant coelacanth lung during ontogenetic development. Nat Commun 6, 8222; 10.1038/ncomms9222 (2015).
Menke, D. B., Guenther, C. & Kingsley, D. M. Dual hindlimb control elements in the Tbx4 gene and region-specific control of bone size in vertebrate limbs. Development 135, 2543–2553 (2008).
Kumar, M. E. et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science 346, 1258810 (2014).
Nikaido, M. et al. Coelacanth genomes reveal signatures for evolutionary transition from water to land. Genome Res 23, 1740–1748 (2013).
Zhang, W. et al. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol 11, 111; 10.1186/1741-7007-11-111 (2013).
Gregory, T. R. Animal genome size database. (Date of access:20/05/2016) http://www.genomesize.com. (2016).
Kerr, J. G. The development of Polypterus senegalus Cuvier. In J. G. Kerr (ed.): Budgert Memorial. (Cambridge University Press, 1907).
Liem, K. F. Form and function of lungs: the evolution of air breathing mechanisms. Am Zool 759, 739–759 (1988).
Lechleuthner, A., Schumacher, U., Negele, R. D. & Welsch, U. Lungs of Polypterus and Erpetoichthys. J Morphol 201, 161–178 (1989).
Perry, S. F. & Sander, M. Reconstructing the evolution of the respiratory apparatus in tetrapods. Respir Physiol Neurobiol 144, 125–139 (2004).
Lauder, G. V. L. & K, L. The evolution and interrelationships of the actinopterygian fishes. Bull Mus Comp Zool 150 (1983).
Nelson, J. S., Grande, T. C. & Wilson, M. V. H. Fishes of the world. 5th edn, (John Wiley & Sons, 2016).
Near, T. J. et al. Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci USA 109, 13698–13703 (2012).
Near, T. J. et al. Boom and bust: Ancient and recent diversification in bichirs (Polypteridae: Actinopterygii), a relictual lineage of ray-finned fishes. Evolution 68, 1014–1026 (2014).
Britz, R. & Johnson, G. D. On the homologies of the posterior most gill arch in polypterids (Cladista, Actinopterygii). Zool J Linn Soc 138, 495–503 (2003).
Britz, R. Polypterus teugelsi, a new species of bichir from the upper cross river system in Cameroon (Actinopterygii: Cladistia: Polypteridae). Ichtyol Explor Freshwaters 15, 179–186 (2004).
Chiu, C. H. et al. Bichir HoxA cluster sequence reveals surprising trends in ray-finned fish genomic evolution. Genome Res 14, 11–17 (2004).
Standen, E. M., Du, T. Y. & Larsson, H. C. Developmental plasticity and the origin of tetrapods. Nature 513, 54–58 (2014).
Raincrow, J. D. et al. Hox clusters of the bichir (Actinopterygii, Polypterus senegalus) highlight unique patterns of sequence evolution in gnathostome phylogeny. Journal of experimental zoology Part B, Molecular and developmental evolution 316, 451–464 (2011).
Bartsch, J., Gemballa, S. & Piotrowski, T. The embryonic and larval development of Polypterus senegalus Cuvier, 1829: its staging with reference to external and skeletal features, behavior and locomotory habits. Acta Zool 78, 309–328 (1997).
Takeuchi, M., Okabe, M. & Aizawa, S. The genus Polypterus (bichirs): a fish group diverged at the stem of ray-finned fishes (Actinopterygii). Cold Spring Harbor protocols 2009, pdb emo117; 10.1101/pdb.emo117 (2009).
Shono, T., Kurokawa, D., Miyake, T. & Okabe, M. Acquisition of glial cells missing 2 enhancers contributes to a diversity of ionocytes in zebrafish. PLos One 6, e23746; 10.1371/journal.pone.0023746 (2011).
Sakiyama, J., Yamagishi, A. & Kuroiwa, A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development 130, 1225–1234 (2003).
Minoo, P., Su, G., Drum, H., Bringas, P. & Kimura, S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol 209, 60–71 (1999).
Sekine, K. et al. Fgf10 is essential for limb and lung formation. Nat Genet 21, 138–141 (1999).
Arora, R., Metzger, R. J. & Papaioannou, V. E. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLos Genet 8, e1002866; 10.1371/journal.pgen.1002866 (2012).
Cebra-Thomas, J. A. et al. T-box gene products are required for mesenchymal induction of epithelial branching in the embryonic mouse lung. Dev Dyn 226, 82–90 (2003).
Park, W. Y., Miranda, B., Lebeche, D., Hashimoto, G. & Cardoso, W. V. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol 201, 125–134 (1998).
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. J Morphol 88, 49–92 (1951).
Friedman, M. & Brazeau, M. D. A reappraisal of the origin and basal radiation of the Osteichthyes. J Vertebr Paleontol 30, 36–56 (2010).
Friedman, M. The early evolution of ray-finned fishes. Palaeontology 58, 213–228 (2015).
Zhu, M. & Yu, X. Stem sarcopterygians have primitive polybasal fin articulation. Biol Lett 5, 372–375 (2009).
Qu, Q., Zhu, M. & Wang, W. Scales and dermal skeletal histology of an early bony fish Psarolepis romeri and their bearing on the evolution of rhombic scales and hard tissues. PLos One 8; 10.1371/journal.pone.0061485 (2013).
Zhu, M. et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502, 188–193 (2013).
Lu, J., Giles, S., Friedman, M., den Blaauwen, J. L. & Zhu, M. The oldest actinopterygian highlights the cryptic early history of the hyperdiverse ray-finned fishes. Curr Biol 26, 1602–1608 (2016).
Choo, B., Zhu, M., Zhao, W., Jia, L. & Zhu, Y. The largest Silurian vertebrate and its palaeoecological implications. Sci Rep 4, 5242; 10.1038/srep05242 (2014).
Weaver, M., Dunn, N. R. & Hogan, B. L. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, 2695–2704 (2000).
Rankin, S. A. et al. A Molecular atlas of Xenopus respiratory system development. Dev Dyn 244, 69–85 (2015).
Shu, W., Yang, H., Zhang, L., Lu, M. M. & Morrisey, E. E. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem 276, 27488–27497 (2001).
Tebar, M., Destree, O., de Vree, W. J. & Ten Have-Opbroek, A. A. Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech Dev 109, 437–440 (2001).
Rajagopal, J. et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development 135, 1625–1634 (2008).
Keijzer, R. et al. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 128, 503–511 (2001).
Zhu, Y., Li, Y., Jun Wei, J. W. & Liu, X. The role of Sox genes in lung morphogenesis and cancer. International journal of molecular sciences 13, 15767–15783 (2012).
Cass, A. N., Servetnick, M. D. & McCune, A. R. Expression of a lung developmental cassette in the adult and developing zebrafish swimbladder. Evol Dev 15, 119–132 (2013).
Zheng, W. et al. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLos One 6, e24019; 10.1371/journal.pone.0024019 (2011).
Braasch, I. et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet 48, 427–437 (2016).
Bian, C. et al. The Asian arowana (Scleropages formosus) genome provides new insights into the evolution of an early lineage of teleosts. Sci Rep 6, 24501; 10.1038/srep24501 (2016).
Inoue, J. G., Miya, M., Tsukamoto, K. & Nishida, M. Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the “ancient fish”. Mol Phylogenet Evol 26, 110–120 (2003).
Amores, A. et al. Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714 (1998).
Taylor, J. S., Braasch, I., Frickey, T., Meyer, A. & Van de Peer, Y. Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 13, 382–390 (2003).
Qu, Q., Haitina, T., Zhu, M. & Ahlberg, P. E. New genomic and fossil data illuminate the origin of enamel. Nature 526, 108–111 (2015).
Mashima, J. et al. DNA data bank of Japan (DDBJ) progress report. Nucleic Acids Res 44, D51–D57 (2016).
Riddle, R. D., Johnson, R. L., Laufer, E. & Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416 (1993).
Frazer, K. A., Pachter, L., Poliakov, A., Rubin, E. M. & Dubchak, I. VISTA: computational tools for comparative genomics. Nucleic Acids Res 32, W273–W279 (2004).
Loots, G. G., Ovcharenko, I., Pachter, L., Dubchak, I. & Rubin, E. M. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res 12, 832–839 (2002).
Acknowledgements
Ptk-EGFP vector was a kind gift from Drs. Hisato Kondo and Masanori Uchikawa (Osaka University, Japan). We thank Mr. T. Kimura and Ms. M. Takazawa for the technical support. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas 20017024 (M.O.), Grant-in-Aid for Scientific Research (C) 15K08145 (M.O.), Grant-in-Aid for JSPS Fellows 11J09181 (K.F.) and Grant-in-Aid for Young Scientists (B) 15K21006 (K.F.). Also Research Grants in the Natural Sciences, The Mitsubishi Foundation (Mitsubishi Zaidan) (M.O.).
Author information
Authors and Affiliations
Contributions
N.T., K.F. and M.O. conceived and designed the experiments. N.T., R.K., T.Y., M.N. and K.F. performed the experiments. N.T., R.K., K.F. and M.O. analyzed the data. N.T., K.F., T.Y., M.N., K.F., N.O. and M.O. contributed reagents/materials/analysis tools. N.T., T.Y., K.F. and M.O. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tatsumi, N., Kobayashi, R., Yano, T. et al. Molecular developmental mechanism in polypterid fish provides insight into the origin of vertebrate lungs. Sci Rep 6, 30580 (2016). https://doi.org/10.1038/srep30580
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30580
This article is cited by
-
Expanded olfactory system in ray-finned fishes capable of terrestrial exploration
BMC Biology (2023)
-
Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates
Nature Ecology & Evolution (2018)
-
Evolution of Shh endoderm enhancers during morphological transition from ventral lungs to dorsal gas bladder
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.