Abstract
Approximately 20% of HER2 positive breast cancer develops disease recurrence after adjuvant trastuzumab treatment. This study aimed to develop a molecular prognostic model that can reliably stratify patients by risk of developing disease recurrence. Using miRNA microarrays, nine miRNAs that differentially expressed between the recurrent and non-recurrent patients were identified. Then, we validated the expression of these miRNAs using qRT-PCR in training set (n = 101), and generated a 2-miRNA (miR-4734 and miR-150-5p) based prognostic signature. The prognostic accuracy of this classifier was further confirmed in an internal testing set (n = 57), and an external independent testing set (n = 53). Besides, by comparing the ROC curves, we found the incorporation of this miRNA based classifier into TNM stage could improve the prognostic performance of TNM system. The results indicated the 2-miRNA based signature was a reliable prognostic biomarker for patients with HER2 positive breast cancer.
Similar content being viewed by others
Introduction
Breast cancer is the leading cause of cancer-related death in women worldwide1, and approximately a quarter of breast cancer present with HER2 gene amplification2,3,4. Trastuzumab, which targets the HER2 protein and thus inactivates the downstream signal pathway, has been demonstrated to significantly reduce the possibility of tumor relapse by 50% in patients with HER2 positive breast cancer after complete resection5,6,7. However, despite this remarkable efficacy, there are still 15–22% of patients developing tumor recurrence or metastasis after receiving adjuvant trastuzuamb, most of which are incurable and fatal. This represents a key challenge and thus a robust prognostic model that can effectively identify patients with poorer outcome is urgently required.
Limited prognostic information is provided by traditional clinical factors. Recent studies reported that higher T stage, lack of hormonal receptor (HR) expression may be useful to assess the risk of disease development for patients receiving adjuvant trastuzumab8. On the other hand, abundant tumor-infiltrating lymphocytes and the absence of lymphovascular invasion were found to be favorable, independent prognostic factors for disease free survival (DFS) in patients with HR−/HER2+ breast cancer9. Besides, in the HR+/HER2+ subgroup, the regional heterogeneity affected DFS10. Nevertheless, the robustness of those clinicpathological factors is inadequately examined. Therefore there is still a need to improve the prognostic value of the current staging system, which may be accomplished by the use of molecular biomarkers.
Several studies investigate the molecular predictors of patients with HER2 positive breast cancer. It was reported that single nucleotide polymorphism (SNP) of metastasis-associated in colon cancer-1 (MACC1) gene, a key regulator of the HGF/MET pathway, was significantly associated with clinical outcome in HER2 positive breast cancer. Increased risk for progression or death was observed in carriers of the G-allele of rs1990172 and T-allele of rs975263, respectively. While C-allele of rs3735615 showed a significant protective impact on event-free survival as well as overall survival11. In another study, the presence of HER2/HER3 heterodimers and the loss of p21 expression were also discovered to predict a significantly poorer clinical outcome in patients when submitted to adjuvant chemotherapy and trastuzumab. But these biomarkers still require validation and are not part of standard clinical practice.
miRNAs are evolutionally conserved, small (18–25 nucleotides), endogenously expressed RNAs, which has emerged as critical modulators involved in malignant activity12. Furthermore, several miRNAs have been reported to be aberrantly expressed in HER2 positive breast cancer cell lines and associated with the resistance to anti-HER2 treatment13,14,15,16,17. However, the prognostic value of miRNA expression in clinical tumor tissue is not fully tested in this specific population.
In this study, patients with HER2 positive breast cancer who had undergone radical resection and completed adjuvant chemotherapy and trastuzumab were enrolled. We performed the comprehensive miRNA analysis and generated a multi-miRNA based signature to predict DFS. Prognostic accuracy of this classifier was assessed in training set and internal testing set, and further confirmed in an independent testing set. We also compared the prognostic efficacy with traditional clinical factors.
Results
Development of the miRNA prognostic classifier
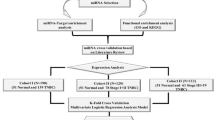
A total of 211 patients who have undergone radical surgical resection with histologically negative resection margins followed by adjuvant chemotherapy and trastuzumab were included. Table 1 showed clinicopathological characteristics of the training set (101 patients), internal testing set (57 patients), and external independent testing sets (53 patients). HR positive tumor comprised 50.5%, 65.0%, and 68% of patients in each cohort, respectively. The median follow-up time was 58.4 months (IQR 42.8–76.9), and 49 of 211 patients (23.2%) developed tumor relapse during the follow-up period.
Firstly, we compared the global and targeted miRNA expression profiling in another 14 FFPE primary breast tumor specimens, including seven non-recurrent cases (group A) and seven recurrent cases (group B). There was no statistically difference between the two groups in terms of clinical characteristics, except the DFS (Supplementary Table 1). The median DFS was 81.5 ± 37.0 and 24.2 ± 7.1 months in group A and group B, respectively. As a result, nine miRNAs that were significantly differentially expressed between the two groups were identified. (Table 2; Supplementary Figure 1)
Then, the expression of the nine miRNAs was confirmed using qRT-PCR analysis in the training set. The optimum cutoff values for these candidate miRNAs were generated by X-tile plots, which translate miRNA data from continuous variable into categorical variable (high expression or low expression). (Supplementary Figure 2A–I)
After that, we put each miRNA status (high or low expression), together with DFS data, into COX regression formula, and thus identified 2 miRNA (mir-150-5p and mir-4734) that were independently significantly associated with DFS. (Table 3) Next, a formula based on the two miRNA status and their impact on DFS (represented by theβcoefficient in COX regression model) was generated to calculate the risk score for the hazard of disease recurrence. The risk score = (1.642* status of miR-150-5p)−(1.721* status of miR-4734), where low expression status equals 0 and high expression status equals 1.
The risk score of each patient in the training set was calculated, and then took into X-tile plot. As a result, −0.1 was selected as the optimal cut-off value of risk score. (Supplementary Figure 2J) Therefore we classified those patients with risk score ≥ −0.1 as high-risk group, and those with risk score < −0.1 as low-risk group.
We further compared the prognostic predict performance of 9-miRNA model with 2-miRNA model in the training set using time-ROC curve. As a result, 2-miRNA set was significantly superior to the 9-miRNA set (p = 0.034) (Supplementary Figure 3A) Besides, the kaplan-meier curve also suggested 2-miRNA set had an advantage over 9-miRNA set. (Supplementary Figure 3B), which supporting the establishment of 2-miRNA signature.
Validation of the miRNA prognostic classifier
Patients in the low-risk group generally had better disease free survival than those in the high-risk group. There was no significant difference in the distribution of clinicopathological features between the high-risk and low-risk group in each set (Table 1). In the training set, 5-year disease-free survival was 59.0% for the high-risk group and 89.2% for the low-risk group (hazard ratio [HR] 5.35, 95% CI 2.13–13.44; p < 0.001) (Fig. 1B).
Data are AUC (95% CI) or hazard ratio (95% CI). ROC = receiver operator characteristic. AUC = area under the curve. Training cohort.(A,B) internal testing cohort. (C,D), external independent validation cohort (E,F) and combination set(G,H). We used AUCs at 5 years to assess prognostic accuracy, and calculated p values using the log-rank test.
We did the same analysis using in the internal testing cohort. Five-year disease-free survival was 45.8% for the high-risk group and 87.5% for the low-risk group (HR 3·71, 95% CI 1.08–12.74; p = 0.025). (Fig. 1D)
To confirm that 2-miRNA based classifier had consistent prognostic value in different populations, we applied it to the external independent testing set. Five-year disease-free survival rate was 38.9% for the high-risk group and 80.1% for the low-risk group (HR 3·43, 95% CI 1.35–8.69; p = 0.006) (Fig. 1F).
Univariate analysis showed that 2-miRNA signature was significantly associated with DFS in each cohort. (Supplement Table 2) After multivariable adjustment by other prognostic factors including HR status, TNM stage, tumor grade and age, the 2-miRNA based model remained a powerful and independent factor in the entire cohort of 211 cases (HR 4.63, 95% CI 2.45–8.74, p < 0.0001) (Table 4).
When stratified by clinicopathological risk factors, the 2-miRNA based classifier still showed clinically and statistically significant prognostic effect in all subgroups (Fig. 2).
Comparing the prognostic performance of miRNA classifier with other clinicopathological factors
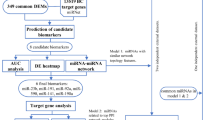
To further evaluate the prognostic performance of the miRNA signature, we assessed the prognostic accuracy of the 2-miRNA based classifier with time-dependent ROC analysis at five years, and calculated the AUC of the ROC curves for disease recurrence in all 211 patients.
The 2-miRNA signature showed an AUC of 0.693 (95% CI 0.563–0.823), 0.729 (95% CI 0.561–0.897), and 0.674 (95% CI 0.522–0.827) in the training, internal and external independent set, respectively (Fig. 2A,C,E). In the whole cohort of 211 patients, the 2-miRNA based classifier showed higher prognostic accuracy than other clinicopathological risk factor, including TNM stage, HR status, tumor grade and age (Fig. 3).
ROC = receiver operator characteristic. AUC = area under curve. HR = hormonal receptor. (A) Comparisons of the prognostic accuracy by the two-miRNA-based signature (high risk vs low risk), TNM stage (Stage1 vs Stage2 vs stage 3), pathological tumor grade (high vs low), and the miRNA signature and TNM stage combined. (B) Comparisons of the prognostic accuracy by the two-miRNA-based signature (high vs low risk), the miRNA signature and TNM stage combined, the miR-4734 alone (high vs low expression) and miR-150-5p alone (high vs low expression).
Eventually, the combination of TNM stage and 2-miRNA based classifier demonstrated the highest prognostic accuracy than any other prognostic factors or miRNA alone, with an AUC of 0.711 (95% CI 0.634–0.787). In addition, the performance of 2miRNA+TNM stage was statistically superior to TNM stage alone (AUC: 0.711 vs.0.609, p = 0.027), supporting the addition of 2miRNA to traditional TNM stage as a prognostic factor (Fig. 3).
Collectively, our results demonstrate that expression of a small set of miRNA, measured from primary breast cancer tissues at initial diagnosis, was a valid prognostic indicator and will improve the prognostic capacity of AJCC stage for the development of disease recurrence.
Discussion
In this study, we developed and confirmed, for the first time, a novel prognostic model based on 2-miRNA expression to improve the prediction of disease recurrence in patients with HER2 positive breast cancer who completed standard treatment. Our results clearly demonstrated that this classifier can successfully stratified patients into two groups by their risk of tumor recurrence, regardless of the clinical features. Furthermore, this signature predicted the five year DFS better than other clinicopathological factors, and added prognostic value to the TNM staging system.
The value of miRNA as prognostic biomarkers has been increasingly explored18,19,20. However, it have not been comprehensively studied in patients with primary HER2 positive breast cancer. Jung et al.14 showed that circulating miR-210 levels were associated with trastuzumab sensitivity, tumor presence, and lymph node metastases in patients who received neo-adjuvant trastuzumab based chemotherapy. In contrast to short-term efficacy, the present study focused on the association between miRNA and long-term benefit of trastuzumab based treatment. We accessed to large numbers of primary tumor tissues with extensive clinical follow-up in distinct study cohorts to confirm the robustness of this miRNA based signature as a useful predictor of long-term prognosis.
Our results suggested that incorporation of miRNA signature into the conventional clinical factors can provide more accurate prognostic information. The miRNA signature successfully distinguished patients with similar clinical features into distinct groups depending on their risk of tumor recurrence. Besides, combination of miRNA signature and TNM system improved the prognostic predict performance than other models including miRNA alone or TNM stage alone. Therefore, the novel miRNA classifier is valuable, in supplement of current TNM system, to define more accurate prognosis, and had the potential to improve the management of HER2 positive breast cancer patient.
Our data suggested that patients with high risk of recurrence may be inadequately treated with the currently available treatment. Presently, strategies of escalating adjuvant anti-HER2 treatment have been pursued. Neratinib significantly reduced the risk of recurrence by 33% versus placebo at first two years, in patients who have completed the chemotherapy and one year of trastuzumab21. The effect of combination of trastuzumab and pertuzumab in adjuvant setting was also examined in phase III APHINITY trial. Therefore, development of this miRNA based prognostic assay will contribute to identify patients who may benefit most from more extensive adjuvant therapies, and thus help to tailor the adjuvant anti-HER2 treatment.
The biologic function of the two miRNAs in breast cancer still needs to be established. Increased expression of mir150 was reported to correlate with poorer clinical outcome in intrahepatic cholangiocarcinoma22 and non-small cell lung cancer23. Besides, recent evidence showed that mir150 was member of a miRNA based prognostic model for primary melanoma, and was associated with CD45+ TILs in tumor tissues18. On the other hand, mir4734 is a newly identified miRNA in breast cancer by extensive next-generation sequencing analysis. It encodes within the ERBB2/Her2 gene, which is amplified in HER2 positive breast cancer and cause the clinically genomic aberration24. Our work provided new evidence revealing the association between mir4734 expression and clinical outcome of HER2 positive breast cancer, which may aid further exploration of potent biological function.
The major limitation of this work is the small sample size of the original cohort selected for microarray study. Be aware of this issue, several efforts were made to make up for the deficiency. Firstly, the original groups of patients for miRNA screening were carefully selected, matching all the clinical prognostic factors well, and making DFS being the only significantly different factor. Secondly, the preliminary result of microarray experiment was strictly validated with larger sample size using appropriate methods. Besides, a large, multicenter prospective study to assess the robustness of prognostic signature in the general HER2 positive breast cancer population is required.
In summary, the described miRNA signature represented the first step to develop a molecular prognostic assay for HER2 positive breast cancer. We believe such a model has the potential to improve the management of this specific population.
Methods
Study population
We used formalin-fixed paraffin-embedded (FFPE) tissue samples from 211 patients with stage I-III HER2 positive breast cancer. For the training and internal testing set, data were obtained from 158 patients in Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China, between June 1, 2000, and June 30, 2015. We used computer-generated random numbers to assign 101 of these patients to the training set, and 57 patients to the internal testing set. We enrolled another 53 patients, with the same criteria as above, from other 10 hospitals in China as the independent validation set.
HER2-positive was defined as 3+ on immunohistochemical [IHC] analysis or 2+ with gene amplification by fluorescence in situ hybridization [FISH]. Informed consent was obtained from all patients and approval acquired by the Institutional Review Board. Clinical information relevant to this study include date of diagnosis, date of recurrence or last follow-up or death, age, HR status, tumor grade, TNM stage for primary tumors and menopausal status. We defined disease free survival as the time from the date of surgery to the date of confirmed tumor relapse or the date of last follow-up visit for disease-free patients. We excluded patients who had no FFPE tumor sample from initial diagnosis, or insufficient RNA (less than 100 ng/μL) available. All the patients provided informed consent. The study was performed in accordance with Declaration of Helsinki and approved by ethics committee in Cancer Hospital, Chinese Academy of Medical Sciences.
Clinical specimens
All specimens were human primary breast cancer samples that were collected, formalin-fixed, and paraffin-embedded at the time of surgery. All tumors were classified according to the 2010 American Joint Committee on Cancer (AJCC) staging system
RNA extraction
All the FFPE tissues comprised at least 80% tumor cells. RNA extraction was performed using the miRNeasy FFPE Kit (Qiagen) following manufacturer’s recommendations, using the Xylene/Ethanol method for deparaffinization/rehydration.
microRNA microarray expression profiling and data preprocessing
To generate miRNA expression profiles, we selected another panel of FFPE tumor samples from 14 patients including seven relapsed disease and seven non-relapsed. The miRNA profiling was performed using Agilent miRNA array, which contained probes interrogating 2006 human mature miRNAs from miRBase R19.0. Microarray experiments were conducted according to the manufacturer’s instructions. Briefly, the miRNAs were labeled using the Agilent miRNA labeling reagent. Total RNA (100 ng) was dephosphorylated and ligated with pCp-Cy3, the labeled RNA was purified and hybridized to miRNA arrays. Images were scanned with the Agilent microarray scanner (Agilent), gridded, and analyzed using Agilent feature extraction software version 10.10. The miRNA array data were analyzed for data summarization, normalization and quality control by using the GeneSpring software V12 (Agilent). To select the differentially expressed genes, we used threshold values of 2-fold change and a Benjamini-Hochberg corrected p vlaue of 0.05. The data was Log2 transformed and median centered by genes using the Adjust Data function of CLUSTER 3.0 software then further analyzed with hierarchical clustering with average linkage. Finally, we performed tree visualization by using Java Treeview (Stanford University School of Medicine, Stanford, CA, USA).
microRNA real-time qPCR and data processing
On the basis of the miRNA microarray results, we further examined miRNA expression using qRT-PCR to analyze the 211 FFPE samples to validate the prognostic value of every candidate miRNA. One microgramme of RNA was reverse-transcribed in 25-mL reactions using the Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was conducted using the SYBR Premix Ex TaqTM II (TliRNaseH Plus) kit (TaKaRa, Japan) with the Bio-Rad (USA) machine. U6 small nuclear RNA was used as internal normalized references. Expression levels of individual miRNA were determined by −ΔCT approach (ΔCT = CT miRNA − CT U6 RNA).
Statistical analysis
We compared two groups using χ2 test for categorical variables. For survival analyses, we used the Kaplan-Meier method to analyze the correlation between variables and disease-free survival, and the log-rank test to compare survival curves. We used the Cox regression model to do the multivariable survival analysis. Statistical significance was set at 0.05. All these statistical tests were done with SPSS version 17.0 (SPSS Inc., Chicago. USA).
We selected the optimum cutoff score for the expression of every miRNA using X-tile plots (version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA) based on the association with the patients’ disease-free survival. X-tile plots provide a single and intuitive method to assess the association between variables and survival25.
We investigated the prognostic accuracy of each feature and multi-miRNA based classifier using time-dependent receiver operating characteristic (ROC) analysis26. We used the area under the curve at 5 years to measure prognostic accuracy. We used R software version 3.1.3 and the “time ROC” package to do the time-dependent ROC curve analysis.
Additional Information
How to cite this article: Du, F. et al. A miRNA-based signature predicts development of disease recurrence in HER2 positive breast cancer after adjuvant trastuzumab-based treatment. Sci. Rep. 6, 33825; doi: 10.1038/srep33825 (2016).
Change history
14 October 2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J Clin 66, 7–30 (2016).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 100, 8418–8423 (2003).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283 (2011).
Gianni, L. et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 12, 236–244 (2011).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Lee, H. J. et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res. Treat. 145, 615–623 (2014).
Lee, H. J. et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes and the Tertiary Lymphoid Structures in HER2-Positive Breast Cancer Treated With Adjuvant Trastuzumab. Am. J. Clin. Pathol. 144, 278–288 (2015).
Lee, H. J. et al. Clinicopathologic Significance of the Intratumoral Heterogeneity of HER2 Gene Amplification in HER2-Positive Breast Cancer Patients Treated With Adjuvant Trastuzumab. Am. J. Clin. Pathol. 144, 570–578 (2015).
Muendlein, A. et al. Significant survival impact of MACC1 polymorphisms in HER2 positive breast cancer patients. Eur. J. Cancer 50, 2134–2141 (2014).
Esquela-Kerscher, A. & Slack, F. J. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 (2006).
Gong, C. et al. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J. Biol. Chem. 286, 19127–19137 (2011).
Jung, E. J. et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 118, 2603–2614 (2012).
Huynh, F. C. & Jones, F. E. MicroRNA-7 inhibits multiple oncogenic pathways to suppress HER2Delta16 mediated breast tumorigenesis and reverse trastuzumab resistance. PLoS ONE 9, e114419 (2014).
Bai, W. D. et al. MiR-200c suppresses TGF-beta signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int. J. Cancer 135, 1356–1368 (2014).
Ye, X. et al. MiR-221 promotes trastuzumab-resistance and metastasis in HER2-positive breast cancers by targeting PTEN. BMB Rep 47, 268–273 (2014).
Hanniford, D. et al. A miRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin. Cancer Res. 21, 4903–4912 (2015).
Kleivi, S. K. et al. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin. Cancer Res. 21, 1207–1214 (2015).
Zhang, J. X. et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 14, 1295–1306 (2013).
Chan, A. et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2016).
Wang, S. et al. Upregulated circulating miR-150 is associated with the risk of intrahepatic cholangiocarcinoma. Oncol. Rep. 33, 819–825 (2015).
Yin, Q. W., Sun, X. F., Yang, G. T., Li, X. B., Wu, M. S. & Zhao, J. Increased expression of microRNA-150 is associated with poor prognosis in non-small cell lung cancer. Int J Clin Exp Pathol 8, 842–846 (2015).
Persson, H. et al. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res. 71, 78–86 (2011).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10, 7252–7259 (2004).
Heagerty, P. J., Lumley, T. & Pepe, M. S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56, 337–344 (2000).
Acknowledgements
The following institutions participated in this study: Fudan University Shanghai Cancer Center; Zhejiang Cancer Hospital; Guangdong General Hospital; Tongji Hospital, Tongji Medical College Huazhong University of Science and Technology; Nanfang Hospital, Southern Medical University; Sun Yat-Sen University Cancer Hospital; West China Hospital, Sichuan University; Harbin Medical University Cancer Hospital; Henan Cancer Hospital, Zhengzhou University; Peking Union Medical College Hospital. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0466), and funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3344).
Author information
Authors and Affiliations
Contributions
F.D., P.Y., Y.L., F.M., J.Y.W., Y.F., R.G.C., P.Z., Q.L., Y.S. and B.X. conceived, designed, performed the experiments, wrote the manuscript and checked the English grammars in manuscript. Z.T.Z., Z.Y., T.W. and J.D.Z. performed the experiments, wrote the manuscript and checked the English grammars in manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Du, F., Yuan, P., Zhao, Z. et al. A miRNA-based signature predicts development of disease recurrence in HER2 positive breast cancer after adjuvant trastuzumab-based treatment. Sci Rep 6, 33825 (2016). https://doi.org/10.1038/srep33825
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33825
This article is cited by
-
MiR-195-5p inhibits proliferation and invasion of nerve cells in Hirschsprung disease by targeting GFRA4
Molecular and Cellular Biochemistry (2021)
-
Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer
Cancer Chemotherapy and Pharmacology (2020)
-
Serum microRNA-21 predicted treatment outcome and survival in HER2-positive breast cancer patients receiving neoadjuvant chemotherapy combined with trastuzumab
Cancer Chemotherapy and Pharmacology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.