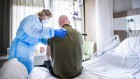
Clinical research has been disrupted as hospitals devote more resources to caring for people critically ill with COVID-19.Credit: Alberto Pizzoli/AFP/Getty
When 2020 began, Neena Nizar and her family were poised to harvest the fruit of a decade of hard work and sacrifice: a clinical trial of an experimental treatment for her two sons’ rare genetic disorder that was slated to start before the year’s end.
“I can’t even put into words what we’ve been able to do to get to this point,” she says. “My kids have given bone biopsies; I gave up my job and moved to a new country. We’ve just been going, going, going.”
Then came COVID-19. Now, Nizar wipes away tears in her Nebraska home as she reads a message from researchers at the US National Institutes of Health. Work to assess the toxicity of the experimental therapy in animals has stalled because laboratories have been forced to close. The same might be true, she has heard, of the firm hired to manufacture the drug for clinical testing.
Latest news on the coronavirus
Nizar’s sons have a painful degenerative disorder called Jansen’s disease, which has hampered their bodies’ ability to regulate calcium and phosphate, causing kidney damage and bone deformations. Her older son, who is 11, has had at least one operation every year for the past five years, and Nizar knows that the longer he has to wait to receive the experimental treatment, the less likely it is to work.
“My son asks me all the time, ‘So when are we doing this trial? When can I? I don’t want to feel this pain anymore,’” she says. “I feel like we were chugging along on a train and then somebody dropped a huge boulder on it.”
Trials suspended
Scientists are rushing to launch clinical trials of experimental vaccines against the coronavirus, and treatments for COVID-19. But as hospitals brace for an onslaught of critically ill patients and laboratories worldwide are disrupted, researchers have had to shelve clinical trials of therapies for other illnesses.
“We’re going to see a nearly complete close-down in clinical research,” says Tim Dyer, chief executive of Addex Therapeutics, a biotechnology company based in Geneva, Switzerland. “The health-care systems will simply be overloaded.” On 18 March, Addex announced that it would delay the start of a clinical trial to treat involuntary movements in people with Parkinson’s disease.
The following week, the pharmaceutical giant Eli Lilly in Indianapolis, Indiana, announced that it would halt enrolment in ongoing studies and delay the launch of new trials. “The COVID-19 situation is dynamic,” a spokesperson for Swiss pharmaceutical company Roche in Basel told Nature. “We are now seeing impacts on clinical-trial continuity in all the regions where we conduct clinical studies.”
At Yale University in New Haven, Connecticut, lung-cancer researcher Roy Herbst says clinical trials for cancer have been cut to “almost zero” and are allowed only when a participant is deemed to have exceptional need.
The coronavirus pandemic in five powerful charts
“It’s hard to believe that just a month ago, I’d never seen cancer clinical trials better,” says Herbst, citing a list of experimental treatments that were showing promise against some of the deadliest lung cancers. “Now the whole process has really ground to a halt, and I feel bad because there are patients who might have benefited from those trials.”
But the measures are necessary, he adds. Many people with advanced cancer are vulnerable to infection, and trips to the clinic for treatments and assessments could be deadly if patients are exposed to the coronavirus. Some cancer treatments weaken the immune system, and advanced treatments that use genetically modified cells require intensive medical monitoring — something that might not be possible in the middle of the outbreak.
Herbst has had to ask three-quarters of his colleagues in the oncology department at Yale to stay away from the hospital to minimize their risk of infection. Instead, they are held in reserve to treat people with COVID-19 in case the first round of clinicians become infected. Even routine procedures such as biopsies, sometimes required for enrolment in a clinical trial, are now difficult to obtain as hospitals struggle with personnel and equipment shortages.
Agencies adapt
Government agencies have released guidance for investigators who need to suspend or modify trials. The US Food and Drug Administration, for example, has issued guidance for trials that might have to pause, change their study plans or make do with incomplete data because of the COVID-19 pandemic. Ethics committees are working overtime as researchers file requests to alter their clinical-trial plans in ways that minimize how often participants need to venture into the clinic, says Barbara Bierer, who directs the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard in Boston, Massachusetts.
Why does the coronavirus spread so easily between people?
Agencies and clinical-trial funders have shown remarkable flexibility, says Charles Blanke, an oncologist at Oregon Health & Science University in Portland and leader of the publicly funded SWOG Cancer Research Network. The US National Cancer Institute announced on 23 March that it would allow the investigators it funds to assess trial participants' health remotely where possible. Some doctors’ assessments may be carried out over video calls instead of in person, and some audits of clinical-trial procedures will be conducted virtually, with inspectors examining the paperwork online rather than physically visiting the clinic to assess standards.
The rapid release of these guidelines is a particular relief because many clinical-trial sites did not plan for a pandemic such as COVID-19, says Blanke, despite warnings from experts that an outbreak was inevitable. “Did we do enough thinking in advance in terms of how research would be affected? My answer is no, we didn’t,” he says. “We never even talked about it.” But after this outbreak, he says, clinical researchers will be better prepared, and the increased emphasis on and capacity for virtual visits will be a lasting boon to both researchers and patients.
Uncertain future
For now, it’s unclear what long-term effects the outbreak will have on drug regulation. “There will be a disruption, obviously,” says Bierer. “And whether that delay manifests in delaying final approvals is unknowable today.”
It’s that uncertainty that haunts Nizar. She worries that her concerns might sound selfish in the face of the global suffering caused by the pandemic. But she also knows that the delay to her clinical trial could last well beyond the months of social isolation and lockdowns.
Her best hope now, she says, is that regulators will learn from the speed and urgency with which a candidate vaccine for the virus that causes COVID-19 has been rushed into clinical trials, forgoing some of the pre-trial animal tests that regulators typically require. Nizar wants to see therapies for rare diseases treated with the same urgency.
“Our lives have always been in panic mode,” she says. “Now the world has a glimpse into what our reality is.”

 Covert coronavirus infections could be seeding new outbreaks
Covert coronavirus infections could be seeding new outbreaks
 Coronavirus vaccines: five key questions as trials begin
Coronavirus vaccines: five key questions as trials begin
 The race to unravel the biggest coronavirus outbreak in the United States
The race to unravel the biggest coronavirus outbreak in the United States
 How Facebook and Twitter could be the next disruptive force in clinical trials
How Facebook and Twitter could be the next disruptive force in clinical trials