Abstract
The enhanced light-harvesting capacity and effective separation of photogenerated carriers in fantastic hierarchical heterostructures enjoy striking attention for potential applications in the field of solar cells and photocatalysis. A three-dimensional (3D) dandelion-shaped hierarchical Bi2S3 microsphere compactly decorated with wing-shaped few layered MoS2 lamella (D-BM) was fabricated via a facile hydrothermal self-assembly process. Especially, polyethylene glycol (PEG) has been proven as the vital template to form D-BM microsphere. Importantly, the as-prepared D-BM microsphere presents pH-dependent superior adsorption behavior and remarkable visible light photocatalytic activity for degradation of organic dyestuffs (Rhodamine B/RhB and Methylene blue/MB), far exceeding those for the pure Bi2S3 and MoS2. It is understandable that D-BM with high surface area possesses more active sites and promotes light utilization due to the unique porous structure with outspread wings. Besides, based on the experiments and theoretical calculations, the staggered type II band alignment of D-BM permits the charge injection from Bi2S3 to MoS2, subsequently accelerates the separation and restrains the recombination of carriers, leading to excellent photocatalytic activity, as well as the photoconductance and photoresponse performance (with Ilight/Idark ratio 567).
Similar content being viewed by others
Introduction
The intense demand for photocatalysis applying to the pollutants degradation and the effective solar energy conversion keeps moving due to the worsening environment and energy crisis1,2,3,4,5,6,7,8,9. It is believed that the desired photocatalysis should have high light-harvesting capability and retardative recombination of photoexcited carriers10,11,12. A promising approach to enhance the photoactivity is to increase the light absorption range, the surface area and active site of the catalysts. Numerous studies have shed light on the importance of the semiconductor-based composites with certain modification in improving the performance, such as photovoltaic conversion, catalytic, and electrochemical properties13,14,15,16. Thus, studying the hierarchical semiconductor nanocomposite with controllable morphologies becomes the frontier strategy. Among these semiconductors, mater sulfides (ZnS, CdS) have been extensively surveyed for the generation of new optoelectronic performance with effective electron-hole separation and transport5,14.
Typically, as a eco-friendly lamellar-structured semiconductor, Bi2S3, with a typical band gap (1.3–1.7 eV) for solar photovoltaic, has attracted lots of research interests17,18,19. In recent years, reported Bi2S3 nanocrystal with various morphologies have promising applications in solar cells, photodetectors, gas sensors, electrochemical hydrogen storage and X-ray computed tomography imaging due to the suitable band gap20,21,22,23. Besides, it also has been used as a stability sensitizer and photocatalyst derived from the broad absorption of visible light up to 800 nm24,25. However, the photocatalytic performance of Bi2S3 has been restricted because of the toilless recombination of photogenerated carriers and therewith the low quantum yield. Hence, as a feasible method so far to prolong the lifespan of electron-hole pairs, establishing composite structures with metal oxides, metal chalcogenides or 2-dimensional (2D) hexagonal graphene, for instance Bi2S3/Bi2WO6, Bi2S3/TiO2 and Bi2S3/Pd4S, has been paid significant attention12,26,27,28,29. In particular, the 3-dimensional (3D) porous architecture with Bi2S3 skeleton, keeping superiority in large active contact area, is considered to have potential in synergistically enhancing the photocatalytic features.
The wide researches and successful applications of graphene recently have triggered great attention on 2D-layered materials. The 2D transition metal dichalcogenides (TMD) exhibit ultrathin physical characteristic, excellent optical, electronic, mechanical properties and potential applications in field-effect transitions (FET) and sensing devices30,31,32,33,34. As a representative 2D TMD, consisted of the weakly coupled S-Mo-S atoms sandwich layers, MoS2 nanostructures have been synthesized with plentiful morphologies of nanosheet, nanoribbon and nanosphere35,36,37,38. Different from graphene, the controlled band gap (1.2–1.9 eV) and thermal stability, make MoS2 be widely applied to biosensors, memory, capacitors, logic circuit devices, and lithium batteries39,40,41. Importantly, 2D-layered MoS2 possesses a large surface area and massive active sites, which can provide sufficient contact and effective reactions42. Hence, forming hierarchical composites with MoS2 has been proven to be promising and it also opens opportunities for optoelectronic applications. Zhou et al. have successfully fabricated MoS2 nanosheet-coated TiO2 nanobelt heterostructures, which showed a high photocatalytic hydrogen production and strong photocatalytic degradation of the dye molecules31. It is believed that the formation of this MoS2-TiO2 nanocomposite forcefully retard the photogenerated electron-hole recombination. Moreover, Chen et al. have put forward a bottom-up strategy of solvothermal method for 2D MoS2 nanosheets composites, which exhibited preeminent properties in biomedicine and realized considerable applications in photothermal regression of tumor43,44.
In this work, we propose a novel heterostructure of 3D-dandelion Bi2S3@MoS2 microsphere using a facile hydrothermal method for the first time. Intriguingly, the layers of MoS2 coating likes the wring of the dandelion. This unique architecture offered a high light utilization as a result of large surface area and massive active sites for reactions. Moreover, the formation of the staggered type II band alignment in D-BM made a prolonged lifespan of electron-hole pairs, based on calculated energy band positions and the corresponding electronic structures. Obviously, evaluated by the degradation of Rhodamine B (RhB) and Methylene blue (MB) under visible light, the as synthesized heterostructure exhibits more remarkable adsorption and visible light photocatalytic properties than the pristine Bi2S3 and MoS2. Also, it presents improved photoresponse property with a high Ilight/Idark ratio of 567.
Results and Discussion
Phase structure and Raman studies
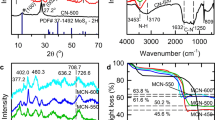
As shown in Fig. 1a, the phase information and purity of D-Bi2S3, MoS2 nanoflowers and their composites were characterized by XRD analysis. Pattern I corresponded well to the lattice planes of orthorhombic Bi2S3 (JCPDS#65-2431 a = 11.290 Å, b = 3.978 Å, and c = 11.150 Å) without some impurity as Bi2O3. And pattern II can be indexed to the standard peaks of hexagonal MoS2 (JCPDS#37-1492; a = 3.16 Å, b = 3.16 Å, and c = 12.30 Å). As for hierarchical D-BM structure (5 MBS, pattern III), the weak diffraction peak at 2θ = 14.2°, corresponding to the MoS2 lattice plane of (002), can be inferred that the few-layered MoS2 petals have been broken by D-Bi2S3. Raman spectroscopy was further utilized to identify the hybrid production. In Fig. 1b, two characteristic peaks of MoS2 can be observed at 375 and 402 cm−1 from the local amplification, which correspond to the E2g and A1g vibration modes, respectively41. The vibrational modes of pure Bi2S3 microspheres are located at 107 cm−1 and 260 cm−1, which can be assigned as the Ag and B1g modes21. Obviously, all the characteristic Raman spectral signatures of Bi2S3 and MoS2 both are presented in the result of their hybrids, demonstrating the successful incorporation of D-BM structures. Note that there is a blue shift of the B1g mode compared to the pure Bi2S3. It probably suggests the surface strain is due to the novel coated MoS2 on each Bi2S3 nanorod.
Morphology analysis
The morphology and micro-structure of all the samples have been investigated by SEM images. Figure 2a,b display the pure D-Bi2S3 microspheres in different magnifications. It can be seen that the Bi2S3 structures are distributed in the shape of irregular microspheres with the average diameter of 5 μm. Apparently (inset of Fig. 2b), these D-Bi2S3 micropheres were composed of a large number of acicular crystalline nanorods with uniform diameters of about 80 nm. Moreover, the broken D-Bi2S3 microsphere shown in Fig. 2c indicates that the acicular Bi2S3 nanorods radiate from the center and stack uniformly. It is extremely vital that the interstices between Bi2S3 nanorods can provide particular framework for the embedded of layered MoS2 petals. As presented in Fig. 2d, the pure MoS2 flowers reveal the diameter of 4 μm, with number of thinnish petals aggregated closely. The inset of Fig. 2d provides a chapped MoS2 flowers, and it illustrates that these disordered petals grown from a common center to form the spherical structure. Figure 2e exhibits the morphology of the hydrothermal synthesized D-BM (5 MBS) hetero-microspheres. Detailedly, some other SEM information on Bi2S3, MoS2, and hybrids are shown in Figs S1 and S2 (ESI). Generally, the whole D-Bi2S3 microspheres are uniformly covered with 2D MoS2 nanosheets. A high magnification top view SEM image shown in the inset of Fig. 2e, the composites present apparent differences from the pristine D-Bi2S3 or MoS2. Iconically, almost each Bi2S3 nanorod are compactly decorated with a pair of expanding wings of MoS2 nanosheets, thus forming the MoS2 coated Bi2S3 heterostructure. In the inset of Fig. 2f, a section of the broken composites elucidates that MoS2 nanosheets have grown along the Bi2S3 nanorods and deeply rooted in the center of Bi2S3 spheres, which expects that this unique hierarchical architecture can provide more effective activity sites and enhance the photoelectric properties.
(a,b) SEM images of the dandelion-shaped Bi2S3 microspheres under different magnifications. The inset shows the high magnification SEM image of vimineous Bi2S3 nanorods. (c) The broken Bi2S3 microspheres. (d) SEM image of MoS2 nanoflower and the inset is the several chapped MoS2 nanoflowers. (e,f) SEM image with different magnifications of the synthesized D-BM heterostructures. The insets are a local amplification from the side views and a broken section.
In addition, a sequence of TEM and HRTEM images of D-BM nanocomposite have been employed to reveal more specific structural information. In Fig. 3a, homogeneous MoS2 sheets are detected at the edge of each Bi2S3 nanorod from the low magnification TEM survey. Not only Fig. 3b,c clarify the intimate interfacial contact between MoS2 sheets and elongated Bi2S3 rods, but also they indicate that the MoS2 sheets are ultrathin compared with the pure MoS2 flowers. In the HRTEM image (Fig. 3e), the lattice fringes of d = 3.54 Å and d = 3.74 Å, corresponding to the (130) and (101) planes of Bi2S3, respectively28. The coated MoS2 exhibits the lattice spacing of 6.19 Å, which matches well with the (002) planes of hexagonal MoS231. Furthermore, the related cleaved crystal structure in theoretical section indicates the distance between adjacent Bi atoms on the (130) crystal surface of Bi2S3 (3.4148 nm), which is eleven intervals of the adjacent S atoms on the (001) crystal surface of MoS2 (0.3169 nm × 11)45. It is believed that Bi2S3 nanorods might be available for the growth of the MoS2 nanosheets to form the heterostructure between the mutual effect of S and Bi atoms. Thus, it can be inferred that the MoS2 sheets, with about 5–8 layers, embellished at the surface of the Bi2S3 rods. Moreover, the selected area electron diffraction (SAED) pattern (inset of Fig. 3b) further proves the mixed-phase nature of single crystal Bi2S3 (bright diffraction spots) and layered superimposed MoS2 sheets (diffraction rings). In order to accurately confirm the elemental composition and spatial distribution, energy dispersive X-ray spectrometry (EDS) analysis in Fig. 3e have been performed. The well-proportioned distributions of S, Bi, and Mo can be obtained from the mapping results. Besides, the EDS line scan (Fig. 3f) of the marked region sheds light on the unique hierarchical heterostructure, as well as in agreement with SEM and TEM observation.
XPS analysis
We also performed XPS analysis to elucidate the surface chemical composition and valence states of the pristine Bi2S3, MoS2, and D-BM (5 MBS) heterostructures. The Fig. 4a shows the survey XPS spectra of Bi2S3 and MoS2 (Note that C element acts as reference and O element comes from the absorbed oxygen). After the second hydrothermal reaction, the overall XPS spectra in Fig. 4b indicates the main constituent of Bi, S, and Mo elements. Figure 4c,d display the spin-orbit components of Bi 4f (158.46/163.76 eV) and Mo 3d (228.84/231.99 eV) for pure Bi2S3 and MoS2, respectively. In addition, the XPS spectrum of the hybrids in Fig. 4e can be well fitted into several dominate peaks with binding energies of 158.75 eV, 162.05 eV, 163.30 eV, and 164.05 eV, which are assigned to Bi 4f7/2, S 2p3/2, S 2p1/2, and Bi 4f5/2, respectively. The high resolution Mo 3d (Fig. 4f) binding energies of the hybrids are located at around 229.23 eV and 232.38 eV, corresponding to Mo 3d5/2 and Mo 3d3/2, respectively. Notablely, the Bi 4f and Mo 3d peaks shift toward the high banding energy, with dotted lines mark. Hence, it could be deduced that chemical bonds of Bi-S-Mo formed probably, related to the electronic shielding effect46. A weak peak at 226.31 eV has also be found (Fig. 4f), which is attributed to the S 2s47. Take the spin orbit separation into consideration, the phenomenon among Bi 4f (5.30 eV), S 2p (1.25 eV), and Mo 3d (3.15 eV) peaks disclose the existence of Bi3+, S2−, and Mo4+, based on the reported results.
(a,b) The overall spectra of Bi2S3, MoS2, and 5 MBS, respectively. The Bi 4f and Mo 3d spectra for Bi2S3 (c), MoS2 (d), and D-BM (e,f), respectively. Note that the scatter and solid lines indicate the experimental data and fitting results, respectively. The dotted lines mark the peak shifts of Bi 4f and Mo 3d.
Growth mechanism investigation
The growth mechanism of the D-BM nanomaterials has been studied for potential applications and controllable synthesis of other novel structures. For D-Bi2S3, the formation process has been investigated by SEM at different reaction times, as shown in Fig. 5a–f. At the early reaction stage (1 h), the half-baked micropheres (diameter of 2 μm) have been formed and proven to be orthorhombic Bi2S3 by XRD analysis (Fig. S3), which indicate the fast nucleation of Bi2S3. As the reaction proceeded (3 h), some actinomorphic-shaped Bi2S3 (diameter of 5 μm) can be captured in Fig. 5b. From the close inspection (Fig. S4), however, the surface of the radial Bi2S3 nanorods are rough and inhomogenous, indicating a rapid growth of Bi2S3 microspheres. By prolonging the reaction time to 8 h, massive intact D-Bi2S3 microspheres have been successfully synthesized. Then the products under hydrothermal condition have been collected at 5 h, 8 h, and 12 h. The SEM images in Fig. 5d–f manifest that the coated MoS2 nanosheets become increasing and orderly along with the prolonged reaction process. It can also be verified through the related XRD survey (Fig. S4). At the beginning, the characteristic peak of MoS2 at 2θ = 14.2° is indeed indetectable. With prolonging the reaction time, the emergence of MoS2 (002) plane provides the convincible proof for the formation of the MoS2 coated Bi2S3 nanocomposites.
Based on the results of the time-dependent experiments and analysis, the probable morphology evolution process of the hetero-Bi2S3@MoS2 structure is illustrated in Fig. 5g. In our experiments, thiourea was chosen to act as the sulfide source, for constituting the Bi3+-thiourea complexes, initially. Under elevated temperature, these complexes decomposed, accompanied by shaping into needle-like nanospheres with nucleated Bi2S348,49. As time went on, Bi2S3 grew gradually to improve the rough surface, at the expense of Bi2S3 particles or rods. It can be attributed to the typical Ostwald ripening process, which could significantly reduce the total surface free energy50. Besides, the solution of PEG is necessary to build a suitable viscous surroundings, which promotes the unique geometrical patterns. For comparison, the morphology and crystallinity of the product obtained without PEG also have been surveyed. As a result (Fig. S5), the regular Bi2S3 microspheres with short tomentum ascertain the role of PEG as the important template. Finally, the 3D D-Bi2S3 microspheres could provide available high active sites for the growth and insertion of MoS2 nuclei, leading to the hierarchical and porous framework.
BET surface areas and photocurrent response
As shown in Fig. 6a, the nitrogen adsorption-desorption measurements have been performed to ascertain the surface area and the porous structure of the as-synthesized samples. According to the Brunaner-Deming-Deming-Teller (BDDT) classification, the isotherms of D-BM displays a typical hysteresis loops as type IV. The specific Brunauer-Emmett-Teller (BET) surface area have been estimated to be about 19.48 m2/g for the hybrid. Obviously, the characteristics with enlarged surface area and porosity of the composite recognized the embedded structure of MoS2 nanosheets into Bi2S3 microspheres. By the Barrett-Joyner-Halenda analysis, the pore-size distribution of Bi2S3@MoS2 (inset of Fig. 6a) indicates the main mesopores with about 4.5 nm.
Before moving toward the photocatalytic study of the as-synthesized products, the separation of charge carriers, as a crucial factor, needs to be investigated indispensably. Hence, the photocurrent transient response measurements of pure Bi2S3, MoS2, and their mesoporous composites have been carried out to verify the extended lifespan of the photogenerated charges. Under visible-light irradiation, Fig. 6b records the fast and consistent photocurrent responses over several on-off cycles, illustrating that all three samples are reproducible and stable. Apparently, pure Bi2S3 and MoS2 both present low photocurrent densities, consisted with the low quantum efficiency. However, the photocurrent density of the D-BM electrode is enlarged about one order of magnitude higher than the pure Bi2S3 electrode. It can be interpreted that this novel heterostructure possess particular tunnel for transformation of photogenerated carries, subsequently retards the recombination and extends the lifetime of carries.
Adsorption and photocatalytic properties
To demonstrate the photocatalytic ability of the D-BM heterostructures, photodegradation of RhB in aqueous solution has been investigated under visible-light irradiation. In Fig. 7a, at pH = 7, the corresponding decomposition rate of bare Bi2S3 (26%) and MoS2 (50%) are indistinctive after 40 min. Whereas, when the MoS2 was introduced to Bi2S3, the decomposition rate significantly increases to near 92%. The time-dependent absorption spectra of RhB solutions by D-BM and others were shown in Fig. 7e and Fig. S6. It could conclude that the hierarchical D-BM structure owns outstanding photocatalytic performance than two others. As it has been proposed by Daage, the “rim-edge” mode of MoS2 have massive dege active sites, where strong interaction occurs with dye molecules51,52,53. Also, these ultrathin and wrinkled surfaces of MoS2 may lead to several internal intersections, which make it possible to bring much more effective area for absorption. Likewise, it is favoring to transfer excited carries between MoS2 and Bi2S3 heterostructure, leading to reduce the recombination efficiency and prolong the lifetime of carries. In addition, the photocatalytic activity can also be related to the amount of the coated MoS2 nanosheets, which highlights the optimization of 5 MBS. As for 2 MBS samples, fewer MoS2 nanosheets cannot provide enough multiplex refraction for the incident path, as well as the more active site for the adsorption of reactant molecules. On the other hand, 8 MBS samples with superabundant MoS2 nanosheets impeded the transformation of photogenerated carries in reverse, thus facilitating their recombination. To make a more specific comparison, the reaction kinetics of all catalysts have been linear fitted as ln (C0/C) = kt according to Landmuir-Hinshelwood mechanism shown in Fig. 7b 54,55,56. Note that C and C0 are the real-time concentrations and initial concentration of RhB, t and k denote the irradiation time and the overall photodegradation rate constant, respectively. The rate constant of pristine Bi2S3 and MoS2 are 0.007 and 0.016 min−1. Nevertheless, a dramatic improvement (0.073 min−1) of 5 MBS can be achieved. Specifically, the increased reaction rates were attributed to the unique porous structure, with faster mass transport and more accessible active sites, resulting in an increased reaction rate. The stability and reusability of the composites (sample 5 MBS at pH = 7) also have been evaluated under irradiation by collecting and reusing them over 3 cycles. As shown in Fig. 7f, the insignificant decline in photocatalytic activity after three runs (90%) confirms the stability of the catalysts, excepting the incomplete collection. The constancy can be recognized through the XRD result after photocatalytic tests (Fig. S7).
The adsorption (in dark) and photodegradation (visible light irradiation) effects (C/C0) of RhB aqueous solution (10 mg/L) at (a) pH = 7 by different catalysts and (c) pH = 3–11 by D-BM. The plots of ln(C0/C) versus irradiation time of RhB aqueous solution at (b) pH = 7 by different catalysts and (d) pH = 3–11 by D-BM. The corresponding adsorption spectra (e) and photodegradation ((f), cycled catalyst) of RhB solution after 60 min in dark and 40 min irradiation with D-BM at pH = 7. The adsorption (in dark) and photodegradation (visible light irradiation) effects (C/C0) of MB aqueous solution (10 mg/L) at (g) pH = 3–11 by D-BM and (h) the corresponding decomposition rate by different catalysts. (i) The absorption spectra of the catalysts. (j) Relevant kinetics mechanism of adsorption and photocatalysis for D-BM in RhB solution. (k) Schematic diagram of the charge transfer and separation mechanism for the D-BM heterostructure.
It is believed that the effective photocatalysis need handle both the mass transfer and the light transfer issues. The adsorption capacity of catalyst surface for dyes during illumination is a defining factor in photodegradation. In addition, the initial pH plays a dominant role to the adsorption process. Therefore, based on the above adsorption behavior of the D-BM product in dark and neutral surroundings, the pH-dependent adsorption (3.0–11.0) and photocatalysis survey have been conducted. Note that the initial pH of the RhB solution was adjusted by HCl and NaOH solution (1 M). The Fig. 7c reveals that the adsorption capacity of D-BM hybrids has been promoted under acidic conditions and pH = 5 appears to the most beneficial. The adsorption efficiency reaches approximately 76% (Fig. S8) and photodegradation rate k = 0.157 min−1 (Fig. 7d) at pH = 5. For verifying the adsorption behavior and photocatalytic activity of D-BM hybrid, the colors of the degraded MB solutions (Fig. S8) and photodegradation of MB solution under pH of 3, 5, 7, 9, and 11 have been obtained in Fig. 7g,h. Obviously, D-BM shows a superior adsorption and photodegradation properties compared with pure Bi2S3 and MoS2, especially when pH < 7. Accordingly, although the initial pH affects the adsorption process of dyes onto the catalyst, the photodegradation process of D-BM remains resultful at a large range of pH values. It indicates that D-BM hybrid can serve as a high efficiency catalyst for wastewater treatment, which contained a mild acid commonly.
In order to further investigate the stability of the catalyst, the XRD and EDS mapping results after adsorption and photodegradation tests have been shown in Fig. 8a,b. Compared with the XRD results (Fig. 1a) and EDS mapping (Fig. 3e) before photocatalysis, there exist minor change among the elemental composition, spatial distribution, and the phase characterization results. Therefore, the composite has presented relative stability in view of its unique heterostructure and synergistic effect attributed to the intense interaction between Bi2S3 and MoS2, which favors the separation of the photoinduced carriers. However, a small percentage of the samples might had undergone hydrolysis which was unavoidable, taking the slight decrease through cycling experiments and photocorrosion into consideration.
Relevant kinetics mechanism
In Fig. 7j,k, the related adsorption and photocatalysis kinetic mechanism of the D-BM heterostructure under visible light irradiation has been put forward. In mild acidic environment (pH < 7), the surfaces of the catalyst is exposed to acidic (positive) conditions and positively charged, as shown in Fig. 7j. In addition, both the π-bond of RhB and MB can beneficially excite electrons with irradiation, as well as the existence of the functional groups (-COO−) as the shown molecular structure of RhB in Fig. 7j. Thus, we can conclude that the dyes might be adsorbed by D-BM hybrid through complex relationship involving electrostatic attraction or hydrogen bonds. Subsequently, these adsorbed dyes on the surface of D-BM could be in situ degraded promptly (Fig. 7j). The mass transfer and the chemical reaction process can be guaranteed by the opposite concentration difference and the visible light irradiation, which help to accomplish the degradation synergistically. In order to make it clear, the right part of the Fig. 7j illustrates the complete process simply, which contains both the adsorption and the photocatalysis behaviors of the D-BM hybrid.
On the other hand, since the band-edge potential levels play a crucial role in determining the migration directions of the photoexcited carriers in a heterojunction, the relative band positions of the two semiconductors have been investigated to approach the mechanism of the enhanced photocatalytic activity of their composites. Significantly, a staggered type-II configuration formed at the interface of D-BM structures by the calculated band edge positions, according to the empirical formula: EVB = X − Ee + 0.5Eg12. Note that EVB is the valence band (VB) edge potential and Ee refers to the free electrons energy based on the hydrogen scale (4.5 eV). Based on previous studies, X is the electronegativity of the corresponding semiconductor (5.28 eV for Bi2S3 and 5.32 eV for MoS2), expressed as the geometric mean of the electronegativity for the component atoms, and the band gap energy Eg of Bi2S3 and MoS2 are 1.33 eV and 1.3 eV, respectively12,19. Correspondingly, the EVB and ECB (CB, the conduct band) of Bi2S3 are estimated to be 0.12 eV and 1.45 eV, both higher than those of MoS2, with ΔEVB = 0.02 eV and ΔECB = 0.05 eV, respectively. Compared with the pure samples, the heterostructure of D-BM presents an obvious enhanced absorption (Fig. 7i), both in range and intensity. As illustrated in Fig. 7k, under visible-light irradiation, excited electrons-holes are generated facilely in both Bi2S3 and MoS2. With the suitable type II band alignment, the transfer of carries are also performed from the CB of Bi2S3 to MoS2, as well as the reflux of holes mainly from the VB of MoS2 to Bi2S3. Therefore, more vacancies have been left at the bottom of CB and top of VB for Bi2S3/MoS2, resulting in pronging the lifetime of carries and boosting more available separation. After the photoexcited carries fleetly flow to the CB of MoS2, the reaction of O2 → O2•− has been further promoted through one-electron reducing. Then hydroxyl radicals OH•, the dominating active species of RhB photodegradation, can be generated by the reaction between water and the unstable superoxide radical anion O2•−57. Meanwhile, the remaining holes in the valence band of Bi2S3 also participate in the degradation of RhB as the trapped active species.
Photoconductance and photoresponse performances
Besides photocatalysis, the as-synthesized D-BM hybrid is expected to have improved photoconductance and photoresponse properties compared with the pure Bi2S3 structure. In order to spin coating, the photodetector devices (Fig. 9a) were fabricated by these disrupt NR-BM and Bi2S3 nanorods (NR-Bi2S3). Figure 9b plots the typical current-voltage (I–V) curves of NR-BM based nano-photodetector exposed to 650 nm illumination (1.0 mW cm−1) and in dark, respectively. It is obvious that the quasilinearity of the I–V curves indicates the formation of an good Ohmic contact between Au electrode and NR-BM interface. With illumination, the NR-BM shows a remarkable photocurrent reaches about 0.703 μA under −1.5 V bias voltage, resulting a higher Ilight/Idark ratio to about 567, which compared to the NR-Bi2S3 of 126 with the shortened lifetime of the electron-hole pairs (Inset of Fig. 9b). The electrical characteristics of the NR-BM based device have been investigated in dark and at increasing illumination intensities from 0.25 mW cm−1 to 5 mW cm−1 (Fig. 9c). Clearly, the photocurrent increases with the enlarged light intensity and exhibits a high dependence on it, and their relationship can be described by a simple power law as I = APθ58,59. Where A and θ represent a constant for a certain wavelength and the response of photocurrent to light intensity, respectively. The fitting curve leads to θ = 0.89, which suggests that there exists little trap states (Inset of Fig. 9c) in the NR-BM photodetector. In addition, Fig. 9d provides the time dependent photoresponse of the D-BM device at a bias voltage of 1 V, which sheds light on a readily switch between high- and low-conduction states by illumination on/off. It is known that the responsivity (R) serves as a critical metric to the photodetector sensitivity, which is defined as R(AW−1) = Ip/Popt60. Where Ip and Popt are the photocurrent and incident light power, respectively. Based on the results, the R of the D-BM device is estimated to be 13.3 AW−1. Consequently, the improvement of photoconductivity and photoresponse performance supports the efficient carries separation resulting from the heterostructures of dandelion-shaped Bi2S3@MoS2.
(a) Schematic illustration of the fabricated photodetector. The I-V curves of the NR-BM (Inset of Fig. 8b for NR-Bi2S3) photodetectors illuminated by 650 nm with (b) 1.0 mW/cm2 and (c) different incident light intensity. Inset of Fig. 8c is the photocurrent measured versus light density at a bias voltage of 0.1 V. (d) The time dependent of the on-off photocurrent response of the device at a bias of 1 V by 650 nm illumination.
Theoretical investigation
The obtained band structures and the density of states (DOS) of Bi2S3 and MoS2 have been shown in Fig. 10a–f, respectively. As we can see, the top of VB contains S 3p and few contributions of Bi 6s, while the bottom of CB are mostly Bi 6p and S 3p for Bi2S3, with calculated Eg = 1.44 eV. As for MoS2 (with calculated Eg = 1.29 eV), the top of VB originates from Mo 4d, and the bottom of CB are Mo 4d and some hybridization with S 3p. Note that Fig. 10f shows the crystal surface matching of MoS2 (top) and Bi2S3 (down). By aligning the Fermi level relative to the vacuum energy level, the obtained work functions for Bi2S3 and MoS2 are 4.88 and 5.00 eV, respectively. Due to the differential work functions, a built-in electric field from Bi2S3 to MoS2 can be established near the interface. Thus the built-in electric field of the composites is expected to facilitate the separation of photo-generated carriers. It can be concluded that the theoretical calculations of the band energy positions (Fig. 10e) keep highly unanimous with the experimental results. In consequence, the MoS2 nanosheets uniformly layered-coated the Bi2S3 microspheres sufficiently absorb visible-light and retard the electron-hole recombination, eventually leading to improvement of the photocatalytic and optoelectronic properties. Moreover, the unique novel architecture can provide valuable references to take advantage of solar energy in the future.
Conclusion
In summary, a green strategy based on the hydrothermal method has been developed for the fabrication of hierarchical 3D dandelion-shaped Bi2S3 microspheres coated with layered MoS2 nanosheets. The novel heterostructure with unique porosity and intimate interfacial contact can provided efficient visible-light utilization and penetrable paths for reactant molecules to reach the inner structure. Compared with the pristine Bi2S3 or MoS2, the as-synthesized D-Bi2S3@MoS2 composite has exhibited much higher adsorption behavior and photocatalytic activity under visible-light irradiation. The formed staggered type II band alignment of Bi2S3@MoS2 has expected to promote the separation of carries, accelerate the transportation and prolong lifespan of electron-hole pairs, which can be verified by the excellent photoconductance and photoresponse properties. Correspondingly, the Bi2S3@MoS2-0.5 mol (5 MBS) has been proven to achieve an optimal photocatalysis performance, highlighting the importance of novel core/shell heterostructures for environmental remediation and solar energy harvesting applications in the future.
Methods
Synthesis
Generally, the D-BM heterostructure were synthesized by a two-step hydrothermal method. Firstly, the dandelion-shaped Bi2S3 microspheres were prepared at 180 °C19. Then, for forming the hybrids, another hydrothermal process was employed to embed MoS2 nanosheets onto Bi2S3 microspheres (molar ratios of Mo4+ to Bi3+ were 20%, 50%, and 80%, with the labels of 2 MBS, 5 MBS, and 8 MBS, respectively). Further information about the experimental details are available in the ESI.
Characterization
The crystallinity and purity of the resulting products were assessed by X-ray diffraction (XRD, Bruker D8 Advance diffractometer) equipped with Cu Kα radiation (λ = 1.5418 Å). Field emission scanning electron microscopy (FESEM, JEOL-JSM-6700F) was employed to investigate the morphologies of the synthesized samples, equipped with an energy dispersive X-ray spectroscopy (EDS). For detailed insight into the 2D-layered MoS2 coated Bi2S3, Transmission Electron Microscope (TEM) and high resolution transmission electron microscopy (HRTEM) studies were analyzed at the accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS, RBD upgraded PHI-5000C ESCA system, Perkin-Elmer) measurements were carried out with Mg-Kα radiation (hν = 1253.6 eV). Raman spectroscopy experiments were implemented by Jobin-Yvon LabRAM HR 800 micro-Raman spectrometer using a 532 nm line from a He-Cd laser. The absorption spectra have been obtained using the PerkinElmer Lambda 950 spectrophotometer in dilute solution. The specific surface area were calculated by the Brunauer-Emmett-Teller (BET) method (TriStar II 3020, America).
Photocatalytic test
The photocatalytic performance of the as-synthesized D-BM composites were evaluated by degrading RhB and MB (organic pollutant of dye wastewater) under visible light irradiation. More details about the photocatalytic tests have been provided in ESI. In addition, transient photocurrent responses for the as-prepared catalysts were performed over an electrochemical analyzer (CHI660D Instruments, China) in a standard three electrode system. Under the irradiation of simulated sunlight (500 W Xe lamp with a cutoff filter), the prepared samples acted as working electrode (ITO as supporter). A Pt wire worked as counter electrode, and Ag/AgCl (saturated KCl) as reference.
Measurement of photoconductance
The photoconductance and photoresponse behavior of the D-BM was studied based on the nano-photodetectors. Typically, the disrupt Bi2S3@MoS2 nanorods (NR-BM) from D-BM microspheres by oscillating (Sonics VCX800, America) were drop-deposited on the SiO2 (300 nm)/Si substrate. Then the Au electrodes (100 μm × 150 μm) were defined on the NR-BM film by photolithography and high-vacuum electron beam (EB) evaporation process. The optoelectronic properties of the fabricated device were measured by a semiconductor parameter analyzer system (Keithley 4200-SCS) with the illumination laser of 650 nm.
Theoretical calculation
In the present work, the first-principles calculations of the heterogeneous structure have been performed, to provide the theoretical basis of the promoted photocatalytic activity. In details, the plane-wave pseudopotential calculations with the generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE) have been carried out using the Materials Studio 7.0 Package61,62,63. The cutoff kinetic-energy (Bi2S3 and MoS2) are 600 eV/450 eV and the 3 × 8 × 3/5 × 5 × 1 Monkhorst-Pack k-point mesh have been employed for the Brillouin-zone integration. The electronic structures were calculated with the optimized lattice geometries. Based on the convergence criteria, both energy and force were less than 10−5 eV and 0.01 eV Å−1, for the fully relaxed initiating structures, respectively.
Additional Information
How to cite this article: Li, M. et al. Superior adsorption and photoinduced carries transfer behaviors of dandelion-shaped Bi2S3@MoS2: experiments and theory. Sci. Rep. 7, 42484; doi: 10.1038/srep42484 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Zou, Z. G., Ye, J. H., Sayama, K. & Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 21, 625–627 (2001).
Bian, T. et al. Spontaneous Organization of Inorganic Nanoparticals into Nanovesicles Triggered by UV Light. Adv. Mater. 26, 5613–5618 (2014).
Feng, X. L. et al. Hollow Mesoporous Aluminosilica Spheres with Perpendicular Pore Channels as Catalytic Nanoreactors. ACS Nano 6, 4434–4444 (2012).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 6, 269–271 (2001).
Qin, N. et al. One-Dimensional CdS/TiO2 Nanofiber Composites as Efficient Visible-Light-Driven Photocatalysts for Selective Organic Transformation: Synthesis, Characterization, and Performance. Langmuir 6, 1203–1209 (2015).
Pu, Y. C. et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-visible Region for Photoelectrochemical Water Splitting. Nano Lett. 6, 3817–3823 (2013).
Li, W. et al. Hydrothermal Etching Assisted Crystallization: A Facile Route to Functional Yolk-Shell Titanate Microspheres with Ultrathin Nanosheets-Assembled Double Shells. J. Am. Chem. Soc. 133, 15830–15833 (2011).
Zhu, C. B. et al. A General Strategy to Fabricate Carbon-Coated 3D Porous Interconnected Metal Sulfides: Case Study of SnS/C Nanocomposite for High-Performance Lithium and Sodium Ion Batteries. Adv. Sci. 2, 1500200 (2015).
Li, L., Li, P. F., Lu, N., Dai, J. & Zeng, X. C. Simulation Evidence of Hexagonal-to-Tetragonal ZnSe Structure Transition: A Monolayer Material with a Wide-Range Tunable Direct Bandgap. Adv. Sci. 2, 1500290 (2015).
Wang, M. G., Han, J., Xiong, H. X. & Guo, R. Yolk@Shell Nanoarchitecture of Au@r-GO/TiO2 Hybrids as Powerful Visible Light Photocatalysts. Langmuir 31, 6220–6228 (2015).
Alessanodro, L. T. et al. Assembly, Growth, and Catalytic Activity of Gold Nanoparticles in Hollow Carbon Nanofibers. ACS Nano 6, 2000–2007 (2012).
Zhang, Z. J., Wang, W. Z., Wang, L. & Sun, S. M. Enhancement of Visible-Light Photocatalysis by Coupling with Narrow-Band-Gap Semiconductor: A Case Study on Bi2S3/Bi2WO6 . ACS Appl. Mater. Interfaces 4, 593–597 (2012).
Guo, S. Q. et al. Mesoporous Bi2S3 nanorods with graphene-assistance as low-cost counter-electrode materials in dye-sensitized solar cells. Nanoscale 6, 14433 (2015).
Elena, K. et al. Suppression of the Plasmon Resonance in Au/CdS Colloidal Nanocomposites. Nano Lett. 11, 1792–1799 (2011).
Hong, T. et al. Anisotropic photocurrent response at black phosphorus-MoS2 p-n heterojunctions. Nanoscale 7, 18537 (2015).
Zhang, J., Liu, S. W., Yu, J. G. & Jaroniec, M. A simple cation exchange approach to Bi-doped ZnS hollow spheres with enhanced UV and visible-light photocatalytic H2-production activity. J. Mater. Chem. 21, 14655 (2011).
Malakooti, R. et al. Shape-Controlled Bi2S3 Nanocrystals and Their Plasma Polymerization into Flexible Films. Adv. Mater. 18, 2189–2194 (2006).
Cademartiri, L. et al. Cross-Linking Bi2S3 Ultrathin Nanowires: A Platform for Nanostructure Formation and Biomolecule Detection. Nano Lett. 9, 1482–1486 (2009).
Nambiar, S., Osei, E. K. & Yeow, J. T. W. Bismuth Sulfide Nanolflowers for Detection of X-rays in the Mammographic Energy Range. Sci. Rep. 5, 9440 (2015).
Rath, A. K., Bernechea, M., Martinez, L. & Konstantatos, G. Solution-Processed Heterojunction Solar Cells Based on p-type PbS Quantum Dots and n-type Bi2S3 Nanocrystals. Adv. Mater. 23, 3712–3717 (2011).
Tahir, A. A. et al. Photoelectrochemical and Photoresponsive Properties of Bi2S3 Nanotube and Nanoparticle Thin Films. Chem. Mater. 22, 5084–5092 (2010).
Chen, G. H. et al. Fabrication of Ultrathin Bi2S3 Nanosheets for High-Performance, Flexible, Visible-NIR Photodetectors. Small 11, 2848–2855 (2015).
Mesa, F., Dussan, A. & Gordillo, G. Study of the growth process and optoelectrical properties of nanocrystalline Cu3BiS3 thin films. Phys. Status Solidi C 7, 917–920 (2010).
Chen, J. S. et al. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol. Dalton Trans. 42, 15133 (2013).
Gao, X. H. et al. Formation of Mesoporous Heterostructured BiVO4/Bi2S3 Hollow Discoids with Enhanced Photoactivity. Angew. Chem. Int. Ed. 53, 5917–5921 (2014).
Yu, H. J., Huang, J., Zhang, H., Zhao, Q. F. & Zhong, X. H. Nanostructure and charge transfer in Bi2S3-TiO2 heterostructures. Nanotechnology 25, 215702 (2014).
Long, L. L., Zhang, A. Y., Huang, Y. X., Zhang, X. & Yu, H. Q. A robust cocatalyst Pd4S uniformly anchored onto Bi2S3 nanorods for enhanced visible light photocatalysis. J. Mater. Chem. A 3, 4301 (2015).
Manna, G., Bose, R. & Pradhan, N. Photocatalytic Au-Bi2S3 Heteronanostructures. Angew. Chem. Int. Ed. 53, 6743–6746 (2014).
Jia, Y. N., Zhan, S. H., Ma, S. L. & Zhou, Q. X. Fabrication of TiO2-Bi2WO6 Binanosheet for Enhanced Solar Photocatalytic Disinfection of E. coli: Insights on the Mechanism. ACS Appl. Mater. Interfaces 8, 6841–6851 (2016).
Low, J. X., Cao, S. W., Yu, J. G. & Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 50, 10768–10777 (2014).
Zhou, W. J. et al. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 9, 140–147 (2013).
Gong, Y. J. et al. Tellurium-Assisted Low-Temperature Synthesis of MoS2 and WS2 Monolayers. ACS Nano 9, 11658–11666 (2015).
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Hong, T. et al. Polarized photocurrent response in black phosphorus field-effect transistors. Nanoscale 6, 8978–8983 (2014).
Gao, Q. S., Giordano, C. & Antonietti, M. Electronics Oxygen Activation by MoS2/Ta3N5 Nanocomposites for Selective Aerobic Oxidation. Angew. Chem. Int. Ed. 51, 11740 (2012).
Perea-López, N. et al. CVD-Grown Monolayered MoS2 as an Effective Photosensor Operating at Low-Voltage. 2D Mater. 1, 011004 (2014).
Min, Y. L., He, G. Q., Xu, Q. J. & Chen, Y. C. Dual-functional MoS2 sheet-modified CdS branch-like heterostructures with enhanced photostability and photocatalytic activity. J. Mater. Chem. A 2, 2578–2584 (2014).
Zhang, Y. et al. In Situ Fabrication of Vertical Multilayered MoS2/Si Homotype Heterojunction for High-Speed VisibleCNearInfrared Photodetectors. Small 8, 1062–1071 (2016).
Yoo, Y. D., Degregorio, Z. P. & Johns, J. E. Seed Crystal Homogeneity Controls Lateral and Vertical Heteroepitaxy of Monolayer MoS2 and WS2 . J. Am. Chem. Soc. 137, 14281–14287 (2015).
Xiao, J. et al. Exfoliated MoS2 Nanocomposite as an Anode Material for Lithium Ion Batteries. Chem. Mater. 22, 4522–4524 (2010).
Wang, Y. C. et al. Electrochemical Control of Photoluminescence in Two-Dimensional MoS2 Nanoflakes. ACS Nano 7, 10083–10093 (2013).
Fan, X. B. et al. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano lett. 15, 5956–5960 (2015).
Wang, S. G. et al. A Facile One-Pot Synthesis of a Two-Dimensional MoS2/Bi2S3 Composite Theranostic Nanosystem for Multi-Modality Tumor Imaging and Therapy. Adv. Mater. 27, 2775–2782 (2015).
Wang, S. G. et al. Biocompatible PEGylated MoS2 nanosheets: Controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials 39, 206–217 (2015).
Li, Y. T. et al. Co-nucleus 1D/2D heterostructures with Bi2S3 nanowire and MoS2 monolayer: One-step growth and defect-induced formation mechanism. ACS Nano 7, 10083–10093 (2013).
Cui, W. Q., Shao, M. Y., Liu, L., Liang, Y. H. & Rana, D. Enhanced visible-light-responsive photocatalytic property of PbS-sensitized K4Nb6O17 nanocomposite photocatalysts. Applied Surface Science 276, 823–831 (2013).
Ren, X. P. et al. A Se-doped MoS2 nanosheet for improved hydrogen evolution reaction. Chem. Commun. 51, 15997–16000 (2015).
Shen, G. Z., Chen, D., Tang, K. B., Li, F. Q. & Qian, Y. T. Large-scale synthesis of uniform urchin-like patterns of Bi2S3 nanorods through a rapid polyol process. Chem. Phys. Lett. 370, 334–337 (2003).
Tian, Y., Ding, T. T., Zhu, X. L., Tu, Y. F. & Zheng, G. Bi2S3 microflowers assembled from one-dimensional nanorods with a high photoresponse. J. Mater. Sci. 50, 5443–5449 (2015).
Krichevsky, O. & Stavans, J. Correlated Ostwald Ripening in Two Dimensions. Phys. Rev. Lett. 70, 1473 (1993).
Perera, S. D. et al. Hydrothemal Systhesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity. ACS Catal. 2, 949–956 (2012).
Rauf, A. et al. Facile Synthesis of Hierarchically Structured Bi2S3/Bi2WO6 Photocatalysts for Highly Efficient Reduction of Cr(VI). ACS Sustainable Chem. Eng. 3, 2847–2855 (2015).
Daage, M. & Chianelli, R. R. Structure-Function Relations in Molybdenum Sulfide Catalysts: The “Rim-Edge” Model. J. Catal. 149, 414–427 (1994).
Kumar, S., Khanchandani, S., Thirumal, M. & Ganguli, A. K. Achieving Enhanced Visible-Light-Driven Photocatalysis Using TypeII NaNbO3/CdS Core/Shell Heterostructures. ACS Appl. Mater. Interfaces 6, 13221–13233 (2014).
Romão, J. & Mul, G. Substrate Specificity in Photocatalytic Degradation of Mixtures of Organic Contaminants in Water. ACS Catal. 6, 1254–1262 (2016).
Gao, Y. W., Wang, Y. & Zhang, H. Removal of Rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation. Appl. Catal., B: Environ. 178, 29–36 (2015).
Zhou, J. et al. Growth rate controlled synthesis of hierarchical Bi2S3/In2S3 core/shell microspheres with enhanced photocatalytic activity. Sci. Rep. 4, 4027 (2014).
Luo, L. B. et al. Near-Infrared Light Photovolatic Detector Based on GaAs Nanocone Array/Monolayer Graphene Schottky Junction. Adv. Funct. Mater. 24, 2794–2800 (2014).
Jie, J. S. et al. Photoconductive characteristics of single-crystal CdS nanoribbons. Nano Lett. 9, 1887–1892 (2006).
Zhai, T. et al. Fabrication of High-Quality In2Se3 Nanowire Arrays toward High-Performance Visibe-Light Photodetectors. ACS Nano 4, 1596 (2010).
Feng, N. et al. Magnetism by Interfacial Hybridization and p-type Doping of MoS2 in Fe4N/MoS2 Superlattices: A First-Principles Study. ACS Appl. Mater. Interfaces 6, 4587–4594 (2014).
Thomson, J. W. et al. Ultrathin Bi2S3 Nanowires: Surface and Core Structure at the Cluster-Nanocrystal Transition. J. Am. Chem. Soc. 132, 9058–9068 (2010).
Wang, Y. et al. Syntheses, Growth Mechanism, and Optical Properties of [001] Growing Bi2S3 Nanorods. J. Phys. Chem. C 113, 16009–16014 (2009).
Acknowledgements
One of the authors (M. J. Li) would like to thanks Prof. L. B. Luo, Y. Q. Yu, Dr. C. Li and H. L. Li for constructive discussions. This work was financially supported by Major State Basic Research Development Program of China (Grant No. 2013CB922300), the Natural Science Foundation of China (Grant Nos 11374097, 61376129, and 61504156), Projects of Science and Technology Commission of Shanghai Municipality (Grant Nos 15JC1401600, 14XD1401500, 13JC1402100, and 13JC1404200), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Author information
Authors and Affiliations
Contributions
M.L. and Q.D. analysed data and prepared the manuscript and figures. M.L., P.Z., J.Z., and K.J. prepared the samples and performed experiments. M.L. and J.W. performed the first-principles calculations. The project was initiated and conceptualized by Z.H. and J.C. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, M., Wang, J., Zhang, P. et al. Superior adsorption and photoinduced carries transfer behaviors of dandelion-shaped Bi2S3@MoS2: experiments and theory. Sci Rep 7, 42484 (2017). https://doi.org/10.1038/srep42484
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42484
This article is cited by
-
Development of chitosan@Fe2O3/rGO/Bi2S3 as a new eco-friendly photocatalyst for enhancing the catalytic stability and superior degradation of organic pollutants
Research on Chemical Intermediates (2023)
-
Bi2S3 nanoparticles/ZnO nanowire heterojunction films for improved photoelectrochemical cathodic protection for 304 SS under visible light
Journal of Applied Electrochemistry (2022)
-
Photocatalytic Degradation of Rhodamine B by Highly Effective Heterostructure Pd/Bi2MoO6 Nanocomposites Synthesized by Photoreduction Deposition Method
Journal of Inorganic and Organometallic Polymers and Materials (2021)
-
Controllable interlayer space effects of layered potassium triniobate nanoflakes on enhanced pH dependent adsorption-photocatalysis behaviors
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.