Abstract
We undertook an RNA sequencing (RNAseq)-based transcriptomic profiling study on lymphoblastoid cell lines of a European ancestry sample of 529 schizophrenia cases and 660 controls, and found 1058 genes to be differentially expressed by affection status. These differentially expressed genes were enriched for involvement in immunity, especially the 697 genes with higher expression in cases. Comparing the current RNAseq transcriptomic profiling to our previous findings in an array-based study of 268 schizophrenia cases and 446 controls showed a highly significant positive correlation over all genes. Fifteen (18%) of the 84 genes with significant (false discovery rate<0.05) expression differences between cases and controls in the previous study and analyzed here again were differentially expressed by affection status here at a genome-wide significance level (Bonferroni P<0.05 adjusted for 8141 analyzed genes in total, or P<~6.1 × 10−6), all with the same direction of effect, thus providing corroborative evidence despite each sample of fully independent subjects being studied by different technological approaches. Meta-analysis of the RNAseq and array data sets (797 cases and 1106 controls) showed 169 additional genes (besides those found in the primary RNAseq-based analysis) to be differentially expressed, and provided further evidence of immune gene enrichment. In addition to strengthening our previous array-based gene expression differences in schizophrenia cases versus controls and providing transcriptomic support for some genes implicated by other approaches for schizophrenia, our study detected new genes differentially expressed in schizophrenia. We highlight RNAseq-based differential expression of various genes involved in neurodevelopment and/or neuronal function, and discuss caveats of the approach.
Similar content being viewed by others
Introduction
Schizophrenia, a common (~1%) and often severe psychiatric disorder, typically has a late adolescent or early adult onset of overt psychotic symptoms.1 A number of etiological contributions have been proposed,2 in particular genetic predisposition,3 but there is also some evidence for immunological and inflammatory mechanisms.4, 5 Genome-wide association studies (GWASs) of schizophrenia and their meta- and mega-analyses with increasing sample sizes have yielded over 100 genome-wide significant loci.6, 7, 8, 9, 10 Most common single-nucleotide polymorphism (SNP) GWAS variants lie outside of genes and/or are not in linkage disequilibrium with polymorphisms affecting amino-acid sequence,9, 10 and functional consequences of candidate variants remain largely unclear. Disease-associated copy-number variants (CNVs) and trait-associated SNPs from GWAS on disorders with complex genetics (including schizophrenia) have been found to be enriched for regulatory sequences (DNase I hypersensitive sites, ENCODE11) and for expression quantitative trait nucleotides,12, 13, 14, 15 which suggests a likely importance of gene regulation for such variants. Therefore, we have conducted an RNAseq-based genome-wide gene expression study to investigate genetic mechanisms involved in schizophrenia.
Our previous effort consisted of a transcriptional analysis of lymphoblastoid cell lines (LCLs) from 268 schizophrenia cases and 446 controls from the Molecular Genetics of Schizophrenia (MGS) European ancestry GWAS sample.16 In that first study, instead of RNAseq, we used the Illumina HT12-v4 microarray16 and found differential expression by affection status for 95 transcripts from 89 genes, among which we noticed immune-related gene enrichment.16 Interestingly, the results from a later large GWAS meta-analysis strongly supported immunological activation in schizophrenia, especially in B-lymphocyte lineages involved in acquired immunity.10 We present here the transcriptomic profiles of a larger, non-overlapping set of 529 schizophrenia cases and 660 controls, using RNAseq technology. Here we report our primary RNAseq gene expression findings and the results of a combined analysis of both cross-platform data sets (totaling 797 cases and 1106 controls) by the use of a sample size-weighted meta-analysis of P-values.
Materials and methods
Subjects
The Institutional Review Board from NorthShore University HealthSystem reviewed and approved the protocol, and informed consent was previously been obtained for all subjects. The MGS sample ascertainment, assessment, composition and characteristics have been previously described.6, 16, 17 Briefly, the MGS case–control collection is a large data set previously collected at 10 locations to study the genetic etiology of schizophrenia. As with our previous study,16 we included only MGS European ancestry GWAS-studied samples. The selected individuals did not overlap with the previously array-studied expression sample,16 that is, consisted of entirely non-overlapping subjects. We chose approximately equal proportions of males and females in each group, and roughly matched cases and controls based on 5-year age brackets, to reduce potential confounder effects. Our studied sample is described in Table 1, and the case and control components matched quite well on the measured covariates. Subject (sample) quality control for identity verification included ensuring concordance between known sex (that is, dosages of X and Y chromosomes) and RNAseq expression levels of sex-dimorphic expressed genes on chromosomes X (XIST) and Y (RPS4Y1, ZFY, USP9Y, DDX3Y, UTY, KDM5D and EIF1AY). We also compared RNAseq-called genotypes (using SAMtools mpileup function,18 requiring >8 reads at a called SNP site) with previously determined SNP genotypes (Affymetrix 6.0, Santa Clara, CA, USA) from the MGS GWAS6 for informative SNPs (mean 76 per sample), requiring at least 95% concordance (mean 99.5% concordance).
LCLs
The standardized careful methods for obtaining, reviving, growing, assaying (growth rate and energy status) and harvesting the LCLs for RNA, as well as the use of appropriate (culture/biological and RNA/technical) replicates, RNA isolation and processing are the same as described for the previous microarray-based expression study.16 In the current study, all LCLs were transformed at Rutgers University Cell and DNA Repository. As with our previous array-based study, we collected LCL characteristics data that may confound the relationship between schizophrenia status and gene expression levels,19 namely, Epstein–Barr virus (EBV) load (copy number), cell count at harvest (LCL growth rate) and LCL energy status (indexed by ATP levels adjusted by cell count).
Transcriptome sequencing
We shipped samples, always intercalating cases and controls (as was done in the LCL-growing protocol, to minimize any cryptic case–control differences that might still exist besides affection status), in five large batches to the University of Minnesota Genomics Center, which performed next-generation sequencing (RNAseq), using Illumina TruSeq SBS v3 (San Diego, CA, USA) reagents and Illumina HiSeq instruments, and obtaining 50 bp single-end reads, obtaining average read quality (Q-score >30) across the entire read length, and achieving >8 million read depth for the analyzed samples. (If more than one run was required, we combined the reads across RNAseq runs, including all runs with >2 million reads. In all such instances, the RNAseq runs were done in the same sequencing batch on the same RNA harvest, that is, we did not regrow LCLs from a study participant multiple times, harvest RNA multiple times and then run RNAseq on these multiple RNA harvests. Similarly, we did not combine RNAseq results across different RNAseq runs.)
RNAseq data processing
We used TopHat20 (v2.0.12, based on Bowtie2,21 v2.2.3) for read alignment, and CuffLinks22 (v2.2.1) for counting the number of mapped alignments and calculating the measure of gene expression, FPKM (fragments per kilobase of exon per million reads mapped). We normalized the data by square root transformation as a variance-stabilizing transformation, as FPKM may be viewed as following a Poisson distribution, that is, of ‘rare events’. For human genome annotation, we used GenCode v.20 based on human genome assembly GRCh38 (hg38), and program parameters had default values. The total number of reads per sample averaged 14 040 233 (range 9 003 327–64 218 868), excepting 32 LCLs from carriers (23 cases and 9 controls) of known risk CNVs for schizophrenia. These samples were batched together and sequenced at a higher depth (mean=71 758 557, range 11 921 205–107 065 076). Excluding these 32 high-depth CNV carrier samples, there was no difference between the RNAseq coverage between cases and controls (see footnote to Table 1).
Differential expression analyses
For the primary analysis (RNAseq, full sample), we focused on the 21 146 genes with detectable (that is, non-zero) expression in at least 80% of the samples. The average correlation of square root transformed gene expression levels across these 21 146 genes between 46 technical replicates, 63 culture replicates and all unrelated samples was in the expected order (r=0.99, r=0.98 and r=0.97, respectively). As in the array study before,16 we used the same measured covariates (sex, age, age2, genotypic principal components, batch (four dummy variables for five RNAseq batches), EBV load, growth rate and ATP) jointly applied (to account for their possible confounding) to a multivariate linear regression model with affection status. For the comparison to the previous array results, we limited the study to genes detected well with both array and RNAseq (detectable expression in ⩾80% of samples for both platforms). The RNAseq sample is a fully independent sample with no overlap of subjects with the array-studied sample. To combine results across the two studies (array and RNAseq), we used extension of Stouffer’s Z method,23 for which we (1) selected the 8141 genes expressed in >80% of samples by both methods, (2) used the P-values and beta-coefficients for differential expression by schizophrenia status to generate Z-scores, (3) weighted the Z-scores by sample sizes (array 714 consisting of 268 cases and 446 controls; RNAseq 1189 consisting of 529 cases and 660 controls), (4) combined the Z-scores for the meta-analytic result, and then (5) reverted the combined Z-scores back to P-values corresponding to the combined P-values of RNAseq and array, using an R package.24
Pathway and network analyses
We performed gene ontology-term and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses using the Database for Annotation, Visualization and Integrated Discovery tool25 for our list 1058 differentially expressed (Bonferroni P<0.05) genes, using all of the analyzed genes (21 146 genes with detectable expression in at least 80% of the samples) for the background. We also submitted the same gene list to Disease Association Protein-Protein Link Evaluator26 to evaluate their network connectivity, using 5000 permutations to estimate significance. For the 459 input genes (of the 1058) in the same direct network identified by Disease Association Protein-Protein Link Evaluator, we also performed directed gene ontology-term enrichment analysis and Kyoto Encyclopedia of Genes and Genomes pathway analysis using the Database for Annotation, Visualization and Integrated Discovery tool.
Data sharing
For the results of primary RNAseq expression analyses, we are sharing the data by depositing it into the database of Genotypes and Phenotypes (dbGaP, dbgap.ncbi.nlm.nih.gov). The array expression data have already been deposited into dbGaP (phs000775). GWAS and phenotypic data for all subjects have already been deposited into dbGaP (phs000021 and phs000167), and LCLs (and phenotypic data) are available through the National Institute of Mental Health repository (www.nimhgenetics.org) contractors (rucdr.rutgers.edu and zork.wustl.edu, respectively), with MGS drawing from National Institute of Mental Health repository schizophrenia studies 0 (sites 30–32), 6 (sites 40–49) and 29 (sites 139–150).
Results
Sample
We present an outline of the overall study design and data processing steps in Supplementary Figure S1 and present the basic characteristics of the study participants by affection status in Table 1. Cases had chronic, usually unremitting schizophrenia (88%) or schizoaffective disorder (12%) with an average age of onset of 21.6 years old. Controls were screened for psychosis, and both cases and controls were collected under the unified MGS GWAS protocol.6 The current RNAseq-studied sample of 529 schizophrenia cases and 660 controls were fully independent of the previous array-studied sample.16 Case and control study participants matched well for most primary epidemiological characteristics of study subjects and their LCLs (Table 1). RNA quality was high throughout, and RNAseq quantity and quality scores were comparable between cases and controls (Table 1).
RNAseq preparative analyses
We aligned RNAseq data to hg38 using TopHat, with 93% of reads being aligned on average, with no significant differences observed between cases and controls (Table 1). We estimated gene-based expression levels as FPKM. We observed positive (non-zero) FPKM expression for 26 337 genes on average (median: 25 920) per sample (range: 22 660–36 325). The number of expressed genes detected increased with the number of reads per sample (Supplementary Figure S2). There is a near-linear relationship between the number of genes with detectable expression and the read count on a logarithmic scale, suggesting that non-detection of expression of genes with low average expression level was primarily due to limited read depth rather than truly absent expression. As expected, the expression levels varied widely between different genes. A histogram of the expression levels (after square root transformation, and averaged across samples) across genes is shown in Supplementary Figure S3. Not all genes were detectably expressed (non-zero FPKM) in all samples. Supplementary Figure S4 is a scatter plot of gene expression levels plotted against the proportion of samples yielding detectable expression. These two quantities are highly positively correlated (r=0.72), which indicates again that non-detection of a given gene in a given sample likely is caused by limited read depth rather than actual non-expression. To focus on the genes with reliably estimated expression (that is, moderately to highly expressed), and reduce the multiple testing burden, we retained for analysis genes (N=21 146) that had detectable expression in ⩾80% of the 1189 samples. Supplementary Figure S5 shows an overview of the numbers of genes expressed at different FPKM thresholds and the mean correlation of a given sample with all other samples, and shows a lack of any apparent sample outliers.
Differential expression analyses
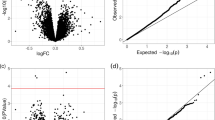
We performed multiple linear regression analysis to identify genes whose average expression level varied significantly between cases and controls. Although many of the examined characteristics of cases and controls were comparable to one another (Table 1), we nonetheless included as nuisance parameters in our regression model those 16 covariates that we considered to be potential confounders or noise contributors, namely, subject characteristics (sex, age, age2 and genotypic principal components 1–5 to index sample ancestry), LCL characteristics (EBV load, growth rate and energy status) and RNAseq main batch (five batches, that is, four dummy variables). A total of 1058 genes (5.0%) were differentially expressed by affection status after Bonferroni P<0.05 adjustment for 21 146 separately analyzed genes, or P<~2.36 × 10−6 (Figure 1). Among these genes, 361 were downregulated and 697 were upregulated in cases compared to controls (Supplementary Tables S1 and S2).
Manhattan plot of differential expression by schizophrenia status. The −log10 of the P-values for the differential expression by schizophrenia status is plotted against the chromosomal location for the 21 146 genes with detectable expression in at least 80% of the studied samples. The black bar corresponds to Bonferroni P⩽0.05.
Gene set enrichment analyses
We conducted gene set enrichment analysis (GSEA) to investigate the overall characteristics of the differentially expressed genes from our RNAseq study. These 1058 differentially expressed genes were enriched (Supplementary Table S1) for genes involved in immunity (as a percentage of analyzed genes: 31% vs 13%, Fisher P=5 × 10−50). The immune enrichment was more pronounced in the upregulated (697) vs downregulated (361) genes (34% vs 25%, Fisher P=1.7 × 10−3). As most of the lines of evidence suggest immune contributions to schizophrenia point towards immune activation (see discussions, Schizophrenia Working Group of the Psychiatric Genomics Consortium,10 Eaton et al.,27 Eaton et al.,28 Brown and Derkits,29 and Brown30), we performed GSEA on the genes upregulated and downregulated in the schizophrenia cases separately, similar to the directional GSEA approaches used by others, (for example, Lin et al.31 and Huan et al.32). GSEA of the 697 upregulated genes using the Database for Annotation, Visualization and Integrated Discovery tool25 showed gene ontology-term enrichment (false discovery rate<0.05) for categories including multiple immune categories (response, activation and regulation), apoptosis regulation and cellular components (cell surface and cytosol; Table 2). Pathway analyses of the 361 downregulated genes showed gene ontology-term enrichment for different (not immune or apoptosis categories) and many more categories, including those related to transport/localization (intracellular, vesicular and membrane), multiple protein and glycosylation categories, and cellular components and molecular functions related to the aforementioned biological processes (Supplementary Table S3). In addition, the downregulated genes were enriched for the Kyoto Encyclopedia of Genes and Genomes pathway term of N-glycan biosynthesis (hsa00510; major constituents of glycoproteins, which often are involved in cell–cell interactions; Supplementary Table S3). We note that an increasing number of studies are finding alterations in glycan biosynthesis, glycan levels and glycosylation in schizophrenia, both in the brain and blood, suggesting dysregulation of glycosylation in schizophrenia (see review in Kippe et al.33). Recently, protein levels of important glycosylation enzymes, B3GNT8 and MGAT4A, were found decreased in the prefrontal cortex in schizophrenia (12 case–control pairs),33 whereas in our study B3GNT1, B3GNT3 and MGAT1 transcripts were downregulated in schizophrenia cases (Supplementary Table S1). Protein–protein interaction network analyses using Disease Association Protein-Protein Link Evaluator26 of the 697 upregulated genes showed significantly higher network connectivity than expected, both direct (P=2.2 × 10−4) and indirect (P=2.2 × 10−4), as it did for the 361 downregulated genes (direct P=2.0 × 10−4, indirect P=2.0 × 10−4).
We have performed a number of additional analyses on all 1058 differentially expressed genes (that is, the combination of upregulated and downregulated genes), as follows. As cases were 50% male and controls were 54% male, and as males are affected with schizophrenia more often and more severely than females, we checked for enrichment of sex chromosome genes. We observed no enrichment for chromosome X (3.0% vs 2.9%, Fisher P=0.44) or chromosome Y (0% vs 0.09%, Fisher P=1.00). Similarly, as cases had a slightly higher average EBV load, we checked results for enrichment of genes known to be associated with EBV copy number,34 and found modestly significant statistical evidence for enrichment (1.2% vs 0.6%, P=0.01). Genes expressed in the adult brain were not enriched (91% vs 90%, Fisher P=0.14), although 84% of differentially expressed immune-related genes are expressed in the brain. More specifically, for the analyzed genes (21 146 genes with detectable expression in at least 80% of the samples), 10 774 (90%) were expressed in the brain and 1156 (10%) were not (via hbatlas.org as for Supplementary Table S1), whereas for the 1058 differentially expressed by affection status genes (Supplementary Table S1) for which there was adult brain expression data, 779 (91%) were expressed in the brain and 73 (9%) were not (via hbatlas.org, the remainder being unlisted which we designated as ‘NA’ in Supplementary Table S1).
Comparison to microarray results
We previously had conducted a gene expression study of LCLs from European ancestry cases and controls from the same MGS cohort, using non-overlapping samples.16 The previous microarray study16 and the current RNAseq study used the same laboratory methods for growing cells and harvesting RNA, and used consistent criteria for which genes to include in the analysis (expressed in ⩾80% of samples). We therefore wondered whether the findings in both samples, which were studied by different methods (microarray and RNAseq), were consistent with one another overall. Supplementary Figure S6 shows a scatter plot of the results from both studies for the 8141 genes investigated in both studies (on the scale of the sign of the estimated beta regression coefficient multiplied with the −log10 of the differential expression P-value). We found a highly significant positive correlation over all genes (r=0.29, P<10−16) between the array and the RNAseq findings. The correlation grows stronger among genes whose expression level is significantly associated with schizophrenia. For the P-value tails of the array study (array P<0.05, P<0.005, P<0.0005, P<0.00005), the correlation increases (r=0.47, r=0.55, r=0.57, r=0.68, respectively). We observe a similar pattern of increasing correlation of array and RNAseq findings when moving into such tails of the RNAseq study (RNAseq P<0.05: r=0.38, P<0.005: r=0.43, P<0.0005, r=0.47, P<0.00005, r=0.50). Thus, despite fully independent samples and different technologies (microarray and RNAseq), we found notable consistency in the detected expression levels and direction over all overlapping genes, which was more pronounced for those genes differentially expressed in schizophrenia.
Of the 89 genes that we reported to be significantly differentially expressed in our previous array study (false discovery rate<0.05),16 84 were analyzed in this RNAseq study. Fifteen (18%) of those 84 were differentially expressed by affection status here at a genome-wide significance level (Bonferroni P<0.05 adjusted for 8141 analyzed genes in total, or P<~6.1 × 10−6), all with the same direction of effect (Supplementary Table S4). At a less stringent significance level applying a Bonferroni correction only for the 84 examined array false discovery rate<0.05 significant genes (that is, P<~6.0 × 10−4), 29 (35%) were significant in this RNAseq study, with 28 (97%) out of these 29 with the same direction of effect.
Meta-analysis of RNAseq and array data sets
As we have confirmed differential expression by affection across two different technologies (microarray vs RNAseq) and two different (independent) sample sets, we combined the results across the two studies (array and RNAseq, for the 8141 genes expressed in >80% of samples by both methods) using Stouffer’s Z method.23 Briefly, we used the differential expression P-values and beta-coefficients to generate Z-scores, which we weighted by the respective sample sizes and combined and then reverted back to differential expression P-values and beta-coefficients for the combined result on these 8141 jointly expressed genes. Although the primary RNAseq analysis on 21 146 genes yielded 1058 (5.0%) genes differentially expressed by affection status (Bonferroni P<0.05; Supplementary Tables S1 and S2), the meta-analysis of 8141 genes (expressed in >80% of samples by both methods) yielded 647 (7.9%) such genes (Bonferroni P<0.05; Supplementary Table S5). There was a great deal of overlap in these two genes lists, with 478 (74%) of the 647 significant genes from the meta-analysis having been in the list of 1058 significant genes from the primary RNAseq analysis. We note that similarly to the main RNAseq analysis, these 647 differentially expressed genes were enriched for genes involved in immunity (as a percentage of analyzed genes: 38% vs 22%, Fisher P=1.4 × 10−18). Among the 169 new genes from the meta-analysis (that is, not significant in the primary RNAseq analysis), are several previously discussed in our array paper16 and several others discussed below.
Discussion
Using RNAseq to quantify gene expression in LCLs, we have detected 1058 genes differentially expressed by affection status in 529 schizophrenia cases and 660 controls. We have provided further support for some genes detected by other methods (for example, the Psychiatric Genomics Consortium for schizophrenia and GWAS10) and platforms (for example, our previous microarray expression study16), and we have detected novel genes (Supplementary Tables S1 and S2). These 1058 differentially expressed genes were markedly enriched for genes involved in immunity (Supplementary Table S1). GSEA showed enrichment for categories related to immunity, apoptosis, messenger RNA and protein processing and cell growth (Table 2; Supplementary Table S3). We also provide substantial corroborative evidence for our previous array-based gene profiling study16 (Supplementary Table S4; Supplementary Figures S6A and B).
Genes differentially expressed in RNAseq data set
All 1058 differentially expressed genes are listed in Supplementary Table S1, and ~10% of those genes are highlighted in Supplementary Table S2. We selected to further discuss here four genes involved in immune function (CR1, C3, TGFB1 and TGM2) with various additional support from the literature (expression, genetic association, human post-mortem brain, pharmacological and so on), and four genes (PIK3CD, PDE4B, SHANK2 and NDE1) involved in neurodevelopment and/or neuronal function, also with various types of additional support from the literature (expression, genetic association, human post-mortem brain, rodent modeling, pharmacological and so on). Some of the genes discussed below and in Supplementary Tables S1 and S2 (and even Supplementary Table S5 below) have both neuronal and immunological roles and/or expression (for example, C3, TGFB1, PIK3CD, PDE4B, FAM69A, IFITM1 and PPP3CC). Although we discuss the immune aspects below, these examples also serve here as reminders of various bridges between the two systems (neural and immune) relevant for schizophrenia, such as the general area of neuroinflammation,35 immune dysfunction and/interventions sometimes affecting psychotic symptoms (for example, co-administration of antipsychotic and anti-inflammatory drugs augmenting the former’s antipsychotic effects36, 37), various schizophrenia treatments also having immune effects (for example, clozapine, risperidone and so on, reviewed in Muller and Schwarz38 and O'Sullivan et al.39), and many brain disorders also having immune or inflammatory aspects (for example, schizophrenia as below, but also depression, Alzheimer disease, multiple sclerosis and so on40, 41, 42).
Immune-related differentially expressed gene examples
We found differential expression in components of the complement system, though not for C4A, which was recently reported to associate with schizophrenia in proportion to each allele’s tendency to generate greater expression of C4A43 and also resides in the most strongly associated region of the genome (the major histocompatibility complex (MHC) region).10 Of the ~50 complement system genes,44 23 were well expressed (in ⩾80% of samples), 4 of which were upregulated in their expression in schizophrenia cases, which was a modest enrichment of differential expression in the complement system genes (0.4% vs 0.1%, P=0.026). The four complement components for which we found evidence of upregulation in schizophrenia cases were: CR1, CR2, CD55, and C3. CR1 is expressed on most blood cells and has a high affinity for complement components (C1q, C3 and C4).45 CR1 expression on the surface of lymphocytes, monocytes and neutrophils was found to be higher in schizophrenia cases than in controls,46 as its messenger RNA was in the current study. In addition, CR1 has achieved genome-wide significance in GWAS for Alzheimer’s disorder.47, 48 C3 plays a central role in the activation of complement system, which is required for both classical and alternative complement activation pathways. C3 and other complement components have been suggested to influence risk for schizophrenia (see review Mayilyan et al.49). C3 also plays roles in neurodevelopment (synaptic remodeling).50, 51 C3 has been reported as being upregulated in blood from schizophrenia cases versus controls,52 consistent with our findings of C3 messenger RNA upregulation. However, a recent study found no significant difference in C3 expression in blood for drug-naive schizophrenia cases versus controls.53
TGFB1 is a cytokine that regulates proliferation, differentiation, adhesion, migration and other functions in many cell types.54, 55 Besides its importance in the maintenance of immune homeostasis (strongly inhibiting the production of pro-inflammatory cytokines),54, 55 TGFB1 also has relevant neurodevelopmental effects (for example, trophic effects on midbrain dopaminergic neurons).56, 57 TGFB1 protein has been reported to be elevated in blood from unmedicated schizophrenic cases compared to controls in Korea, with normalization after antipsychotic treatment.58 A small Polish case–control study showed nominal significance (P=0.03) for a functional SNP (T29C; Leu10Pro59) being associated with schizophrenia.60 In the Polish study, TGFB1 protein was also upregulated in schizophrenia cases versus controls,60 consistent with our findings of increased messenger RNA expression in schizophrenia cases.
TGM2 catalyzes the crosslinking of proteins by epsilon-gamma glutamyl lysine isopeptide bonds, appears to be involved in apoptosis and is the autoantigen implicated in celiac disease (gluten-sensitive enteropathy).61 TGM2 SNPs and a haplotype showed nominal association (P~0.0004) to schizophrenia in a family-based study of 131 British family trios,62 though no association was found in a Chinese case–control study.63 TGM2 has been reported (in a patent, US20070015152) to be upregulated in the brain (anterior cingulate cortex) of schizophrenia cases versus controls. Celiac disease has an epidemiological association with schizophrenia; gluten-free diets occasionally reduce psychotic symptoms; and circulating gliadin antibodies are elevated in schizophrenia cases versus controls (reviewed in Wang et al.63).
Neuronal-related differentially expressed gene examples
PIK3CD has been shown to be involved in axonal outgrowth during neurodevelopment.64 The catalytic subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase (that is, PIK3CD)65 was previously reported to have higher expression in human LCLs (β=0.41),66 consistent with our finding (β=0.28). Administration of haloperidol to rats led to specific reductions of PIK3CD brain expression; and furthermore, specific blocking of PIK3CD blocked amphetamine-induced hyperlocomotion (a rodent model of psychosis-like behavior, related to a hyperdopaminergic state) in mice.66 PIK3CD has also been shown to be important for the biochemical function of the NRG1–ERBB4–PI3K signaling pathway, that is, involving two leading candidate genes for schizophrenia (NRG1 and ERBB4).66 Dysregulation of PI3K activity has been implicated in both autism spectrum disorders and schizophrenia.67, 68, 69
PDE4B plays a role in signal transduction by regulating cellular concentrations of cyclic nucleotides (for example, cyclic AMP), and also has a central role in inflammation.70, 71, 72 Studies of Pde4b knockout mice demonstrated decreased striatal dopamine and serotonin activity associated with decreased prepulse inhibition, decreased baseline motor activity and an exaggerated locomotor response to amphetamine.73 There is also a case report of this gene being disrupted by a balanced t(1:16) translocation in a subject diagnosed with schizophrenia and a cousin with chronic psychiatric illness.72 Post-mortem cerebellum showed decreased PDE4B expression in schizophrenics versus controls,74 though other studies have not found differential brain expression.75 Increased expression in peripheral blood has been seen for depression versus controls.76 Several studies have reported nominal association of variants at this gene with schizophrenia,74 with a meta-analysis (Psychiatric Genomics Consortium for schizophrenia) reporting P=~2 × 10−7.10
SHANK2 is a component of molecular scaffolds in the postsynaptic density of excitatory glutamatergic synapses, which have important roles in neurodevelopment and the adult brain.77, 78 A recent sequencing study of SHANK2 in 481 schizophrenia cases and 659 controls found an over-representation of rare (minor allele frequency<0.01) missense SNPs in schizophrenia cases.79 In addition, variants (CNVs and SNPs) in SHANK2 have been associated with autism and mental retardation.78, 80, 81 Mouse mutant lines for SHANK2 (as well as SHANK1 and SHANK3) have shown behavioral changes and dysfunction of glutamatergic synapses.82 SHANK2 is upregulated in brains of Alzheimer’s disorder cases (whereas SHANK1 and SHANK3 are downregulated).81
NDE1 is a member of the nuclear distribution E family of proteins, and plays an essential role in neuronal migration.83 Mutations in NDE1 cause lissencephaly 4, (lissencephaly, severe brain atrophy, microcephaly and severe intellectual deficiency).84 NDE1 interacts with schizophrenia risk genes, most notably, DISC1.85, 86 NDE1 is also within a chromosome 16p13.11 CNV reported as associated with schizophrenia (also for autism, attention deficit hyperactivity disorder, seizures and intellectual deficiency).87, 88, 89 A rare variant in NDE1 has also been implicated by re-sequencing for rare coding variants (finding S214F), association testing (nominal P=0.04), and functional analyses (finding the mutation affected axonal outgrowth and the interaction between NDE1 and the neurodevelopmental regulator, YWHAE).90 In a Finnish schizophrenia family cohort, interaction was reported between NDE1 genotypes and high birth weight increasing schizophrenia susceptibility.91
Support for differentially expressed genes detected by array
There were 15 genes differentially expressed by affection status in both our previous array study (false discovery rate<0.05)16 and the current RNAseq study (Bonferroni P<0.05, all with the same direction of effect, Supplementary Table S4). Notable genes among these repeated findings were: FAM69A, a member of the FAM69A-EVI-RPL5 gene cluster implicated in the autoimmune disorder multiple sclerosis by GWAS92, 93, 94 and which also had some association support in the MGS European ancestry GWAS;6 XBP1, which is a transcription factor known to be a key regulator of MHC class II genes95 and has a functional promoter variant reported as nominally associated with schizophrenia in some studies on Asian samples but not in other studies;96, 97, 98, 99, 100 and SYT11, which is genome-wide significantly associated with Parkinson’s disease.101 We note that the extended MHC region histones found differentially expressed in schizophrenia previously in our array-based study16 were not differentially expressed in our current RNAseq-based transcriptional profiling. This increases the likelihood that the array-based finding for histones was a technical artifact that RNAseq may be better able to avoid, for example, as the sequence similarity within the histone gene family may reduce specificity more for short microarray sequences than on a gene-wide basis as with RNAseq (see discussion in Sanders et al.16).
Meta-analysis of RNAseq and array data sets
As is common with a large data set being studied by rapidly developing technologies, different parts of the MGS data set have been studied for gene expression by different technologies, that is, array and RNAseq. Although array expression studies were more prevalent prior to reductions in RNAseq costs, the advantages of RNAseq over array measurements are many,102, 103 including that RNAseq: (1) detects transcription of unknown transcripts, exons and transcript isoforms (alternatively spliced variants); (2) measures both gene-wide and exon-specific expression levels; (3) assays allele-specific expression; (4) provides sequence information; (5) provides gene expression levels that are highly correlated with measures of absolute expression level (as assayed by quantitative PCR) across a wide dynamic range; and (6) processing of the obtained read counts is less critical and complicated than with array data. Although a number of methods for meta-analysis of array data sets have been developed (reviewed in Xia et al.104), due to the technology differences, direct data merging of array vs RNAseq data sets is impractical, leaving approaches such as combination of P-values, rank orders or votes as meta-analytic options.104 We used an extension of Stouffer’s Z method23 for our meta-analytic approach to combine the RNAseq and array data sets, allowing us to study the 8141 genes expressed in >80% of samples by both methods, and found 647 genes to be differentially expressed by affection status (Supplementary Table S5). Among the 169 new genes from the meta-analysis (but not significant in the primary RNAseq analysis) are several previously discussed in our array paper (B3GNT2, MOXD1, DBNDD2 and S100A10),16 and we also chose to further highlight three additional genes here: VAMP4, PPP3CC and IFITM3.
VAMP4 is a main component of a protein complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane,105 and is critical for neuron outgrowth. A Swedish family-based candidate gene association study found the cSNP rs15655 in the 3′-untranslated region of VAMP4 to be nominally associated in both their discovery (77 trios, two-tailed P=0.004) and replication (190 trios, two-tailed P=0.019 for same allele) samples of suicide attempters.106
PPP3CC is one of the regulatory subunits for calcineurin, which is a calcium-dependent, calmodulin-stimulated protein phosphatase involved in the downstream regulation of dopaminergic signal transduction. PPP3CC is localized to presynaptic terminals in hippocampal neurons, and RNA interference-mediated knockdown disrupts synaptic vesicle cycling.107 PPP3CC has been reported as associated with schizophrenia in families,108 but a number of other studies (for example, Sanders et al.109) have not detected association. Decreased hippocampal expression has been found in 13 schizophrenia cases versus 12 controls.110 In two European ancestry samples, variants in PPP3CC were found to be nominally associated with treatment-resistant depression, antidepressant treatment response and remission (P-values ranging from 0.04 to 0.0002), and some of these effects were suggested to be through the B-cell receptor signaling pathway via pathway analysis (permutated P=0.03).111
Like IFITM1 (Supplementary Table S2), IFITM3 was also found to be upregulated in post-mortem brain (hippocampus and dorsolateral prefrontal cortex) from schizophrenic cases versus controls.112, 113 In a larger study (55 cases and 55 controls), prefrontal cortex showed increased IFITM3 expression via array in schizophrenia (P<0.01).114 Of relevance for some immunological hypotheses of schizophrenia risk, mouse studies have suggested that IFITM3 expression is a critical mediator of maternal immune activation.115, 116
Immune-related genes
We continue to study LCLs due to their sample size and quality, tractability and substantial overlap with brain expression, coupled with the enhanced possibility of detecting peripheral biomarkers for schizophrenia and their relevance as a model to study immune contributions to schizophrenia, which has been noted previously.16 Briefly, several lines of evidence support a substantial immunological contribution to schizophrenia risk: (1) family history of autoimmune disease is associated with increased schizophrenia risk, and autoimmune disorders modify schizophrenia risk.27, 28 (2) Prospective birth cohort studies with serologically documented gestational infection and immune biomarkers show that specific infections increase the risk of schizophrenia in the offspring.29, 30 (3) GWAS have shown the strongest association at the extended MHC region,10 which is associated with many immune, inflammatory and infectious disorders.117 (4) The Psychiatric Genomics Consortium for schizophrenia recently mapped 108 genome-wide significantly associated loci onto sequences with epigenetic markers characteristic of active enhancers and found strong enrichment at enhancers active in tissues with important immune functions, particularly B-lymphocyte lineages involved in acquired immunity (CD19 and CD20 lines), which remained significant even after excluding the extended MHC region and regions containing brain enhancers.10 Our GSEA showed an enrichment of immune-related genes in the differentially expressed genes (as a % of analyzed genes: 31% vs 13%), and this enrichment was more pronounced in the upregulated vs downregulated genes (34% vs 25%). Our directional GSEA finding that multiple immune categories (response, activation and regulation) were enriched in the 697 upregulated genes (Table 2), but not the 361 downregulated genes (Supplementary Table S3), is consistent with most of the lines of evidence pointing towards immune activation contributing to schizophrenia (see discussions, Schizophrenia Working Group of the Psychiatric Genomics Consortium,10 Eaton et al.,27 Eaton et al.,28 Brown and Derkits,29 and Brown30).
Caveats and limitations
The tissue to study is always a challenge for psychiatric disorders due to limited access to brain or neural tissue; we chose to study LCLs, that is, B-cells transformed by EBV. The EBV transformation itself may influence gene expression, although we attempted to minimize some (for example, by using LCLs only transformed at one site by a unified protocol) and to adjust for other aspects (such as including EBV load as a nuisance parameter in our regression models). Some genes are regulated differently in LCLs than in brain, and other brain expressed genes are not detectably expressed in LCLs and hence not assayed here. However, many genes expressed in the brain are expressed also in LCLs, and LCLs present other advantages including: availability of large numbers (MGS European ancestry sample, a widely shared repository sample, with GWAS genotypes available), ease of experimental manipulation, living tissue, high-quality RNA, far removed from environmental ‘state’ influences (for example, diet, diurnal rhythms, exercise and medications), and arguably a particular suitability for the study of immune/infection hypotheses of schizophrenia. Other limitations of the current study include the exclusive focus on European ancestry samples (though that made it more comparable to the previous array-based study), lack of stronger correlation between array- and RNAseq-based results, analysis limited to gene expression, and focus on the full distribution (means) of expression (though please refer to our separate study focusing on extreme upper and lower tails of the expression distribution, that is, outliers118).
References
APA Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th edn. American Psychiatric Association: Washington, DC, USA, 2000.
Brown AS . The environment and susceptibility to schizophrenia. Prog Neurobiol 2011; 93: 23–58.
Gejman PV, Sanders AR, Duan J . The role of genetics in the etiology of schizophrenia. Psychiatr Clin North Am 2010; 33: 35–66.
Khandaker GM, Dantzer R . Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology 2016; 233: 1559–1573.
Cox D, Chan MK, Bahn S . The potential of immune biomarkers to advance personalized medicine approaches for schizophrenia. J Nerv Ment Dis 2015; 203: 393–399.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460: 753–757.
Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. Common variants conferring risk of schizophrenia. Nature 2009; 460: 744–747.
Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet 2011; 43: 969–976.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74.
Gamazon ER, Nicolae DL, Cox NJ . A study of CNVs as trait-associated polymorphisms and as expression quantitative trait loci. PLoS Genet 2011; 7: e1001292.
Gamazon ER, Badner JA, Cheng L, Zhang C, Zhang D, Cox NJ et al. Enrichment of cis-regulatory gene expression SNPs and methylation quantitative trait loci among bipolar disorder susceptibility variants. Mol Psychiatry 2013; 18: 340–346.
Below JE, Gamazon ER, Morrison JV, Konkashbaev A, Pluzhnikov A, McKeigue PM et al. Genome-wide association and meta-analysis in populations from Starr County, Texas, and Mexico City identify type 2 diabetes susceptibility loci and enrichment for expression quantitative trait loci in top signals. Diabetologia 2011; 54: 2047–2055.
Richards AL, Jones L, Moskvina V, Kirov G, Gejman PV, Levinson DF et al. Schizophrenia susceptibility alleles are enriched for alleles that affect gene expression in adult human brain. Mol Psychiatry 2012; 17: 193–201.
Sanders AR, Goring HH, Duan J, Drigalenko EI, Moy W, Freda J et al. Transcriptome study of differential expression in schizophrenia. Hum Mol Genet 2013; 22: 5001–5014.
Sanders AR, Levinson DF, Duan J, Dennis JM, Li R, Kendler KS et al. The internet-based MGS2 control sample: self report of mental illness. Am J Psychiatry 2010; 167: 854–865.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N et al. The sequence alignment/Map format and SAMtools. Bioinformatics 2009; 25: 2078–2079.
Choy E, Yelensky R, Bonakdar S, Plenge RM, Saxena R, De Jager PL et al. Genetic analysis of human traits in vitro: drug response and gene expression in lymphoblastoid cell lines. PLoS Genet 2008; 4: e1000287.
Trapnell C, Pachter L, Salzberg SL . TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009; 25: 1105–1111.
Langmead B, Trapnell C, Pop M, Salzberg SL . Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 2009; 10: R25.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010; 28: 511–515.
Darlington RB, Hayes AF . Combining independent p values: extensions of the Stouffer and binomial methods. Psychol Methods 2000; 5: 496–515.
R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2011.
Huang, da W, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57.
Rossin EJ, Lage K, Raychaudhuri S, Xavier RJ, Tatar D, Benita Y et al. Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet 2011; 7: e1001273.
Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006; 163: 521–528.
Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB . Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord 2010; 12: 638–646.
Brown AS, Derkits EJ . Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167: 261–280.
Brown AS . Exposure to prenatal infection and risk of schizophrenia. Front Psychiatry 2011; 2: 63.
Lin M, Zhao D, Hrabovsky A, Pedrosa E, Zheng D, Lachman HM . Heat shock alters the expression of schizophrenia and autism candidate genes in an induced pluripotent stem cell model of the human telencephalon. PLoS ONE 2014; 9: e94968.
Huan T, Esko T, Peters MJ, Pilling LC, Schramm K, Schurmann C et al. A meta-analysis of gene expression signatures of blood pressure and hypertension. PLoS Genet 2015; 11: e1005035.
Kippe JM, Mueller TM, Haroutunian V, Meador-Woodruff JH . Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia. Schizophr Res 2015; 166: 219–224.
Caliskan M, Cusanovich DA, Ober C, Gilad Y . The effects of EBV transformation on gene expression levels and methylation profiles. Hum Mol Genet 2011; 20: 1643–1652.
Muller N, Weidinger E, Leitner B, Schwarz MJ . The role of inflammation in schizophrenia. Front Neurosci 2015; 9: 372.
Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H . Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2010; 71: 520–527.
Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS . Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 2014; 40: 181–191.
Muller N, Schwarz MJ . Immune system and schizophrenia. Curr Immunol Rev 2010; 6: 213–220.
O'Sullivan D, Green L, Stone S, Zareie P, Kharkrang M, Fong D et al. Treatment with the antipsychotic agent, risperidone, reduces disease severity in experimental autoimmune encephalomyelitis. PLoS ONE 2014; 9: e104430.
Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O . Neuroinflammation and psychiatric illness. J Neuroinflammation 2013; 10: 43.
Heppner FL, Ransohoff RM, Becher B . Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015; 16: 358–372.
Chen WW, Zhang X, Huang WJ . Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 2016; 13: 3391–3396.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530: 177–183.
Mayilyan KR . Complement genetics, deficiencies, and disease associations. Protein Cell 2012; 3: 487–496.
Liu D, Niu ZX . The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35). Immunopharmacol Immunotoxicol 2009; 31: 524–535.
Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F et al. Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol 2011; 11: 10.
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet 2009; 41: 1094–1099.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013; 45: 1452–1458.
Mayilyan KR, Weinberger DR, Sim RB . The complement system in schizophrenia. Drug News Perspect 2008; 21: 200–210.
Fourgeaud L, Boulanger LM . Synapse remodeling, compliments of the complement system. Cell 2007; 131: 1034–1036.
Michailidou I, Willems JG, Kooi EJ, van Eden C, Gold SM, Geurts JJ et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol 2015; 77: 1007–1026.
Hakobyan S, Boyajyan A, Sim RB . Classical pathway complement activity in schizophrenia. Neurosci Lett 2005; 374: 35–37.
Ni J, Hu S, Zhang J, Tang W, Lu W, Zhang C . A preliminary genetic analysis of complement 3 gene and schizophrenia. PLoS ONE 2015; 10: e0136372.
Zhang L, Yi H, Xia XP, Zhao Y . Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity 2006; 39: 269–276.
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA . Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006; 24: 99–146.
Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K . TGF-beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J 1995; 14: 736–742.
Krieglstein K, Unsicker K . Transforming growth factor-beta promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience 1994; 63: 1189–1196.
Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 1129–1134.
Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV . Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation 1998; 66: 1014–1020.
Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P et al. Sex differences in TGFB-beta signaling with respect to age of onset and cognitive functioning in schizophrenia. Neuropsychiatr Dis Treat 2015; 11: 575–584.
Sollid LM . Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2: 647–655.
Bradford M, Law MH, Stewart AD, Shaw DJ, Megson IL, Wei J . The TGM2 gene is associated with schizophrenia in a British population. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 335–340.
Wang J, Liu Y, Wang Z, Du W, Hui L, Zhao X et al. Lack of genetic association of the TGM2 gene with schizophrenia in a Chinese population. Psychiatr Genet 2015; 25: 259–262.
Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML et al. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS ONE 2007; 2: e869.
Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA 1997; 94: 4330–4335.
Law AJ, Wang Y, Sei Y, O'Donnell P, Piantadosi P, Papaleo F et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110delta inhibition as a potential therapeutic strategy. Proc Natl Acad Sci USA 2012; 109: 12165–12170.
Levitt P, Campbell DB . The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest 2009; 119: 747–754.
Waite K, Eickholt BJ . The neurodevelopmental implications of PI3K signaling. Curr Top Microbiol Immunol 2010; 346: 245–265.
Gross C, Bassell GJ . Neuron-specific regulation of class I PI3K catalytic subunits and their dysfunction in brain disorders. Front Mol Neurosci 2014; 7: 12.
Wang P, Wu P, Ohleth KM, Egan RW, Billah MM . Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol 1999; 56: 170–174.
Azam MA, Tripuraneni NS . Selective phosphodiesterase 4B inhibitors: a review. Sci Pharm 2014; 82: 453–481.
Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005; 310: 1187–1191.
Siuciak JA, McCarthy SA, Chapin DS, Martin AN . Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology 2008; 197: 115–126.
Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S et al. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res 2008; 101: 36–49.
Rastogi A, Zai C, Likhodi O, Kennedy JL, Wong AH . Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res 2009; 114: 39–49.
Numata S, Iga J, Nakataki M, Tayoshi S, Taniguchi K, Sumitani S et al. Gene expression and association analyses of the phosphodiesterase 4B (PDE4B) gene in major depressive disorder in the Japanese population. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 527–534.
Sheng M, Kim E . The Shank family of scaffold proteins. J Cell Sci 2000; 113 (Pt 11): 1851–1856.
Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED . ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem 2002; 81: 903–910.
Peykov S, Berkel S, Schoen M, Weiss K, Degenhardt F, Strohmaier J et al. Identification and functional characterization of rare SHANK2 variants in schizophrenia. Mol Psychiatry 2015; 20: 1489–1498.
Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 2010; 42: 489–491.
Guilmatre A, Huguet G, Delorme R, Bourgeron T . The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 2014; 74: 113–122.
Schmeisser MJ . Translational neurobiology in Shank mutant mice—model systems for neuropsychiatric disorders. Ann Anat 2015; 200: 115–117.
Feng Y, Walsh CA . Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 2004; 44: 279–293.
Alkuraya FS, Cai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected]. Am J Hum Genet 2011; 88: 536–547.
Hennah W, Tomppo L, Hiekkalinna T, Palo OM, Kilpinen H, Ekelund J et al. Families with the risk allele of DISC1 reveal a link between schizophrenia and another component of the same molecular pathway, NDE1. Hum Mol Genet 2007; 16: 453–462.
Bradshaw NJ, Christie S, Soares DC, Carlyle BC, Porteous DJ, Millar JK . NDE1 and NDEL1: multimerisation, alternate splicing and DISC1 interaction. Neurosci Lett 2009; 449: 228–233.
Ingason A, Rujescu D, Cichon S, Sigurdsson E, Sigmundsson T, Pietilainen OP et al. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry 2011; 16: 17–25.
Liu JY, Kasperaviciute D, Martinian L, Thom M, Sisodiya SM . Neuropathology of 16p13.11 deletion in epilepsy. PLoS One 2012; 7: e34813.
Tropeano M, Ahn JW, Dobson RJ, Breen G, Rucker J, Dixit A et al. Male-biased autosomal effect of 16p13.11 copy number variation in neurodevelopmental disorders. PLoS ONE 2013; 8: e61365.
Kimura H, Tsuboi D, Wang C, Kushima I, Koide T, Ikeda M et al. Identification of rare, single-nucleotide mutations in NDE1 and their contributions to schizophrenia susceptibility. Schizophr Bull 2015; 41: 744–753.
Wegelius A, Pankakoski M, Tomppo L, Lehto U, Lonnqvist J, Suvisaari J et al. An interaction between NDE1 and high birth weight increases schizophrenia susceptibility. Psychiatry Res 2015; 230: 194–199.
Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL . The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet 2008; 9: 516–526.
Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007; 357: 851–862.
Hoppenbrouwers IA, Aulchenko YS, Ebers GC, Ramagopalan SV, Oostra BA, van Duijn CM et al. EVI5 is a risk gene for multiple sclerosis. Genes Immun 2008; 9: 334–337.
He Y, Sun S, Sha H, Liu Z, Yang L, Xue Z et al. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr 2010; 15: 13–25.
Chen W, Duan S, Zhou J, Sun Y, Zheng Y, Gu N et al. A case-control study provides evidence of association for a functional polymorphism -197C/G in XBP1 to schizophrenia and suggests a sex-dependent effect. Biochem Biophys Res Commun 2004; 319: 866–870.
Kakiuchi C, Ishiwata M, Umekage T, Tochigi M, Kohda K, Sasaki T et al. Association of the XBP1-116C/G polymorphism with schizophrenia in the Japanese population. Psychiatry Clin Neurosci 2004; 58: 438–440.
Jonsson EG, Cichon S, Schumacher J, Abou Jamra R, Schulze TG, Deschner M et al. Association study of a functional promoter polymorphism in the XBP1 gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 71–75.
Watanabe Y, Fukui N, Muratake T, Amagane H, Kaneko N, Nunokawa A et al. Association study of a functional promoter polymorphism of the X-box binding protein 1 gene in Japanese patients with schizophrenia. Psychiatry Clin Neurosci 2006; 60: 633–635.
Carter CJ . eIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull 2007; 33: 1343–1353.
Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson's disease genetics: the PDGene database. PLoS Genet 2012; 8: e1002548.
Pickrell JK, Marioni JC, Pai AA, Degner JF, Engelhardt BE, Nkadori E et al. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature 2010; 464: 768–772.
Majewski J, Pastinen T . The study of eQTL variations by RNA-seq: from SNPs to phenotypes. Trends Genet 2011; 27: 72–79.
Xia J, Fjell CD, Mayer ML, Pena OM, Wishart DS, Hancock RE . INMEX—a web-based tool for integrative meta-analysis of expression data. Nucleic Acids Res 2013; 41 (Web Server issue): W63–W70.
Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH . SNAREs contribute to the specificity of membrane fusion. Neuron 2000; 26: 457–464.
Wasserman D, Geijer T, Rozanov V, Wasserman J . Suicide attempt and basic mechanisms in neural conduction: relationships to the SCN8A and VAMP4 genes. Am J Med Genet B Neuropsychiatr Genet 2005; 133B: 116–119.
Cottrell JR, Li B, Kyung JW, Ashford CJ, Mann JJ, Horvath TL et al. Calcineurin agamma is a functional phosphatase that modulates synaptic vesicle endocytosis. J Biol Chem 2016; 291: 1948–1956.
Gerber DJ, Hall D, Miyakawa T, Demars S, Gogos JA, Karayiorgou M et al. Evidence for association of schizophrenia with genetic variation in the 8p21.3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proc Natl Acad Sci USA 2003; 100: 8993–8998.
Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry 2008; 165: 497–506.
Eastwood SL, Burnet PW, Harrison PJ . Decreased hippocampal expression of the susceptibility gene PPP3CC and other calcineurin subunits in schizophrenia. Biol Psychiatry 2005; 57: 702–710.
Fabbri C, Marsano A, Albani D, Chierchia A, Calati R, Drago A et al. PPP3CC gene: a putative modulator of antidepressant response through the B-cell receptor signaling pathway. Pharmacogenomics J 2014; 14: 463–472.
Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry 2013; 3: e321.
Arion D, Unger T, Lewis DA, Levitt P, Mirnics K . Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62: 711–721.
Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E . Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 2007; 7: 46.
Horvath S, Mirnics K . Immune system disturbances in schizophrenia. Biol Psychiatry 2014; 75: 316–323.
Ibi D, Nagai T, Nakajima A, Mizoguchi H, Kawase T, Tsuboi D et al. Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice. Glia 2013; 61: 679–693.
Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK et al. Gene map of the extended human MHC. Nat Rev Genet 2004; 5: 889–899.
Duan J, Sanders AR, Moy W, Drigalenko EI, Brown EC, Freda J et al. Transcriptome outlier analysis implicates schizophrenia susceptibility genes and enriches putatively functional rare genetic variants. Hum Mol Genet 2015; 24: 4674–4685.
Acknowledgements
We thank the GAIN quality control team (GR Abecasis and J Paschall) for making important contributions to the project; S Purcell for assistance with PLINK; personnel at Rutgers University Cell and DNA Repository (JA Tischfield and DA Fugman) for advice and assistance with the LCLs; D He at NorthShore University HealthSystem for technical assistance; T Lehner and G Senthil at the National Institute of Mental Health for their support and scientific advice; MGS, which includes PV Gejman, AR Sanders, J Duan (NorthShore University HealthSystem, and University of Chicago, IL, USA), DF Levinson (Stanford University, CA, USA), J Shi (National Cancer Institute, MD, USA), NG Buccola (Louisiana State University Health Sciences Center, LA, USA), BJ Mowry (Queensland Centre for Mental Health Research, Brisbane and Queensland Brain Institute, The University of Queensland, Australia), R Freedman, A Olincy (University of Colorado Denver, CO, USA), F Amin (Atlanta Veterans Affairs Medical Center and Emory University, GA, USA), DW Black (University of Iowa Carver College of Medicine, IA, USA), JM Silverman (Icahn School of Medicine at Mount Sinai, NY, USA), WF Byerley (University of California at San Francisco, CA, USA), CR Cloninger, DM Svrakic (Washington University, MO, USA); and the study participants and the research staff at the study sites. This work was supported primarily by the National Institutes of Health (NIH grants RC2MH090030 to ARS, R01MH094116 to HHHG and R01MH094091 to PVG); as well as for MGS by NIH grants (R01MH067257 to NGB, R01MH059588 to BJM, R01MH059571 to PVG, R01MH059565 to RF, R01MH059587 to FA, R01MH060870 to WFB, R01MH059566 to DWB, R01MH059586 to JMS, R01MH061675 to DFL, R01MH060879 to CRC, R01MH081800 to PVG, U01MH046276 to CRC, U01MH046289 to C Kaufmann, U01MH046318 to MT Tsuang, U01MH079469 to PVG and U01MH079470 to DFL), the Genetic Association Information Network (GAIN, for genotyping of half of the European ancestry sample and almost all the African American sample) and The Paul Michael Donovan Charitable Foundation. Genotyping was carried out by the Center for Genotyping and Analysis at the Broad Institute of Harvard and MIT (SG and DBM) is supported by NIH grant U54RR020278. Analyses done in San Antonio (EID and HHHG) were conducted in facilities constructed with support from NIH grant RR017515 and a gift from the AT&T Foundation.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sanders, A., Drigalenko, E., Duan, J. et al. Transcriptome sequencing study implicates immune-related genes differentially expressed in schizophrenia: new data and a meta-analysis. Transl Psychiatry 7, e1093 (2017). https://doi.org/10.1038/tp.2017.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.47
This article is cited by
-
Identification of Immune-Linked Hub Genes and Diagnostic Model Construction in Schizophrenia
Journal of Molecular Neuroscience (2023)
-
What genes are differentially expressed in individuals with schizophrenia? A systematic review
Molecular Psychiatry (2022)
-
Schizophrenia risk loci from xMHC region were associated with antipsychotic response in chronic schizophrenic patients with persistent positive symptom
Translational Psychiatry (2022)
-
LncRNA RP5-998N21.4 promotes immune defense through upregulation of IFIT2 and IFIT3 in schizophrenia
Schizophrenia (2022)
-
Peripheral NF-κB dysregulation in people with schizophrenia drives inflammation: putative anti-inflammatory functions of NF-κB kinases
Translational Psychiatry (2022)