Abstract
Bile acids, once considered mere dietary surfactants, now emerge as critical modulators of macronutrient (lipid, carbohydrate, protein) metabolism and the systemic pro-inflammatory/anti-inflammatory balance. Bile acid metabolism and signaling pathways play a crucial role in protecting against, or if aberrant, inducing cardiometabolic, inflammatory, and neoplastic conditions, strongly influencing health and disease. No curative treatment exists for any bile acid influenced disease, while the most promising and well-developed bile acid therapeutic was recently rejected by the FDA. Here, we provide a bottom-up approach on bile acids, mechanistically explaining their biochemistry, physiology, and pharmacology at canonical and non-canonical receptors. Using this mechanistic model of bile acids, we explain how abnormal bile acid physiology drives disease pathogenesis, emphasizing how ceramide synthesis may serve as a unifying pathogenic feature for cardiometabolic diseases. We provide an in-depth summary on pre-existing bile acid receptor modulators, explain their shortcomings, and propose solutions for how they may be remedied. Lastly, we rationalize novel targets for further translational drug discovery and provide future perspectives. Rather than dismissing bile acid therapeutics due to recent setbacks, we believe that there is immense clinical potential and a high likelihood for the future success of bile acid therapeutics.
Similar content being viewed by others
Introduction
Globally, cardiometabolic diseases present a substantial health risk, positively correlating with most, if not all of the top CDC-listed causes of American death.1 The prevalence of these conditions has increased rapidly from an American morbidity of 37.5% in 2011 to 41.8% in 2018, primarily comprised of type 2 diabetes mellitus (T2DM), obesity, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and atherosclerotic cardiovascular disease (ASCVD).2,3 Alongside cardiometabolic diseases, non-metabolic conditions such as inflammatory bowel disease (IBD) and cancer have also been realized as intricate challenges to human health. Despite the apparent heterogeneity, these diseases share the commonality of complex biochemistry, all lack fully deterministic models for disease pathogenesis, and have no well-defined curative treatments. This shared complexity imposes a significant burden on humanity, diminishing quality of life and leading to mortality. Novel treatments are imperative to both better control and potentially cure these diseases.
Bile acids (BAs) are hepatically synthesized cholesterol derivatives that function as amphipathic surfactants and systemic endocrine hormones. Alongside regulating their own synthesis and enterohepatic circulation, BAs are potent modulators of macronutrient (lipid, carbohydrate, protein) metabolism and the systemic pro-inflammatory/anti-inflammatory balance. BAs provide complex physiological modulation by binding to Farnesoid X Receptor (FXR) and Takeda G Protein-Coupled Receptor 5 (TGR5), the canonical BA receptors, while exerting effects at other more recently characterized non-canonical BA receptors.4,5 Alterations in BA physiology are directly correlated to the pathogenesis of cardiometabolic, inflammatory, and neoplastic diseases.6 Hence, the complex role of BAs on physiology provides ample targets for drug discovery.
The most developed BA therapeutic is the FXR agonist candidate Obeticholic Acid (OCA) by Intercept Pharmaceuticals. Although already approved for primary biliary cholangitis (PBC) at lower doses, it was rejected for NASH indication approval by the FDA due to significant dose-dependent toxicities.7 Rather than dismissing BA therapeutics due to recent setbacks, we believe that there is immense clinical potential and a high likelihood for the future success of BA therapeutics. In this review, we provide a bottom-up approach on BAs, mechanistically explaining their biochemistry, physiology, and pharmacology at canonical and non-canonical receptors. Using this mechanistic model of BAs, we explain how abnormal BA physiology drives disease pathogenesis, emphasizing how ceramide synthesis may serve as a unifying pathogenic feature for cardiometabolic diseases. We provide an in-depth summary on pre-existing BA receptor modulators, explaining their shortcomings and theorizing how they be remedied. Lastly, we rationalize novel targets for further translational drug discovery and provide future perspectives.
BA biochemistry
Primary BA synthesis
Pathway overviews
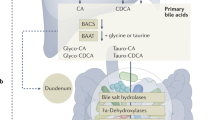
Hepatic BA synthesis tends to follow a common four-stage pattern: 7α-hydroxylation initiation, sterol ring modification, side chain truncation, and phase II conjugation (Fig. 1).8 Each of the previously mentioned metabolic steps takes place in the: endoplasmic reticulum (ER), cytosol, mitochondria, and peroxisome, respectively.9 There are two parallel metabolic pathways, the classical pathway and the alternative pathway, which both perform the first three steps of BA synthesis but use the same enzymes for phase II conjugation. ~90% of human BAs and ~75% of mice BAs are products of the classical pathway. The remaining ~10% in humans and ~25% in mice are synthesized via the alternative pathway, otherwise known as the acidic pathway.10 Metabolic flux through the alternative pathway has been correlated with the upregulation of Cytochrome P450 Family 7 Subfamily B Member 1 (CYP7B1) expression in response to adaptive physiological responses, such as those in response to liver disease, a high fat (HF)/high cholesterol (HC) diet, or cold exposure.11,12,13
Biochemical synthesis and maturation of BAs. Schematic diagram depicting the biochemical synthesis of BAs. A prefix of G represents a glycine conjugate, while a prefix of T represents a taurine conjugate. Far left and far right labels describe the enzymatic reactions shown parallel within the tree. Top numbering keeps track of which conjugated primary BAs become which secondary BAs. This figure was created with BioRender.com
The classical pathway of BA synthesis
The classical pathway for BA synthesis begins with 7α-hydroxylation initiation of cholesterol through the pathway’s rate-limiting enzyme Cytochrome P450 Family 7 Subfamily A Member 1 (CYP7A1).14 For the first branch of the classical pathway, following initiation, C3, C4, and C5 are oxidized to a 4α,5β-enone by 3-Beta-Hydroxysteroid Dehydrogenase Type 7 (HSD3β7) and are 12α-hydroxylated by Cytochrome P450 Family 8 Subfamily B Member 1 (CYP8B1). Subsequently, the A ring is reduced in the 5β and 3β positions by Aldo-keto Reductase Family 1 Member D1 (AKR1D1) and Aldo-keto Reductase Family 1 Member C4 (AKR1C4), respectively. Lastly, C27 is oxidized three times into an alcohol, aldehyde, and eventually a carboxylic acid by Cytochrome P450 Family 27 Subfamily A Member 1 (CYP27A1). This first metabolic branch produces Trihydroxycholestanoic Acid (THCA), the precursor for the BA Cholic Acid (CA). In comparison, after CYP7A1 metabolism, BAs may partake in a second metabolic branch that skips 12α-hydroxylation by CYP8B1. Instead, 7α-hydroxycholesterol is metabolized by HSD3β7, AKR1D1, AKR1C4, and CYP27A1 to produce Dihydroxycholestanoic Acid (DHCA), the precursor for the BA Chenodeoxycholic Acid (CDCA).15
The alternative pathway of BA synthesis
The alternative pathway for BA synthesis begins with C27 oxidation of cholesterol to a spectrum of alcohol, aldehyde, and carboxylic acid metabolites by CYP27A1. Subsequently, the alternative pathway 7α-hydroxylates with CYP7B1, the alternative pathway analogous enzyme to CYP7A1.14 The remainder of the alternative pathway is identical only to the DHCA-synthetic branch of the classical pathway: HSD3β7, AKR1D1, AKR1C4, and CYP27A1.14,15 However, the final CYP27A1 mediated step for the alternative pathway is different compared to the classical pathway, in which only one or two oxidations are performed to complete the oxidation of C27 into a carboxylic acid.14
Convergent synthesis—BA conjugation
Convergently, both the classical and alternative pathways meet at the common conjugation phase of synthesis. The carboxylates of THCA and DHCA are activated to form thioesters with Coenzyme-A (CoA) by BA-CoA Synthase (BACS) and are C25 epimerized from R to S stereochemistry by α-Methylacyl-CoA Racemase (AMACR). The thioester is α,β desaturated at C24 and C25 by Acyl-CoA Oxidase 2 (ACOX2), is oxidized to a β-keto thioester by D-Bifunctional Protein (HSD17β4), and is thiolysed by Peroxisomal Thiolase 2 (SCP2) to release propionyl-CoA.15 These last three reactions heavily mimic the β-oxidation of odd-numbered fatty acids: desaturation, β-keto thioester formation, and thiolysis. The resulting CA-CoA and CDCA-CoA are then C24 phase II conjugated by BA-CoA:amino acid N-acetyltransferase (BAT) to produce C24 glycine or taurine conjugated BAs in humans or only taurine conjugates in rodents.16 In small quantities sulfation at C3 or C6 by Sulfotransferase Family 2 A Member 1 (SULT2A1) or Family 2B Member 8 (SULT2B8) may occur. In addition, small quantities of glucuronidated metabolites at C3, C6, or C24 by UDP-Glucuronosyltransferase Family 1 Member A3 (UGT1A3), Family 2 Member B4 (UGT2B4), or Family 2 Member B7 (UGT2B7) may occur.17,18 Products synthesized after 7α-hydroxylation initiation, sterol ring modification, side chain truncation, and phase II conjugation are denoted as primary conjugated BAs: glycine/taurine conjugates of CA and CDCA in the classical pathway and only CDCA in the alternative pathway.19 CDCA may be further metabolized by Cytochrome P450 Family 3 Subfamily A Member 4 (CYP3A4) to produce Hyocholic Acid (HCA), alternatively known as γ-Muricholic Acid (γMCA), in trace quantities in humans and substantial quantities in rodents. Only rodents metabolize CDCA with Cytochrome P450 Family 2 Subfamily C Member 70 (CYP2C70) to produce α-Muricholic Acid (αMCA), an intermediate whose 7α-alcohol is further epimerized by CYP2C70 to 7β to produce β-Muricholic Acid (βMCA).20,21,22,23
Microbial maturation of primary BAs to secondary BAs
A brief overview of BA maturation
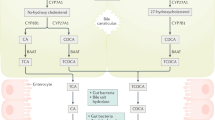
Enterohepatic circulation and gut exposure provide ample opportunities for BA modification and diversification (Fig. 2). The gut microbiome can modify primary BAs by deconjugation with Bile Salt Hydrolase (BSH), 7α/β-dehydroxylation with proteins of the bai operon, and epimerization by the family of Hydroxysteroid Dehydrogenases (HSDHs) to produce what are known as secondary BAs.24 Once systemically reabsorbed, a topic developed in further sections, secondary and primary unconjugated BAs may be reconjugated. Absent in humans, mice express Cytochrome P450 Family 2 Subfamily A Member 12 (CYP2A12) and are able to 7α-rehydroxylate 7α/β-dehydroxylated secondary BAs.25 In humans, the main secondary BAs are Deoxycholic Acid (DCA), Lithocholic Acid (LCA), and Ursodeoxycholic Acid (UDCA), while Hyodeoxycholic Acid (HDCA) is found in trace quantities.19 Similar to humans, rodents synthesize DCA, LCA, but generate larger quantities of UDCA. Rodents additionally metabolize αMCA and βMCA to create substantial quantities of HDCA and ω-Muricholic Acid (ωMCA).26
Microbial maturation of primary BAs to secondary BAs. Schematic diagram depicting the gut maturation of primary conjugated BAs. 1* and 2* are used as shorthand notation to represent primary and secondary, respectively. NAD+ and NADH are the biochemical cofactors Nicotinamide Adenine Dinucleotide (NAD+) and Nicotinamide Adenine Dinucleotide Hydride (NADH), respectively. Red circled regions and atoms highlight the motifs changed by the prior performed metabolic reaction. This figure was created with BioRender.com
Deep-dive, BSH - BA deconjugation & microbially conjugated BAs
BSH is a N-terminal nucleophilic hydrolase that uses a N-terminal cysteine residue to deconjugate primary BAs, commonly thought to serve as gatekeeper reaction for further BA maturation.27,28 Control over BA deconjugation via BSH prevalence is important since the conversion of CA/CDCA conjugates into their free form greatly enhances their antimicrobial nature, restricting Clostridium difficile growth.29,30 BSH is found across most major phyla such as gram-positive Bifidobacterium, Lactobacillus, Clostridium, Enterococcus, Listeria, and gram-negative Stenotrophomonas, Brucella, and Bacteroides, which is suggested to play a substantial role in total deconjugation.31,32,33,34,35,36,37,38,39 This widespread prevalence of BSH suggests that BSH may be horizontally transferable.28,40,41
Contrary to original belief, BA conjugation is not a process that is exclusive to the liver. In addition to deconjugating glycine/taurine conjugates, microbial BSH may also conjugate microbially synthesized (CA/CDCA/DCA/UDCA)-CoA to other amino acids in a microbiota-dependent manner. In addition, BSH may catalyze trans-amidation reactions, where glycine/taurine C24 motifs are exchanged for that of other amino acids.42 All proteinaceous amino acid conjugates have been found except those containing aspartate or proline. This process has been identified in Enterocloster bolteae, Clostridium perfringens, and the resulting molecules are referred to as microbially conjugated BAs (MCBAs).28,43,44
In comparison to the role of glycine/taurine conjugates, MCBAs contain varied lipophilicities and carry modified steric bulk, modulating BA physiochemical and pharmacological properties.45 The more hydrophobic the MCBA, the more potent it acts as an antimicrobial agent, perhaps acting as a regulatory feedback loop against MCBA synthesis.43 MCBAs have already been characterized to both increase and decrease the pharmacological properties of canonical BAs at nuclear transcription factor receptors.44 The differential pharmacological activity of MCBAs compared to classical BAs is strongly species dependent. Comparing humans and mice, some MCBAs provide similar pharmacological effects relative to canonical BAs, while others provide entirely different or neutral effects.44 Microbial conjugates of CA have been found to retain FXR agonism and are enriched in IBD and cystic fibrosis patients, although it is unknown if it is causative or correlative. More research is needed to see how this new form of conjugation modifies the properties of other BAs and impacts disease pathogenesis.
Deep-dive, 7α/β-dehydroxylation and the bai operon
7α/β-dehydroxylation of BAs has been strongly associated with the Clostridium family of bacteria, including C. scindens, C. hylemonae, C. hiranonis, and C. sordellii, encoded by the 9 gene bai operon.46,47,48,49,50 Through the process of 7α-dehydroxylation, CA is converted into DCA and CDCA/UDCA are converted into LCA. C7 hydroxylated BAs such as CA and CDCA/UDCA are transported into bacteria by baiG, and from such are C24 conjugated into a thioester with CoA by baiB.51 BaiA2 then oxidizes the C3 alcohol to form a ketone. BaiCD oxidizes C4/C5 of 7α-hydroxy BAs, while baiH performs the same for 7β-hydroxy BAs, both of which creating 4α,5β-enones, similar in outcome to the reaction performed by HSD3B7 in primary BA synthesis.50,52,53 The enzyme baiF then performs a trans-thioesterification, in which the CoA of the oxidized 7-hydroxy metabolite is transferred to a new substrate BA.46
The rate limiting step of this pathway is performed by the 7-dehydratase baiE, which although not specific for CoA conjugation, is enzymatically more efficient with CoA conjugates.53 Catalytically a D35/H83 catalytic dyad abstracts the 6α-H, allowing for electron delocalization within the adjacent conjugated enone π-system. Through a suspected E1cb mechanism, the 7-hydroxyl (OH) is eliminated as a leaving group which retrieves the proton originally abstracted by H83 and exits the enzyme as water, resulting in the formation of a 4α,5β,6γ,7δ-enone.54 Two steps of reduction are performed by baiN to convert the substrate from a 4α,5β,6γ,7δ-enone to a 4α,5β-enone and to a 3-oxo-BA, while baiA2/baiO performs the final step in reducing C3 back to an alcohol.55,56
Bacteria conserve nine genes to perform BA 7α/β-dehydroxylation
For what reason do bacteria conserve the entire bai operon if it is just baiE/baiN that perform the net reaction? In other words, what value do the other genes add to the operon that cannot be found purely with baiE/baiN? BaiB likely serves as a commitment enzyme analogous to that of thiokinase in the process of β-oxidation. In this case, baiB likely provides the bacteria with a way to regulate pathway activity besides that intrinsic to operon transcriptional regulation. BaiF likely serves multiple valuable functions. In situations of high bacterial BA uptake, it may enhance the steady-state rate at which BAs are committed to the pathway. If baiB does exhibit product inhibition or post-translational regulation, baiF may allow the pathway to continue at a basal rate regardless of baiB activity. Lastly, baiF by performing a trans-thioesterification is energetically thrifty, preventing the bacterial expenditure of more ATP for CoA ligation.
In metabolism it is uncommon if not unseen to see direct deoxygenation of a monofunctional alcohol to an alkane. The rest of the bai operon proves its value by breaking this single complex problem down to multiple standard biochemical reactions. BaiA2 oxidizes the C3 alcohol to a ketone to enhance its electron-withdrawing power, using the standard reversible biochemistry of alcohol to ketone oxidations (Ex: Lactate Dehydrogenase). This new ketone increases the ease at which baiCD/baiH is able to further oxidize, resembling the reverse reaction of enzymes commonly associated with steroid metabolism (Ex: 5α-Reductase). BaiE dehydrates and baiN reduces in close proximity to the baiA2-baiCD/baiH installed electron-withdrawing enone, very similar to fatty acid synthesis (Ex: Fatty Acid Synthase). BaiO/baiA2 finishes the pathway by reversing the initial alcohol to ketone oxidation. At first glance, this pathway seems redundant, but upon further observation it is incredible to see how metabolism repurposes various standard archetypal reactions to obtain new outcomes.
Deep-dive, HSDH epimerization
BAs may be oxidized and epimerized by a two-step reaction, in which the existing alcohol is oxidized to a ketone by the removal of a hydride ion, followed by reduction of the ketone on the opposite face. These two reactions are enzymatically performed by different position-specific HSDHs, likely not originating from the same species of bacteria.57 BAs may be oxidized at the 3, 7, or 12 positions to generate oxo-intermediates and are then reduced to form 3, 7, or 12 epimers. 3-dehydrogenation is associated with Blautia producta, Eggerthella genus, Enterorhabdus mucosicola, and Acinetobacter lwoffii.56,58,59,60,61 Eggerthella lenta 3α-HSDH is a rare exception that is able to metabolize BAs in their conjugate form, challenging the general idea that BSH metabolism is necessary for further maturation into a secondary BA.62 7α-dehydrogenation is confirmed in Clostridium baratii, while 7β-dehydrogenation is found in Ruminococcus gnavus, Clostridium absonum, Stenotrophomonas maltophilia, and Collinsella aerofaciens.63,64,65,66,67 12α-dehydrogenation has been found in Eggerthella lenta, Enterorhabdus mucosicola, Clostridium scindens, Peptacetopacter hiranonis, Clostridium hylemonae, and Bacteroides.41,56,60,61,68 12β-dehydrogenation has been found in Clostridium paraputrificium, Clostridium tertium, and Clostridium difficile.69 Through the process of epimerization, CA may be C7 epimerized to Ursocholic acid (UCA), C3 epimerized to isoCA, or C12 epimerized to Epicholic acid (ECA). CDCA may be C7 epimerized to form UDCA or C3 epimerized to form isoCDCA, while LCA is C3 epimerized to isoLCA.
BA pool composition: structure/functionality
Structural diversity provides varying degrees of hydrophobicity
The tetracyclic fused steroidal backbone of BAs has both a concave hydrophilic α-face and a hydrophobic convex β-face, in which hydrophilicity is correlated to both the number and positioning of OH groups on C6, C7, and C12 (Fig. 3). Experimentation with hydrophilic-hydrophobic indices has revealed that the addition of a single β-OH group to an already existing poly-α-hydroxylated scaffold can greatly increase hydrophilicity. The addition of polar surface area to the hydrophobic β-face disrupts pure hydrophobic interactions, resulting in a BA that is less amphipathic and more hydrophilic. The relative physiochemical hydrophilicities of unconjugated free BAs are as follows: ωMCA > βMCA > HCA > αMCA > UDCA > HDCA > CA > CDCA > DCA > LCA. Relative rankings remain the same regardless of the type of conjugation. As a whole, the hydrophilicities of BA conjugation types are as follows: taurine conjugates > glycine conjugates > free BAs.70
Structural, physiochemical, and pharmacological properties of BAs: Representation of the structural scaffold, conjugates, physiochemical properties, and ranking of endogenous agonist pharmacology at the canonical BA receptors. Sites R1 through R6 represent locations for enzymatic hydroxylation. α-orientation groups are located beneath the steroidal plane, while β-orientation hydroxyl groups are located above the steroidal plane. Subscripted values next to BA names represent the species of origin: (H = Human), (R = Rodent), (Ht = Human Traces), (Rt = Rodent Traces). This figure was created with BioRender.com
Individual BAs work together to produce the BA pool
The BA pool is defined as all the BAs within enterohepatic circulation, disregarding those in systemic circulation. Breaking this down by tissue, ~85–90% of BAs are in the gut, 10–15% in the gallbladder, and 1% in the liver.71 As a guiding principle, BAs found in the liver are strongly conjugated. Those within the biliary tree, small intestine, and systemic circulation are moderately conjugated, while large fractions of free BAs are found in the large intestine and feces.
BA pool composition is defined as the following four metrics: percent occurrence per BA, percent 12α-hydroxylated, percent glycine/taurine conjugated, and percent primary BAs vs. secondary BAs. The BA pool composition of different biological tissues/fluids is frequently measured by liquid chromatography-mass spectrometry analysis (Table 1). The human hepatic BA pool is mostly comprised of CA and CDCA. Once exposed to the gut and excreted as feces, the human BA pool composition shifts strongly more towards the hydrophobic BAs DCA and LCA. The human serum BA composition, derived from hepatic excretion and gut BA reabsorption, is mostly comprised of CA, CDCA, DCA, and UDCA.
It is incredibly important to note that tissue-specific BA pool composition is highly species-dependent. To contrast the BA pool of humans with rodents such as mice, an overwhelming proportion of the mice BA pool in all tissues is composed of the highly polar MCA family. This is imperative to note since as we shall discuss the MCA family of BAs are potent FXR antagonists. Conjugation of human BAs mostly favors glycine conjugation, while in rodents is exclusive to taurine conjugation. Both in MCA prevalence and conjugation preference, interspecies differences between human and rodent BA pools make it incredibly challenging to translate research from rodent models to human applications. From such it is evident that wild-type mice models do not perfectly replicate the properties of the human BA pool.72
A balanced BA pool is essential for gut lipid absorption
The specific physiochemical properties and amphipathic nature of BAs allows for them to act as digestive surfactants, assisting in the solubilization and absorption of lipid nutrients and lipid-soluble vitamins (A, D, E, K). Deviations from a normal BA pool composition by either impaired synthetic or impaired enterohepatic circulation mechanisms will result in an impaired ability to absorb adequate nutrition and have normal growth patterns.14,73,74 Phase II conjugation with glycine or taurine greatly increases the acidity of BA carboxylate groups, lowering their unconjugated pKa from ~5 to ~4 in the case of glycine conjugates and ~1 in the case of taurine conjugates.75 The lower the average pKa of the BA pool, the higher the overall ratio of salt to protonated acid. This effectively allows for the biochemical optimization of BA pool composition to obtain physiological pH solubilization of fat-soluble nutrients by assisting in micelle formation.76 BA pool compositions that are too hydrophobic are hepatotoxic, causative for cholestasis, and are poor surfactants. In comparison, an overly hydrophilic composition cannot stabilize BA-nutrient micelles.77 Therefore, the maintenance of proper species-specific BA pool composition is essential to ensure the optimal surfactant-mediated absorption of lipid-nutrients.
BA pool composition drives balanced pharmacological profiles
Alongside surfactant properties, different BAs have varying pharmacological profiles at the two canonical BA receptors FXR and TGR5. The efficacy and potency rankings for common human BAs as FXR agonists are: CDCA > DCA > LCA > CA >> UDCA/MCAs, and CDCA > LCA > DCA > CA, respectively.78,79,80,81 The efficacy of UDCA and all MCAs are so low that functionally in the presence of other more potent BAs they act as FXR partial-agonists or antagonists.82,83 The analogous rankings for efficacy and potency are identical for TGR5 and is: LCA > DCA > CDCA > CA.84,85 Disregarding trace quantities, within the human BA pool CDCA represents the most potent/efficacious FXR agonist, while DCA and LCA are the most potent/efficacious TGR5 agonists. Both most potent/efficacious TGR5 agonists are secondary BAs, highlighting the importance of BA maturation in modulating TGR5 agonism. In healthy human physiology the BA pool consists of more CDCA than DCA/LCA. Accordingly, any factor that manipulates the relative ratio between classical and alternative pathway metabolic flux, or gut conversion of CA to DCA or CDCA to LCA is key in influencing BA pool composition and hence the BA pool agonist profile. Increasing metabolic flux through the alternative pathway by inhibiting or transcriptionally repressing the classical pathway gatekeeper enzymes CYP7A1 or CYP8B1 will produce a BA pool that has higher FXR agonism and decreased TGR5 agonism.10,86
BA physiology
Mechanisms of enterohepatic circulation & maturation of BAs
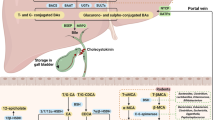
The steady-state BA pool composition is strongly influenced by the tightly regulated physiological processes of enterohepatic circulation, maturation, and fecal excretion of BAs (Fig. 4). Conjugated BAs are secreted from the basolateral surface of hepatocytes into the bile canaliculi for storage in the gallbladder.87 Glycine and taurine conjugated BAs are secreted by Bile Salt Export Pump (BSEP), while sulfate and glucuronate conjugated BAs are secreted by Multidrug Resistance-Associated Protein 2 (MRP2).88 The post-prandial release of Cholecystokinin (CCK) contracts the gallbladder, leading to the release of bile into the duodenum.89 Within the duodenum, BAs assist in the previously mentioned solubilization of fat-soluble nutrients and mature into secondary BAs.88,90 Approximately 95% of intestinal BAs are reabsorbed into the hepatic portal vein due to terminal ileal reuptake by Apical Sodium-Dependent BA Transporter (ASBT) and passive diffusion.91,92 Residual BAs that escape ileal reuptake can be excreted in feces, establishing the only known route for cholesterol excretion.93
Physiological mechanisms behind BA enterohepatic circulation, maturation, and fecal excretion: Schematic diagram representation of the physiology behind BA enterohepatic circulation, maturation, and fecal excretion. BAs are synthesized in the liver and are excreted into the duodenum for maturation. Matured BAs may either be systemically reabsorbed or excreted in feces. This figure was created with BioRender.com
Once within terminal ileal enterocytes, the cytosolic Ileal BA Binding Protein (I-BABP) chaperones BAs from the apical to basolateral membrane, where Organic Solute Transporter α/β (OST (α/β)) and Multidrug Resistance-Associated Protein 3 (MRP3) allow for BA entry into hepatic portal circulation.94,95 In contrast, enteric apical MRP2 effluxes BAs back into the ileal lumen. From the hepatic portal vein, conjugated BAs enter sinusoidal hepatocytes by the use of apical Sodium-Taurocholate co-Co-transporting Polypeptide (NTCP), while free BAs enter by use of apical Organic Anion-Transporting Polypeptide 1 (OATP1), both of which complete the cycle of enterohepatic circulation.75,90 In situations of hepatic BA overload, the liver can efflux BAs into the systemic circulation via basolateral MRP3, Multidrug Resistance-Associated Protein 4 (MRP4), or OST (α/β).10 The hepatic suppression of BSEP/MRP2 expression will limit the quantity of primary BAs that reach the gut for maturation. The ileal suppression of ASBT, MRP3, OST (α/β) expression or induction of MRP2 will enhance fecal loss of BAs and prevent the uptake of gut-modified secondary BAs. Lastly, modifications to the microbial composition of the gut may alter which biosynthetic pathways are utilized for BA maturation.
BA signaling cascades
Canonical BA receptor—FXR, a peripheral signal for nutrient density
Molecular biology of FXR
FXR is a nuclear transcription factor that is encoded by the gene FXRα/NR1H4 and is expressed as four separate isoforms (FXRα1-α4). FXR isoforms dimerize with Retinoid X Receptor Alpha (RXRα) and bind to two distinct FXR Response Elements (FXREs), the Inverted Hexamer Spaced by 1 Nucleotide (IR-1) motif (5’-AGGTCA-X-TGACCT-3’) and the Everted Hexamer Repeat Spaced by 2 Nucleotides (ER-2) motif (5’-TGACCT-XX-GGGTCA-3’) to regulate the transcriptional activity of target genes (Fig. 5a).80,96,97,98 The ENSEMBL database identifies 388 sequence orthologues for the FXR receptor, where the sequence homologies of Mus musculus and Rattus norvegicus compared to the human sequence are 87.3% and 81.64%, respectively.99 Due to sequence divergence between species, results from animal models may not directly correlate to the human FXR receptor. Cumulative reports from the Protein Atlas database identify the main subcellular location of FXR as within the nucleoplasm. mRNA-seq expression reveals that FXR is expressed minorly in most organs. The following list ranks the top 8 FXR-expressing organs in terms of nTPM (normalized mRNA transcripts per million): liver, small intestine, kidney, adrenal glands, ovary, gallbladder, colon, bladder, pancreas.100
Transcriptional and epigenetic roles of FXR and the FXR-SHP axis. a The FXR/RXRα heterodimer binds to either an IR-1 or ER-2 containing FXRE to transcriptionally regulate target gene activity. The FXR/RXRα crystal structure shown is PDB 5Z12.728 b The FXR-SHP axis inhibits LRH-1/HNF4α-driven gene expression. SHP directly dimerizes with LRH-1/HNF4α to prevent their transcriptional activity. In addition, SHP recruits Swi/Snf complex-mediated epigenetic repression. LRH-1 inhibition represses SHP transcription, forming a negative feedback loop. c Epigenetic regulation of FXR target genes by the ASCOM complex. BA agonized FXR/RXRα recruits the ASCOM complex to enhance the expression of FXRE-containing genes. Without FXR agonism, p53 and p53BP1 provide basal SHP expression. This figure was created with BioRender.com
Epigenetic regulation of FXR transcription
Epigenetic methylation has been found to impact FXR activity, in which CpG methylation found in both mouse and human colon cancers directly reduces transcription of FXR.101,102,103 13 sites for CpG methylation have been found in adenomatous polyposis coli deficient mice models.103 A clear relationship has been found between FXR CpG methylation status and the BA pool comparing healthy pregnant women to those with intrahepatic cholestasis, in which patients with reduced methylation and higher FXR transcription were associated with cholestasis, likely due to the repression of trans-biliary bile flux.104
Post-translational and post-transcriptional regulation of FXR activity
The transcriptional activity of FXR at FXREs is directly reduced by acetylation at K217, in which p300 acetylates and Sirtuin 1 (SIRT1) performs NAD+-dependent deacetylation.105,106 SIRT1 further modulates the transcription of FXR by activating Hepatocyte Nuclear Factor 1 Alpha (HNF1α).107 This regulation is further complicated by the expression of miRNA. miR-34a and miR-22 are both known to silence the expression of SIRT1, hence reducing FXR expression. In addition, FXR exhibits negative autoregulation by inducing the transcription of miR-22.108,109,110 Lastly, FXR has been found to be SUMOylated by Small Ubiquitin-Related Modifier 1 (SUMO1) and 2 (SUMO2), inhibiting its ability to trans-activate FXRE-containing genes.111
Molecular biology of the FXR-SHP axis
The nuclear FXR receptor has four main functions: to signal for incoming nutrient density (lipid, carbohydrate, amino acid), to enforce negative autoregulation of BA synthesis to prevent BA overload, to reduce the extent of enteric cholesterol absorption, and to inhibit inflammatory responses. Direct FXR agonism induces the expression of Small Heterodimer Partner (SHP), a corepressor which without a DNA binding domain dimerizes with transcription factors to directly inhibit them and/or epigenetically repress their target genes.112 This is known as the FXR-SHP axis. All FXR isoforms induce SHP expression by binding to a IR-1 motif, while FXRα2 and FXRα4 may additionally bind to a lone ER-2 motif to induce stronger SHP expression.98
The FXR-SHP axis transcriptionally and epigenetically regulates target genes
There are two major epigenetic transcriptional complexes associated with FXR-SHP axis activity. The first of which are the ATP-dependent Swi/Snf chromatin remodeling complexes (Fig. 5b). Swi/Snf chromatin remodeling complexes require an ATPase such as Probable Global Transcriptional Activator SNF2L2 (BRM) or Transcriptional Activator BRG1 (BRG1), other proteins known as BRM or BRG1 associated factors (BAFs), the Paired Amphipathic Helix Protein Sin3a (Sin3a)/Histone Deacetylase 1/2 (HDAC-1/2) corepressor complex, and the histone methyltransferase G9a to modulate chromatin compaction.113,114,115 In such, BA-activated FXR/RXRα dimers recruit the BRG1 Swi/Snf complex, providing chromatin relaxation and allowing for FXR-stimulated transcription, such as that which occurs with SHP. SHP may recruit the BRM Swi/Snf complex, which allows for H3K9/K14 deacetylation, H3K9 methylation, direct inhibition of Liver Receptor Homolog 1 (LRH-1)/Hepatocyte Nuclear Factor 4 Alpha (HNF4α) transcriptional activation, and epigenetic compaction of LRH-1/HNF4α target genes.113,116,117
A secondary epigenetic mechanism is associated with FXR activity, known as the Activating Signal Cointegrator-2 (ASC-2) – Containing Coactivator Complex (ASCOM) (Fig. 5c).118 ASCOM is additionally composed of either Histone Methyltransferase Mixed-Lineage Leukemia Proteins 3 (MLL3) or 4 (MLL4) histone methyltransferases and the histone demethylase Ubiquitously Transcribed Tetratricopeptide Repeat, X Chromosome (UTX). Respectively, these methylate H3K4 and remove methylation at H3K27, cumulatively increasing transcriptional activity.119,120 BA-activated FXR/RXRα dimers recruit the ASCOM complex to enhance the transcription of SHP, BSEP, MRP2, and NTCP.120,121 The ASCOM complex has an additional role with respect to the Tumor Suppressor Transcription Factor p53 (p53). Under normal or stressed conditions the SHP promoter may be bound by p53, which by the scaffolding protein p53 Binding Protein 1 (p53BP1) recruits the ASCOM complex and transcribes SHP.122
Molecular physiology of the FGF15/19-[FGFR4/βK]-FXR axis
Alongside direct BA agonism of FXR, the hepatic FXR signaling axis is also modulated by downstream signaling from a complex of the integral membrane protein β-Klotho (βK) and the receptor tyrosine kinase Fibroblast Growth Factor Receptor 4 (FGFR4) (Fig. 6).123 Gut FXR agonism stimulates the systemic secretion of the FGFR4/βK complex agonists Fibroblast Growth Factor 15 (FGF15 – mice orthologue) and Fibroblast Growth Factor 19 (FGF19 – human orthologue), serving as long-distance signals for high enteric levels of FXR agonists.124,125 Specific to the hunger center of the hypothalamus, a secondary role of FGF15/19 is to suppress appetite by inhibiting Agouti-Related Peptide (AgRP) and Neuropeptide Y (NPY) neurons.126,127
Gut FXR signaling. Gut FXR signaling within enterocytes induces BA/cholesterol efflux, ceramide synthesis, and FGF15/19 secretion. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. Question marks represent unknown mechanisms. This figure was created with BioRender.com
In the liver, FGFR4/βK complex agonism phosphorylates the adaptor protein Fibroblast Growth Factor Receptor Substrate 2 (FRS2) and recruits Growth Factor Receptor Bound Protein 2 (Grb2), Grb2 Associated Binding Protein 1 (Gab1), and Src Homology Region 2 Domain-Containing Phosphatase-2 (SHP2).128 SHP2 binding to GAB1 promotes the dephosphorylation of Phosphoprotein Associated with Glycosphingolipid-Enriched Microdomains (PAG), preventing the membrane anchoring of the Proto-Oncogene Tyrosine-Protein Kinase Src (Src) inhibitory kinase C-Terminal Src Kinase (Csk).129 The release of Csk-mediated inhibition allows for Src to phosphorylate Y67 of FXR and promote nuclear translocation and transcriptional regulation.128,130 This is denoted as the [FGFR4/βK]-FXR axis. Grb2 additionally recruits the Ras guanine nucleotide exchange factor Son of Sevenless (Sos), which in conjunction with Src activity activates the Ras-Raf-MEK-ERK signaling cascade, resulting in the phosphorylation and activation of Extracellular Signal-Related Kinase (ERK).123,131 This is known as the [FGFR4/βK]-ERK axis.
Alongside FGF15/19 secretion, gut FXR agonism induces the systemic secretion of ceramides. Gut FXR transcriptionally enhances the expression of the following ceramide synthetic proteins: Serine Palmitoyltransferase Long Chain Base Subunit 3,4 (SPTLC3, SPTLC4), Sphingomyelin Phosphodiesterase 3,4 (SMPD3, SMPD4), Ceramide Synthase 2,4 (CERS2, CERS4), and Delta 4-Desaturase Sphingolipid 1,2 (DEGS1, DEGS2).132
Master regulators of BA homeostasis—FXR and the FGF15/19-[FGFR4/βK]-FXR axis
Feedback inhibition for BA synthesis is mediated by two mechanisms, one directing from FXR itself, and another originating from agonized FGFR4/βK complex (Fig. 7). BA agonized FXR induces the expression of SHP, while the downstream actions of agonized [FGFR4/βK]-ERK axis mediated phosphorylation of SHP prevents its proteasomal degradation, both leading to increased SHP levels.133,134 SHP dimerizes and directly inhibits the activity of the transcription factors LRH-1 and HNF4α, in addition to inducing the epigenetic repression of their target genes, preventing the transcription of CYP7A1 and CYP8B1. SHP-mediated inhibition and epigenetic repression of LRH-1 target genes represses its own transcription, forming a negative feedback loop.135,136,137,138 HNF4α-induced CYP7A1 and CYP8B1 expression is dependent on the recruitment of COUP Transcription Factor 2 (COUP-TFII), the histone acetyl transferase CREB Binding Protein (CBP), Transcription Factors IID (TFIID), and IIB (TFIIB).139,140,141 In addition, Sos activates Ras, which agonizes Ras-like protein (Ral) and subsequently Mammalian Target of Rapamycin Complex 1 (mTORC1). mTORC1 and ERK phosphorylate and prevent the nuclear translocation of Transcription Factor EB (TFEB), preventing TFEB-induced CYP7A1 expression.142,143
Hepatic FXR signaling—BA autoregulation. Hepatic FXR signaling pertaining to BA autoregulation. Hepatic FXR agonism increases BA/cholesterol efflux, primary BA conjugation, and represses BA synthesis. Dashed mechanism arrows represent the Ras-Raf-MEK signaling cascade, while dashed arrows through a protein represent the direction of substrate flux. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. Question marks represent unknown mechanisms. This figure was created with BioRender.com
Repression of CYP7A1 and CYP8B1 directly lowers metabolic flux through the classic pathway of BA synthesis.137,144,145,146 This two-sided negative-feedback loop lowers the overall BA pool size via CYP7A1 repression and composition via CYP8B1 repression, shunting BA synthesis into the alternative pathway and producing the strong FXR agonist CDCA. This results in a smaller BA pool that is more biased in composition towards FXR agonism. FXR is therefore referred to as the master regulator of BA synthesis.135,147 In addition, FXR agonism can help prevent systemic BA overload-induced toxicity. High concentrations of BAs and FXR-SHP axis agonism represses hepatic NTCP expression and gut ASBT expression to prevent further BA entry, while FXR induces BSEP, MRP2, I-BABP, OSTα (liver-specific), and OSTβ to increase BA fecal excretion.92,148 BACS, BAT, UGT2B4, and SULT2A1 are also upregulated by FXR to increase the rate at which hepatic BAs efflux into the biliary tree.149 Sulfation reduces gut BA reabsorption, assisting in the elimination of excess BAs.17
The FGF15/19-[FGFR4/βK]-FXR axis modulates triglyceride metabolism
Low FXR agonism enhances the rate of cholesterol excretion while high FXR agonism represses the synthesis of triglycerides (Fig. 8). FXR-induced SHP can dimerize with the transcription factor liver X receptor (LXR) and inhibits its induction of sterol regulatory element-binding protein 1C (SREBP-1C), the master transcriptional regulator of triglyceride synthesis.150,151 In addition, [FGFR4/βK]-ERK axis activation represses SREBP-1C activity by inducing ERK phosphorylation of SHP, which recruits DNA Methyltransferase-3A (DNMT3A) to epigenetically repress SREBP-1C target genes.152 Repression of SREBP-1C activity directly reduces the transcription of Fatty Acid Synthase (FAS), Acetyl-CoA Carboxylase (ACC), Stearoyl-CoA Desaturase enzyme 1 (SCD1), and ATP Citrate Lyase (ACLY) genes, key players in triglyceride synthesis. In addition, FXR-SHP axis activation inhibits carbohydrate response element binding protein (ChREBP), another transcription factor which when activated by glucose metabolites induces lipogenesis by upregulating FAS, SCD1, ACC, and ACLY.153 FXR has been shown to induce the expression of Apolipoprotein CII (ApoC-II) and competitively inhibit the HNF4α-mediated transcription of Apolipoprotein CIII (ApoC-III). ApoC-II expression and ApoC-III repression increase the activity of lipoprotein lipase (LPL), allowing for a more efficient removal of triglycerides out of the systemic circulation into peripheral tissue.154,155,156 Furthermore, FXR has been experimentally shown to induce peroxisomal proliferator-activated receptor alpha (PPARα), a key transcription factor responsible for the catabolism of fatty acids.157,158
Hepatic FXR signaling—lipid metabolism. Hepatic FXR signaling pertaining to lipid metabolism represses triglyceride synthesis, VLDL packaging, increases lipid utilization, chylomicron uptake, and promotes a pro-atherogenic serum lipid profile. Dashed mechanism arrows represent the Ras-Raf-MEK signaling cascade, while dashed arrows through a protein represent the direction of substrate flux. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. Question marks represent unknown mechanisms. This figure was created with BioRender.com
FXR modulation of cholesterol metabolism
FXR-SHP axis activation additionally represses very-low density lipoprotein (VLDL) secretion by SHP dimerization with HNF4α, directly inhibiting it and leading to the epigenetic repression of its target genes. This decreases the rate at which the liver distributes cholesterol and triglycerides. When inhibited, HNF4α fails to induce the expression of downstream Apolipoprotein B100 (ApoB100) and Microsomal Triglyceride Transfer Protein (MTTP) genes, both of which are essential to VLDL packaging and synthesis.159 Although VLDL excretion is reduced, FXR-mediated repression of BA synthesis inhibits cholesterol excretion, allowing for it to accumulate hepatically. Resulting from such, the reduced quantity of VLDL produced will now be cholesterol rich, eventually producing cholesterol-rich low-density lipoproteins (LDL). Long-term FXR agonism contributes towards a potentially pro-atherogenic state due to an RXRα-independent negative regulatory FXRE within the Apolipoprotein A1 (Apo-A1) gene. Apo-A1 is the core structural lipoprotein required for high-density lipoprotein (HDL)-mediated scavenging of systemic cholesterol, necessarily fighting against atherosclerotic plaque development.160 FXR activity additionally stimulates the expression of Apolipoprotein E (ApoE) and phospholipid transfer protein (PLTP). Functionally this results in enhanced chylomicron/LDL uptake into the liver, in addition to increasing the rate at which HDL particles uptake phospholipids from VLDL, converting them into LDL.161
FXR-mediated changes to serum cholesterol are further complicated by the FXR-induced expression of HDL Scavenger Receptor Class B Type 1 (SR-B1), which increases the hepatic uptake of HDL cholesterol for biliary excretion, further reducing serum HDL cholesterol.162,163 FXR signaling attempts to ameliorate this systemic cholesterol excess by increasing the expression of hepatic and gut ATP Binding Cassette Subfamily G Members 5 and 8 (ABCG5) (ABCG8), transporters which serve to efflux hepatic cholesterol into the bile canaliculi and to return cholesterol absorbed by enterocytes back into the gut lumen.163,164,165,166 Although this may offset some further cholesterol accumulation, increased cholesterol content within the biliary tree without BAs may increase the risk for gallstones.167 Another compensatory activity of highly agonized FXR-SHP-axis activity is to reduce the expression of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), increasing the activity of the LDL Receptor (LDLr).168,169 However, it needs to be emphasized that even if more LDL cholesterol is absorbed into the liver, the same problem remains, the liver has no active metabolic pathway for large quantity cholesterol excretion.
Physiological implications—double-edged FXR lipid phenotypes
Cumulatively, it is then very helpful to characterize the impact of FXR agonism on lipid metabolism into two different states of FXR activity. Within a state of low total FXR activity SREBP-1C activity is high, HDL synthesis is not repressed, and cholesterol is being metabolized into BAs and excreted, producing a state of high triglycerides, VLDL cholesterol, HDL cholesterol, and low LDL cholesterol. In a state of high total FXR activity, SREBP-1C activity is low, HDL synthesis is repressed, and cholesterol is accumulating, producing a state of low triglycerides, VLDL cholesterol, HDL cholesterol, and high LDL cholesterol.
The FGF15/19-[FGFR4/βK]-FXR axis modulates carbohydrate metabolism
FXR agonism activates glycogenesis and inhibits gluconeogenesis (Fig. 9). FXR agonism through unknown mechanisms has been found to mimic insulin and increase insulin sensitivity by inducing the downstream phosphorylation of S473 on Protein Kinase B (Akt). In addition, the dephosphorylation of S312 and the phosphorylation of Tyr residues on Insulin Receptor Substrates 1/2 (IRS1/2) have been observed.170,171,172,173 FXR activated Akt in addition to [FGFR4/βK]-ERK axis activated Ribosomal-S6-kinase (RSK) phosphorylates and inhibits Glycogen Synthase Kinase 3 Beta (GSK3β).174,175 GSK3β is a physiological inhibitor of glycogenesis, whose inhibition leads to positive glycogenic signaling.
Hepatic/gut FXR signaling—carbohydrate metabolism. Hepatic and gut enteroendocrine L-cell FXR signaling pertaining to carbohydrate metabolism. FXR agonism increases glycogenesis, insulin sensitivity, represses gluconeogenesis, and blunts glycolysis. Gut FXR agonism represses GLP-1 synthesis and secretion. Dashed mechanism arrows represent the Ras-Raf-MEK signaling cascade, while dashed arrows through a protein represent the direction of substrate flux. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. Question marks represent unknown mechanisms. This figure was created with BioRender.com
FXR-SHP axis activation inhibits HNF4α−induced transcription of Phosphoenolpyruvate Carboxykinase (PEPCK), Fructose-1,6-Bisphosphatase (FBPase), and Glucose-6-Phosphatase (G6Pase) genes. FXR-SHP axis activation also inhibits the transcription factor Forkhead Box Protein O1 (FOXO1) and its induced expression of PEPCK and G6Pase.176,177,178 FXR-SHP axis activation therefore has an inhibitory role on the expression of three of the four rate-limiting proteins within hepatic gluconeogenesis (G6Pase, FBPase, PEPCK). In contrast to the general trend of enhancing glucose tolerance, FXR agonism blunts both hepatic and enteroendocrine intestinal L-cell glycolytic flux. This is mechanistically mediated by FXR-SHP axis inhibition of ChREBP, repressing the transcription of Pyruvate Kinase (PK).153 Within enteroendocrine intestinal L-cells this decreases the synthesis and secretion of Glucagon-Like Peptide-1 (GLP-1). Decreased GLP-1 synthesis is explained by reduced ChREBP-induced transcription of GLP-1’s precursor Proglucagon (GCG), while hindered GLP-1 secretion is caused by reduced glycolytic flux and hence reduced ATP-driven exocytosis.179
FGF15/19-[FGFR4/βK] signaling represses hepatic gluconeogenesis
An unknown protein downstream of [FGFR4/βK] complex agonism dephosphorylates the transcription factor cyclic adenosine monophosphate (cAMP) response element binding protein (CREB). The dephosphorylation of pCREB to produce CREB reduces glucagon or adrenergic-induced gluconeogenesis in the short-term by not trans-activating cAMP response element (CRE) mediated expression of G6Pase, PEPCK, and FOXO1. The histone acetyl transferase CBP, Histone Acetyltransferase p300 (p300), and the coactivator CREB regulated transcription coactivator 2 (CRTC2) must be bound by pCREB to epigenetically activate gene expresson.178,180 FGF15/19 serves as a long-distance signal for nutrition by further inhibiting starvation-induced gluconeogenesis, in which inactivated CREB cannot induce the expression of the FOXO1 coactivator peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), drastically suppressing the expression of G6Pase and PEPCK.181,182,183
Viewing carbohydrates, the hepatic FGF15/19-[FGFR4/βK]-FXR axis centrally functions to potentiate insulin-induced glucose uptake, signals for the short-term storage of dietary carbohydrates as glycogen, serves to prevent the unneeded synthesis of carbohydrates in the presence of recently consumed nutrients, hinders glycolytic flux, and intestinally blunts incretin-like activity.
FXR modulation of amino acid metabolism
FXR agonism increases the flux of excess amino acids through the urea cycle and signals for the utilization of recently ingested amino acids within protein synthesis (Fig. 10). In mice, hepatic FXR agonism plays a wide role in sustaining the proper metabolism of amino acids. This occurs by improving glutamate shunting of ammonia via Glutamine Synthetase (GLUL) induction and flux through the urea cycle by inducing Carbamoyl Phosphate Synthetase-1 (CPS1), Arginosuccinate Synthase 1 (ASS1), and Arginosuccinate Lyase (ASL).184,185 [FGFR4/βK]-ERK axis activation phosphorylates and activates RSK and MAP Kinases-Interacting Serine/Threonine-Protein Kinase 1 (MKNK1), resulting in the phosphorylation of Eukaryotic Translation Initiation Factors 4B (EIF4B) and 4E (EIF4E), respectively.175 The phosphorylation of EIF4B and EIF4E are crucial for the initiation of eukaryotic cap-dependent translation.186 RSK also phosphorylates Ribosomal Protein S6 (rpS6), a modulator of ribosomal translation which additionally increases the efficacy of cap-dependent translation.175
Hepatic FXR signaling—amino acid metabolism. Hepatic FXR signaling pertaining to nitrogen and protein metabolism. Hepatic FXR agonism increases urea cycle flux, glutamate shunting, and increases cap-dependent translation. Dashed mechanism arrows represent the Ras-Raf-MEK signaling cascade, while dashed arrows through a protein represent the direction of substrate flux. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. Question marks represent unknown mechanisms. This figure was created with BioRender.com
General mechanisms—FXR dampens hepatic and systemic inflammation
Alongside high expression within the gut and liver, FXR is also expressed within immune cells.187 FXR exhibits potent anti-inflammatory activity in peripheral monocytes, in human and mouse myeloid cells, dendritic cells, and in hepatic natural killer T cells.188,189,190,191 FXR agonism exerts anti-inflammatory properties by physically inhibiting the formation of the NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome (Fig. 11a). This is accomplished by physically preventing the association of NLRP3 and pro-caspase-1, preventing the maturation of the pro-inflammatory cytokines Interleukin 1 Beta (IL-1β) and Interleukin 18 (IL-18).192 In addition, FXR agonism has been found to inhibit the binding of the transcription factor Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cell (NF-κB) to cis-regulatory κB sites of pro-inflammatory genes, such as IL-1β, Interleukin 6 (IL-6), and Tumor Necrosis Factor Alpha (TNFα).146,193 Although FXR mediates hepatic anti-inflammatory properties by the two mechanisms mentioned, it is still unknown if the total anti-inflammatory role of FXR is entirely receptor-mediated or a downstream effect of reducing hepatic lipid content.
FXR signaling is anti-inflammatory, anti-apoptotic, and anti-autophagic. a FXR activity inhibits NLRP3/Caspase-1 and NF-κB induced inflammation. b Non-ligand-bound FXR is anti-apoptotic by preventing extrinsic apoptosis, while ligand-bound FXR prevents BA-overload and deoxysphingolipid induced apoptosis. c FXR activity is anti-autophagic by preventing pCREB/PPARα-mediated autophagic gene expression. d Starvation-induced AMPK enhances BA synthesis and BA mobilization to ensure adequate nutrient absorption. Proteins with a golden outline indicate kinases, while golden arrows represent phosphorylation. This figure was created with BioRender.com
FXR is anti-apoptotic and disrupts autophagy
Hepatocytes under significant cellular stress may be vulnerable to apoptosis, especially under hepatic conditions such as NAFLD or NASH.194 FXR plays a significant hepatoprotective role against apoptosis in which non-ligand-bound FXR binds to the death effector domain of caspase-8, inhibiting extrinsic apoptosis (Fig. 11b).195 FXR additionally prevents apoptosis by inducing Cytochrome P450 Family 4 Subfamily F (CYP4F)-mediated metabolism of pro-apoptotic deoxysphingolipids and by preventing BA-induced apoptosis by repressing CYP7A1/CYP8B1 expression.196,197,198
Under extensive whole body starvation, autophagy recruits intracellular macromolecules to the lysosome to be broken down for energy to ensure physiological survival.199,200 Within the liver pCREB and CRTC2 greatly contribute to the starvation-induced expression of autophagic genes, likely induced by glucagon or adrenergic signaling. Agonized FXR functionally disrupts the pCREB/CRTC2 interaction preventing autophagy, even during fasting (Fig. 11c).201 FXR may also directly bind to autophagic gene promoters and suppress their transcriptional activity. FXR’s suppression over autophagic gene expression is nuanced since the same binding site is regulated by pro-autophagic PPARα, allowing for nutrient-sensitive control over autophagy in addition to that provided by Adenosine Monophosphate (AMP)-Activated Protein Kinase (AMPK) sensing the ATP/AMP balance and mTORC1 sensing free branched chain amino acids.202 Further interplay between FXR and the starvation response has been discovered, in which activated AMPK transcriptionally represses FXR and stimulates Steroid Receptor Coactivator-2 (SRC-2) to coactivate FXR-induced expression of BSEP (Fig. 11d).203,204 In anticipation of upcoming dietary nutrition, this allows for a transient release from BA synthesis autoregulation and increases BA mobilization into the biliary tree, cumulatively increasing the efficacy in which BA-mediated absorption replenishes current depleted nutrient stores.
Canonical BA receptor—TGR5, a metabolic and immunological modulator
Molecular biology of TGR5
TGR5 is a G-protein coupled receptor encoded by the GPBAR1 gene. The Gα subunit for TGR5 is coupled to Adenylate Cyclase (AC), designating TGR5 as a Gαs G-protein.84 The ENSEMBL database identifies 75 sequence orthologues for TGR5, where the sequence homology of Mus musculus and Rattus norvegicus compared to the human sequence are 83.59% and 82.37%, respectively.99 As previously stated for FXR, the sequence divergence between species may not allow for a direct correlation between animal models and results seen in human physiology. The following list ranks the top 8 baseline TGR5-expressing organs in terms of nTPM: adipose tissue, breast, gallbladder, smooth muscle, spleen, stomach, kidney, and heart. Although low in documented nTPM, it is also of use to note that TGR5 is expressed within the small intestine, colon, was recently discovered in mice hepatocytes, and in mice is exercise-inducible via the transcription factor Activating Transcription Factor 6 (ATF6) within skeletal muscle. The membrane-bound TGR5 receptor has many metabolic functions, such as increasing basal metabolic rate, whole-body nutrient tolerance, post-prandial satiety, and via Adiponectin (ADIPOQ) signaling induces insulin-sensitivity, reduces gluconeogenesis, reduces lipogenesis, and increases ceramide metabolism. Non-metabolic functions of TGR5 are to protect against hepatic pathology, reduce hepatic and adipose exposure to inflammatory signaling, and to maintain proper biliary function.205
Epigenetic regulation of TGR5 transcription
CpG methylation of the TGR5 promoter has been found to reduce transcription and is associated with both liver-failure and chronic hepatitis B.206 Hyper-methylation of the TGR5 promoter has also been found associated with hepatocellular carcinoma. Although TGR5 promoter methylation is age-related and higher in patients older than 60, methylation may serve as a potential diagnostic for both acute-on-chronic hepatitis B liver failure and hepatocellular carcinoma.207 Besides direct phenotypic association between hepatic pathology and CpG methylation of the TGR5 promoter, not much research has been conducted directly on the factors driving TGR5 epigenetic regulation. It is currently theorized that a significant portion of TGR5 transcriptional regulation is secondary to FXRE activity within its promoter, being directly driven by the epigenetics, post-transcriptional, and post-translational modifications modulating FXR activity.208 TGR5 agonists have been found to induce SIRT1 and Sirtuin 3 (SIRT3) transcription via activation of PGC-1α.209 TGR5-induced SIRT1 expression may further modulate FXR activity, establishing a mutual regulatory relationship.
TGR5 agonism drives energy utilization and mitochondrial biogenesis
Metabolically relevant TGR5 agonism stimulates AC to produce cAMP and activates Protein Kinase A (PKA) (Fig. 12).85,210,211,212 CREB is phosphorylated by PKA to produce pCREB. pCREB binds to the CRE and induces the adipocyte/skeletal muscle expression of Type 2 Iodothyronine Deiodinase 2 (DIO2) and PGC-1α.213,214 Increased expression of DIO2 directly increases the rate of conversion of Tetraiodothyronine (T4) into Triiodothyronine (T3). When the more active form of the hormone T3 binds to the Thyroid Hormone Receptor (TR), TR acts as a transcription factor for genes with Thyroid Response Elements (TREs). In mice, TGR5-induced TR-agonism represses CYP8B1 expression without the presence of a TRE, while in humans TR-agonism induces the expression of CYP7A1.215,216,217,218,219 In brown adipose tissue (BADT) TR agonism induces Uncoupling Protein 1 (UCP1) which by establishing a futile cycle of mitochondrial proton shuttling increases energy usage via thermogenesis.220,221 In white adipose tissue (WADT) TR agonism induces the expression of β1 and β2 Adrenergic Receptors (β1-AR) (β2-AR) along with UCP1. Spare adrenergic receptors increase WADT sensitivity to adrenergic-induced Hormone-sensitive Lipase (HSL) activity.222,223,224 In skeletal muscle (SKM) Uncoupling Protein 3 (UCP3) is induced by TR agonism along with Sarcoplasmic/ER Calcium-Dependent ATPase (SERCA), resulting in energetically futile cycles of protons at the mitochondria and calcium at the sarcoplasmic reticulum.225,226,227 In all three target tissues, TGR5 agonism increases energy consumption, upregulates mitochondrial oxidative phosphorylation, and hence increases basal metabolic rate.213,228
TGR5 signaling—metabolic effects. Metabolic effects of TGR5 signaling, crystal structure shown is PDB 7CFM.631 TGR5 agonism induces incretin release, upregulates mitochondrial biogenesis and basal metabolic rate, and leads to ADIPOQ synthesis, increasing carbohydrate/lipid tolerance and reducing ceramide concentrations. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Question marks represent unknown mechanisms. Dashed mechanism arrows represent vesicle exocytosis, while dashed arrows through a protein describe the direction of ion flow. Italicized text refers to genes while normal text refers to proteins. This figure was created with BioRender.com
Following the same pattern of energy usage, PGC-1α functions as the master regulator of mitochondrial biogenesis, leads to the adipose exocytosis of ADIPOQ, and enhances adipose UCP1 expression.229,230,231 The coactivator function of PGC-1α in adipose tissue increases the transcriptional activity of Nuclear Respiratory Factor 2 (NRF2), which induces the expression of Nuclear Respiratory Factor 1 (NRF1) by binding to the antioxidant response element (ARE).232 NRF1 and NRF2 induce the nuclear expression of mitochondrial respiratory chain proteins.233 NRF1 induces the mitochondrial transcription of Transcription Factors A (TFAM), B1 (TFB1M), and B2 (TFB2M), resulting in preparation for mitochondrial biogenesis.234,235,236 PGC-1α is additionally a coactivator for Peroxisomal Proliferator-Activated Receptor Gamma (PPARγ)/RXRα dimers. When the ternary complex binds to Peroxisomal Proliferator-Activated Receptor Response Elements (PPREs), adipocytes synthesize and secrete the adipokine ADIPOQ.230,231,237 Systemically, Adiponectin Receptor 1 (AdipoR1) agonism increases whole-body insulin sensitivity by activating Adaptor Protein, Phosphotyrosine Interacting with PH Domain and Leucine Zipper 1 (APPL1), promoting the binding of IRS1/2 to the insulin receptor. In addition, AdipoR1 agonism activates Liver Kinase B1 (LKB1) leading to the phosphorylation and activation of AMPK, which represses hepatic gluconeogenesis, lipogenesis, and cholesterogenesis.238,239,240,241 Adiponectin Receptor 2 (AdipoR2) agonism increases β-oxidation of fatty acids within the liver and adipose tissue by inducing PPARα.242,243 Agonism of both AdipoR1/R2 activates receptor intrinsic ceramidase activity, protecting against the negative metabolic side effects of ceramides.244,245,246,247,248
TGR5 agonism drives incretin release
Within pancreatic β-islets, α-islets, and enteroendocrine L-cells, TGR5 agonism leads to calcium-induced exocytosis by two mechanisms. The first of which involves cAMP activation of Exchange Protein Activated by cAMP (EPAC), the recruitment and activation of Receptor-Associated Protein (RAP), Phospholipase C (PLC) mediated hydrolysis of Phosphoinositide (PIP2) to Inositol Triphosphate (IP3) / Diacylglycerol (DAG), and ER calcium release.249,250 Specific to the pancreas, EPAC additionally stimulates Rab-interacting Molecule 2 (RIM2) and Piccolo to increase calcium-induced exocytotic granule trafficking and fusion.251,252,253 The second mechanism involves PKA-mediated phosphorylation of Sulfonylurea Receptor 1 (SUR1), inhibition of ATP-Sensitive Potassium Channels (KATP), membrane depolarization, Voltage-gated Calcium Channel (VGCC) activation, and calcium-induced calcium release from the ER.254,255,256,257 In β-islets, this leads to the exocytosis of insulin.249,254,258 In enteroendocrine intestinal L-cells this leads to the exocytosis of GLP-1 and Peptide YY (PYY), resulting in enhanced insulin secretion, reduced rates of gastric emptying, and appetite suppression.259,260,261,262,263 By the same two mechanisms pancreatic α-islets secrete GLP-1 instead of glucagon. This transition is explained by α-islet-specific EPAC activity, which induces the expression of proprotein convertase 1 (PC1), biasing alternative splicing of proglucagon mRNA to favor the GLP-1 transcript instead of the glucagon transcript.249 Cumulatively under a metabolic lens, TGR5 agonism enhances basal metabolic rate by increasing thermogenic futile cycling, lipolysis, and mitochondrial biogenesis, while leading to increased nutrient tolerance, insulin sensitivity, satiety, and ceramide metabolism.
TGR5 promotes hepatic mitochondrial function, vasodilation, and biliary flow
TGR5-induced hepatic endothelial cAMP synthesis results in EPAC/RAP-mediated stimulation of Phosphoinositide 3-Kinase (PI3K) and phosphorylation of PIP2 into phosphatidylinositol trisphosphate (PIP3) (Fig. 13).264 PIP3 synthesis activates Pyruvate Dehydrogenase Kinase 1 (PDK1) leading to the phosphorylation of Akt at T308. Two models currently exist, in which either PIP3 or pT308-Akt activate Mammalian Target of Rapamycin Complex 2 (mTORC2), resulting in the phosphorylation of Akt at S473.265 pT308/pS473-Akt along with PKA directly phosphorylate and activate Endothelial Nitric Oxide Synthase (eNOS) at S1117, maintaining hepatic endothelial mitochondrial function and vasodilation.266,267,268 TGR5-induced cAMP-based agonism provides additional benefits by activating Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). CFTR agonism leads to chloride secretion and soon after bicarbonate exchange by Anion Exchange Protein 2 (AE2) into the bile duct, maintaining bile flow and protecting against cholangitis/cirrhosis.269,270,271,272,273
TGR5 signaling—non-metabolic and immunologic effects. Non-metabolic and immunologic effects of TGR5 signaling, crystal structure shown is PDB 7CFM. TGR5 agonism represses macrophage/adipocyte-specific inflammation, enhances hepatic vasodilation, biliary flow, represses pro-inflammatory NF-κB activity, and induces IL-10/TGFβ expression. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Question marks represent unknown mechanisms. Dashed mechanism arrows represent vesicle exocytosis, while dashed arrows through a protein describe the direction of ion flow. Italicized text refers to genes while normal text refers to proteins. This figure was created with BioRender.com
General mechanisms—TGR5 exerts potent anti-inflammatory activity
TGR5 is extensively expressed within cells derived from the myeloid lineage and is anti-inflammatory.274 Immunologically, TGR5-induced pT308/pS473-Akt activates mTORC1, resulting in the differential translation of CCAAT/enhancer-binding protein β (CEBP/β) mRNA to produce Liver-Enriched Inhibitory Protein (LIP).275,276,277 LIP production reduces the expression of pro-inflammatory TNFα and IL-6, reducing both macrophage migration and insulin resistance-associated inflammation within adipose tissue.278 TGR5 agonism inhibits NF-κB pathway activation within macrophages, Kupffer cells, and hepatocytes, reducing the expression of the following cytokines: Interferon Gama (IFNγ), Interleukin-1β (IL-1β), IL-2, IL-6, and TNFα.279,280,281 Mechanistically this is accomplished by inhibiting the phosphorylation of NF-κB Inhibitor Alpha (IκBα) and inhibiting the translocation of the NF-κB transcription complex p50 and p65 (p50/p65). TGR5 activation recruits β-Arrestin 2 and inhibits the phosphorylation and proteasomal degradation of IκBα.282 TGR5 provoked pCREB activation induces the expression of the transcription factor c-Fos (c-Fos), which when phosphorylated by PKA prevents p50/p65 from binding to κB sites.283 In addition, pCREB activation induces the expression of Interleukin 10 (IL-10), and Transforming Growth Factor Beta (TGFβ).284,285 Further reductions in pro-inflammatory cytokines are seen by TGR5-AC-PKA axis activity, leading to the ubiquitination and destruction of the NLRP3 inflammasome.286 NLRP3 cessation directly leads to decreased concentrations of macrophage-relevant pro-inflammatory cytokines IL-1β and IL-18.287,288 Phenotypically, TGR5 agonism has been found to switch macrophages away from the pro-inflammatory M1 phenotype towards the anti-inflammatory M2 phenotype.278,284,289,290,291
Non-canonical BA receptors
Sphingosine-1-phosphate receptor 2
Sphingosine-1-phosphate Receptor 2 (S1PR2) is a Gi coupled G-protein coupled receptor commonly expressed within hepatocytes, cholangiocytes, stellate cells, intestinal epithelial cells, macrophages, and is directly agonized by conjugated BAs.292,293,294,295 Downstream of such S1PR2 induces the expression of Sphingosine Kinase 2 (SphK2). Sphk2 converts sphingosine to Sphingosine-1-phosphate (S1P), inhibiting HDAC-1/2, and enhancing the expression of genes associated with hepatic glucose and lipid metabolism such as: SREBP-1C, FAS, Carnitine Palmitoyl Transferase 1A (CPT-1A), PPARγ, PGC-1α, ApoB100, and FXR. Mice S1PR2−/− and SphK2−/− models found that the lack of S1PR2-mediated induction of ApoB100 and CPT-1A directly enhances the extent of hepatic steatosis, indicating that S1PR2 plays an important modulatory role in NAFLD pathogenesis.296 In addition, S1PR2 may activate ERK, Akt, and c-Jun N-terminal Kinase (JNK) signaling cascades, if bound to glycine conjugated CDCA may induce apoptosis, and stimulates the pro-inflammatory M1 polarization of macrophages via the Gi-PI3K-JNK axis.293,294,297,298,299 Inhibition of S1PR2 activity was found to decrease total serum BAs, reduce cholestatic injury in mice bile duct ligation mice models, restore intestinal mucosal barrier function, and reverse pro-inflammatory M1 macrophage polarization in mice Dextran Sulfate Sodium (DSS)-colitis models.300,301
Muscarinic receptors 2 & 3
Muscarinic receptors 2 (CHRM2) and 3 (CHRM3) are Gi and Gq coupled G-protein coupled receptors, respectively, and both may be agonized by taurine conjugated BAs.302 CHRM2 and CHRM3 overexpression is associated with the initiation and progression of colon, gastric, and pancreatic cancers.303,304,305,306 In addition, taurine conjugated LCA has been found to induce cholangiocarcinoma as a downstream result of muscarinic agonism.307
Vitamin D receptor
The vitamin D receptor (VDR) is a nuclear transcription factor that dimerizes with RXRα, is canonically agonized by vitamin D3, and is expressed extensively within the bones, intestine, kidney, and liver.308,309,310 As time progressed it was discovered that VDR may be agonized by LCA, serving as an intestinal BA receptor.311 LCA agonized VDR suppresses CYP7A1 expression by binding to HNF4α and preventing its association with CYP7A1 or by inhibiting its interaction with COUP-TFII, CBP, TFIID, and TFIIB.136,139,140,141,312 LCA agonized VDR additionally induces the expression of detoxifying metabolic enzymes such as CYP3A4, SULT2A1, MRP3, and ASBT, which cumulatively enhance the metabolism and excretion of BA acids.313,314,315,316 VDR agonism suppresses FXR-SHP-axis activity independent of its DNA binding domain, preventing FXR from transactivating FXRE-containing promoters.317 In addition, VDR agonism transcriptionally represses SHP.318,319 Lastly, VDR expression and polymorphism within VDR has been found to significantly affect microbial beta diversity, directly modulating the efficacy of secondary BA synthesis.320,321 A lack of VDR agonism has been associated with decreased Lactobacillus presence, and enhanced Clostridium and Bacteroidetes presence.
Pregnane X receptor
Pregnane X receptor (PXR) is a RXRα heterodimeric transcription factor that is largely expressed within the liver and gut.322 PXR is commonly referred to as the xenobiotic sensor since it regulates the expression of many classes of enzymes associated with xenobiotic metabolism.323 Upon binding to a xenobiotic agonist, LCA, or 3-oxo-LCA, PXR induces the expression of Cytochrome P450 3 A, 2B, and 2 C subfamilies of phase I metabolic enzymes, phase II enzymes such as glutathione S-transferases, UGTs, and SULTs, and phase III enzymes such as Multidrug Resistance 1 (MDR1), MRP2, and Organic Anion Transporting Polypeptide 2 (OATP2). PXR therefore has a direct role in preventing lipophilic BA-mediated toxicity, such as that which occurs with high LCA concentrations.324,325,326 Activated PXR sequesters HNF4α, preventing it from interacting with PGC-1α, transcriptionally repressing CYP7A1.327,328 PXR agonism enhances de novo lipogenesis by enhancing expression of SCD1 and various long fatty acid elongases, in addition to sequestering the transcription factor Forkhead Box Protein A2 (FOXA2) and preventing the expression of CPT-1A.329,330 Lastly, agonized PXR may directly dimerize with pCREB, reducing its ability to induce gluconeogenesis.331
Constitutive androstane receptor
The hepatically expressed RXRα heterodimeric transcription factor constitutive androstane receptor (CAR) has high homology to that of PXR. Different from that of PXR, CAR is constitutively active, where androstane metabolites were found to be inverse agonists.332,333 Analogous to PXR, CAR induces the expression of enzymes responsible for metabolizing BAs, but it is unsure which BAs modulate CAR transcriptional regulation and to what extent.323,334,335
Liver X receptors
The LXRs are heterodimer transcription factors with RXRα that are endogenously agonized by oxysterols.336 Two genes encode the highly homologous LXR family, NR1H3 for LXRα and NR1H2 for LXRβ.337 LXRα is found extensively within the liver, adipose tissue, the gut, and macrophages, while LXRβ is expressed ubiquitously.338 In rats, LXR activation serves as a sensor for cholesterol over-abundance, enhancing the transcription of CYP7A1, ABCG5, and ABCG8 to increase cholesterol excretion and decrease its absorption.339,340 In humans, LXR activation has similar effects on ABCG5 and ABCG5 but reduces BA synthesis by inducing SHP transcription.341,342,343 HDCA has been found to be a weak LXRα agonist.344
Peroxisomal proliferator-activated receptor alpha
PPARα is a transcription factor that under starved conditions and adipose tissue lipolysis induces fatty acid uptake and catabolism.345,346 HDCA has been found to directly displace Chromosomal Region Maintenance 1 (CRM1) from the Ran GTPase (Ran)/CRM1/PPARα cytosolic shuttling heterotrimer, allowing for PPARα to locate to the nucleus and modulate the transcription of lipid-catabolic genes.347 PPARα agonism has been found to suppress expression of NTCP, OATP1, and upregulate BSEP/MRP3/MRP4 expression, cumulatively enhancing BA efflux from the liver.348,349 In addition PPARα reduces NLRP3 inflammasome activation, caspase-1 cleavage, and downstream maturation of IL-1β by inducing the transcription of lncRNA Gm15441.350
Ceramides, a unifying hypothesis against BA-induced cardiometabolic disease
Abnormal biochemical or physiological changes to the BA pool composition directly alter the ratio between ceramide synthetic gut FXR agonism and ceramide catabolic systemic TGR5 agonism. Resulting increases in ceramide synthesis are directly deterministic for the pathogenesis of cardiometabolic diseases. Ceramides negatively impact the liver by inducing lipogenesis and gluconeogenesis (Fig. 14). Hepatic uptake of gut FXR-induced ceramides has been found to lead to hepatocyte ER stress, the activation of JNK, the phosphorylation of the transcription factor c-Jun, and the inhibition of HNF1α, a key transcription factor responsible for the transcription of the FXR gene. This chain of events prevents proper FXR-mediated SREBP-1C repression, leading to increases in lipogenesis and overall promotion of the steatotic phenotype of NAFLD/NASH.124,351 Hepatic ceramide exposure has also been found to repress the expression of mitochondrial Citrate Synthase (CS), leading to the accumulation of Acetyl-CoA, the allosteric activator of the first enzyme in gluconeogenesis Pyruvate Carboxylase (PC).132 Hence, hepatic ceramide exposure directly enhances the gluconeogenic and hyperglycemic phenotype associated with T2DM. Specific to adipose tissue, ceramide exposure directly represses HSL-mediated lipolysis. The protein SET Nuclear Proto-Oncogene (SET) is generally bound to protein phosphatase 2A (PP2A) and inhibits it, but ceramide exposure releases this inhibition. PP2A activation dephosphorylates and inactivates HSL preventing adrenergic Gαs-cAMP-PKA induced lipolysis.352,353,354 The inhibition of HSL-mediated lipolysis directly predisposes for peripheral adiposity, the hallmark of obesity.
Ceramides, BA-induced culprits behind cardiometabolic disease. Systemic ceramide secretion induced by high gut FXR agonism and low systemic TGR5 agonism increases lipogenesis, gluconeogenesis, pro-atherogenic inflammation, and insulin resistance while inhibiting lipolysis and mitochondrial function. Proteins with a yellow outline represent kinases and phosphatases, while yellow arrows represent phosphorylation/dephosphorylation. Yellow P’s represent phosphate groups. Italicized text refers to genes while normal text refers to proteins. This figure was created with BioRender.com
Ceramide exposure to the liver and adipose tissue results in increased insulin resistance and mitochondrial dysfunction. In the liver and adipose tissue activated PP2A dephosphorylates stimulatory pT308 of Akt.352,353,354,355 In addition, hepatic and adipocyte exposure to ceramides stimulates PKC isoform Zeta (PKC-ξ), leading to the inhibitory phosphorylation of Akt at T34.356,357 The inhibition of the central kinase behind the insulin signaling cascade Akt results in impaired glucose tolerance and insulin resistance, the key phenotype of T2DM. In addition, ceramide synthesis directly stimulates the nuclear translocation of NF-κB, inducing TNFα/IL-1β expression and NLRP3-mediated metabolic inflammation. This inflammatory signaling is a driving factor behind atherosclerotic plaque formation, insulin resistance, and the pathogenesis of obesity and NAFLD/NASH.358,359,360,361 Mitochondrial exposure to ceramides within hepatocytes and adipocytes inhibits oxidative phosphorylation, β-oxidation, and leads to mitochondrial fragmentation, a common finding in T2DM, obesity, and NAFLD/NASH.362,363,364,365 It is then incredibly clear that the relative ratio between gut FXR and central TGR5 agonism determines the rate and extent of ceramide synthesis, which directly induces the hallmark phenotypes associated with T2DM, obesity, NAFLD/NASH, and ASCVD.
BA-mediated pathophysiology of diseases
BA-centric pathophysiology of T2DM
Patients with T2DM appear to have elevated serum BA concentrations in both the fed/fasting states, irrespective of the intensity of insulin therapy (Fig. 15).366,367,368,369 This is thought to occur due to hyperglycemic-induced FXR-independent hyperacetylation of the CYP7A1 promoter allowing for increased expression.370 In addition to an expanded BA pool size, BA-centric mechanisms reinforce the key T2DM characteristics of impaired insulin sensitivity and overactive gluconeogenesis. A direct connection can be made between BA signaling and T2DM by FOXO1, a gluconeogenic transcription factor that should be inhibited by insulin signaling, but in insulin resistance paradoxically remains active. FOXO1 enhances the expression of G6Pase, PEPCK, and in the context of BAs CYP8B1.178,371 In obese patients with insulin resistance higher ratios of serum 12α-OH BAs to non-12α-OH BAs are seen but normalizes in fully established T2DM.372,373 CYP8B1−/− knockout mice were observed to have a lower hydroxylation ratio alongside with increased GLP-1 levels and improved insulin sensitivity, supporting the role that aberrant 12α-OH BA synthesis is associated with reduced incretin release. Overactive CYP8B1 activity leads to an increased BA pool fraction of CA, which increases the speed at which lipid nutrients are absorbed. This results in a smaller quantity of dietary fatty acids that reach enteroendocrine L-cells, lowering ATP generation and ATP-induced GLP-1 secretion.374
FXR-centric pathophysiology of T2DM
The main FXR-centric mechanism for T2DM pathogenesis is the role of overactive gut FXR agonism and the systemic secretion of ceramides. Gut FXR has a twin-fold nature in relation to gluconeogenesis. Gut FXR agonism favors gluconeogenesis by upregulating ceramide secretion and repressing GLP-1 secretion, while opposes gluconeogenesis by [FGF15/19]-[FGFR4/βK]-FXR axis mediated suppression of hepatic FOXO1/PGC-1α/HNF4α activity.375 Systemic ceramide exposure in CA-supplemented diet C57BL/6J mice directly predisposes for T2DM by inhibiting hepatocyte/adipocyte Akt leading to insulin resistance, by repressing the FXR-SHP-[HNF4α/FOXO1] axis, and by stimulating PC activity, leading to increased flux through all four rate-limiting enzymes of gluconeogenesis: G6Pase, PEPCK, FBPase, and PC.176,376 Although serum FGF19 levels are lowered in obese and T2DM patients, future research is needed to distinguish if analogous to insulin a FGF15/19 “resistance” exists, silencing either the secretion of FGF15/19 or its signal transduction within the liver.377 This may be plausible since both the insulin receptor and FGFR4 are receptor tyrosine kinases which are exposed to abnormal levels of their respective agonists. To provide further evidence, human trials in insulin-resistant and non-insulin-resistant NAFLD patients found an inability for insulin-resistant patients to successfully transduce FGF19 binding into CYP7A1 repression.378 Collectively, decreases in incretin release by insulin resistance-induced 12α-OH BAs, ceramide-induced hepatocyte/adipocyte insulin resistance, and ceramide-induced overactivity of all four rate-limiting enzymes of gluconeogenesis create a strong connection between abnormal BA pool compositions, the gut-liver FXR-axis, and the T2DM phenotype.
TGR5-centric pathophysiology of T2DM
A lack of TGR5 signaling can influence both insulin resistance and the inflammatory responses common to the metabolic tissue of T2DM. In the gut, FXR agonism inhibits GLP-1 secretion while TGR5 agonism induces secretion.174,259 A relative increase in gut FXR agonism may lead to compromised GLP-1 release.179,208,379 This viewpoint is supported by TGR5−/− mice which had impaired whole body insulin sensitivity and GLP-1 secretion.380 Low central TGR5 agonism is directly correlated to higher levels of T2DM-specific inflammation, leading to adipose/liver-associated insulin resistance by upregulating the NLRP3 inflammasome, increasing NF-κB/STAT3 signaling, and by decreasing the expression of anti-inflammatory LIP.278,287,381,382 This in addition to the lack of TGR5-induced increases to basal metabolic rate, ceramide metabolism, and TGR5-[PGC-1α]-ADIPOQ-AdipoR1/R2 mediated signaling, puts patients at risk for unregulated gluconeogenesis and insulin resistance. The importance of high gut TGR5 agonism and low gut FXR agonism in preventing the pathogenesis of T2DM is showcased by recent human studies on HCA, which both antagonizes gut FXR and agonizes gut TGR5. HCA is depleted in T2DM patients relative to healthy controls and directly reduces pre-prandial glucose measurements, indicating that its functional absence, an imbalance between gut FXR and gut TGR5 agonism, may be a key contributing factor to the T2DM phenotype.383,384
BA-centric pathophysiology of obesity
Serum BA concentrations in obese patients correlates positively with BMI (Fig. 15).369,385 Similar to obese patients with insulin resistance, patients who lose weight maintain the high 12α-OH BA to non-12α-OH BA ratio despite lowering total BA and GLP-1 concentrations to normal levels.386 Mechanistically the lack of proper non-12α-OH BA synthesis may be a long-term adaptive response to weight-gain that predisposes for further metabolic risk.387,388,389 In addition, human studies identified that hepatic BSEP/NTCP expression was negatively correlated with BMI.390 This indicates that obese patients have reduced hepatic efflux of primary BAs into the gallbladder, in addition to an impaired hepatic reuptake of conjugated secondary BAs from the gut. Subsequently, the hepatic primary BA overload and overactive FXR agonism would directly induce hepatic efflux of primary BAs into the systemic circulation. The lack of primary BA biliary transport will then hinder systemic rates of secondary BA synthesis, reducing the BA pool fraction of potent/efficacious TGR5 agonists.
FXR-centric pathophysiology of obesity
General FXR expression is protective against obesity due to maintaining lipid and carbohydrate metabolism, but overactive hepatic/gut FXR agonism has been found to exacerbate weight gain, dyslipidemias, and reduce glucose tolerance in HF diet ob/ob mice.391,392,393 Analyzing the pathophysiology of obesity, the strong negative autoregulation on BA synthesis may be an adaptive technique to reduce intestinal lipid absorption under a HF diet, but at the cost of reducing BA pool size and hence metabolic impacts at TGR5.394 Human gut FXR agonism within ileal biopsies has been found to directly correlate to BMI.124 Chronic gut FXR agonism and elevated serum ceramide concentrations are associated with diet-induced obesity (DIO) in both humans and mice.358 Gut FXR overactivity and ceramide-induced gluconeogenesis, lipogenesis, and lipid accumulation play key roles in the development of the obese phenotype.124,395 Gut-specific FXR−/− mice were found to be protected against HF DIO and insulin resistance by either enhancing GLP-1 secretion and/or reducing negative ceramide-based metabolic effects.83,124 Whole-body FXR−/− mice had increased HFD-induced dyslipidemias and glucose intolerance if fed a normal chow diet, but were protected against both obesity and glucose intolerance if fed a HF diet.163,392,396,397 The same results were not found in a liver-specific knockout, indicating that the protective role of whole-body FXR knockout may be due to gut-based repression of FXR.398 Analogous to that with T2DM, HCA prevalence was significantly lower in obese patients relative to healthy controls. Similarly, this indicates that the imbalance between gut FXR agonism and gut TGR5 agonism may be a crucial driving factor in the pathogenesis and maintenance of obesity.383
TGR5-centric pathophysiology of obesity
The role and proper agonism of TGR5 is paramount in preventing the onset of obesity.399,400 Mechanisms that shift BA pool composition away from TGR5 agonism may predispose and increase the risk for obesity. Experimentally it was identified that systemic knockout mice for βK (Klb−/−) were protected against HF DIO.401 The lack of βK was found to shift BA pool composition to favor the classical pathway to produce a higher fraction of DCA, a highly potent TGR5 agonist.402 Reduced TGR5 agonism would result in reduced basal metabolic rate and reduced incretin release, predisposing for obesity. This protective role was removed with double knockout Klb−/−/TGR5−/− mice, insinuating that it is not purely the lack of FGFR4/βK activity that is protective, but the increase in potent/efficacious TGR5 agonizing BAs.403,404 Outside of traditional metabolic tissue, TGR5 has been found within the hypothalamus of mice. Within the hypothalamus TGR5 agonism promotes a systemic negative energy balance by increasing systemic sympathetic tone. Following from such, hypothalamic TGR5−/− HF DIO mice were predisposed for obesity.400 Cumulatively, it is then apparent that changes to the BA pool size/composition that reduce TGR5 agonism have a direct effect on the pathogenesis of obesity.
BA-centric pathophysiology of NAFLD/NASH
Fasting and postprandial total/12α-OH BA levels of NASH patients correlate positively with steatosis and histological disease severity (Fig. 15).405,406,407,408 High transcription of CYP7A1 but low CYP7A1 protein is exhibited in NAFLD/NASH, indicating faulty translation or overactive degradation of CYP7A1, along with the inability for hepatic FXR to suppress CYP7A1.409,410 Hepatic FXR activity is protective against steatosis and fibrosis, the two main hallmarks of NAFLD and its disease progression into NASH or hepatocellular carcinoma (HCC).398,411,412,413,414 Whole-body FXR−/− in mice is associated with increased hepatic levels of cholesterol, free fatty acids, triglycerides, and inflammation, where lipid accumulation is inversely correlated with FXR expression in both mice and humans.411,415,416,417 Intestinal FXR−/− in mice improves HF diet-induced steatosis.83,124,132 This is accomplished by reducing ceramide-induced hepatic FXR repression, allowing for proper FXR-SHP axis SREBP-1C silencing. Further supporting this hypothesis is the activity of HDCA, which decreases triglyceride concentrations by activating PPARα-induced β-oxidation and inhibits gut FXR ceramide-induced steatosis. HDCA was significantly depleted in both NAFLD patients and HF high sucrose NAFLD model mice relative to healthy controls, indicating that the lack of BA PPARα agonism and gut FXR antagonism may be pathogenic for NAFLD/NASH.347,418
FXR/TGR5-centric pathophysiology of NAFLD/NASH
The fibrotic risk for a NAFLD/NASH patient is positively correlated to both high conjugated CA concentrations and lower ratios of secondary to primary BAs.408,419 Analogous to obesity, this may be caused by similar inhibition of BSEP/NTCP. Phenotypically this may present with hepatic efflux of primary conjugated BAs into the systemic circulation and reduced entry of BAs into the gut, hence lower rates of secondary BA synthesis. The key cells responsible for hepatic scarring and fibrosis are hepatic stellate cells (HSC), which when agonized by the FXR-SHP axis exhibit less fibrotic activity.420,421,422 HSC FXR-SHP axis agonism represses the expression of and responsiveness to transforming growth factor beta (TGFβ) and its receptor transforming growth factor beta receptor 2 (TGFβR2) in CCl4 mice fibrosis models.423,424 In addition, agonism increases the expression of Perilipin-1 (PLIN1) to stabilize fat droplets and induces Peroxisome Proliferator-Activated Receptor-Gamma (PPARγ) to reduce inflammatory cytokine and collagen synthesis.425,426 However, in situations of HSC activation, such as in NASH, it becomes more challenging to activate FXR due to increasing rates of SUMOylation.427 FXR−/− mice exhibited enhanced hepatic inflammation and necrosis from lipopolysaccharide (LPS) administration or autoimmune hepatitis, highlighting the crucial role of FXR in reducing hepatic inflammatory responses.188,193,428 Further evidence supports the role of the FXR-SHP axis in preventing the inflammatory phenotype of NAFLD/NASH, in which FGF15/19 supplementation prevents hepatic inflammation and cholangiopathies in HF, high fructose, and HC diet NASH mouse models.429,430
TGR5 agonism in relation to NAFLD/NASH is nuanced. Intestinal TGR5 agonism protects against hepatic steatosis by GLP-1 secretion and adipose TGR5 agonism protects against lipotoxicity by ADIPOQ signaling. Systemic TGR5 agonism is potently anti-inflammatory and inhibits NF-κB activity in mouse models of hepatic LPS-induced inflammation, likely reducing hepatic inflammatory burden.280 TGR5’s hepatic immunological response likely plays a strong role in NAFLD/NASH pathogenesis, in which its activity in Kupfer cells directly prevents LPS-induced cytokine production.431 The lack of TGR5 agonism appears to be pathogenic for NAFLD, while TGR5 agonism may be pathogenic for disease progression into NASH, in which TGR5 induces p38MAPK/ERK1/2 activity within HSCs and TGFβ synthesis within mice macrophages, driving profibrotic activity.284,432,433
Gut-liver axis dysfunction suppresses hepatic FXR and induces NAFLD/NASH
NAFLD pathogenesis and disease progression to a good approximation occurs according to the “two-hit” hypothesis: hepatic lipid accumulation followed by inflammation-induced fibrosis.434,435,436 The activity of hepatic FXR is critical for protection against both steatosis and inflammation-induced fibrosis and was found to be repressed by the protein Ying Yang 1 (YY1).437 YY1 is a transcriptional repressor that has roles in cell-cycle progression, mitogenesis, and insulin/insulin-like growth factor (IGF) signaling, but most importantly strongly represses FXR.438,439,440,441 Mechanistically, activated YY1 binds intron-1 of FXR and prevents transcription. YY1 was found to be upregulated in the livers of HF DIO mice, db/db mice, and NAFLD patients due to TNFα-induced activation of NF-κB and NF-κB stimulated transcription of YY1.442,443,444 TNFα exposure may be endogenous due to obesity-induced inflammation or exogenous via c exposure.445,446 Anti-YY1 shRNA containing adenovirus infection was found to rescue FXR levels and decrease hepatic steatosis in obese mice.447 The activity of YY1 hints towards a threshold in which a baseline of obesity-induced inflammation triggers the repression of hepatic FXR, eliminating its important role in protecting against both unregulated SREBP-1C lipogenesis and the activation of profibrotic HSCs; the lack of proper hepatic FXR agonism and overactive gut FXR agonism are both directly associated with the disease onset of NAFLD/NASH. Cumulatively, the proper expression and function of hepatic FXR and repression of gut FXR appear to be key factors in the development of NAFLD/NASH.
BA-centric pathophysiology of cardiovascular disease
BAs have dose-dependent effects on the heart, which if elevated may be cardiotoxic and lead to cardiomyopathy.448 To expand on such, inhibition of FXR has been found to be protective against ischemic cardiac damage, while FXR agonism is pro-apoptotic in cardiomyocytes.449 From the perspective of cardiac arrythmias, conjugated BAs such as taurine conjugated CA have been found to contribute towards atrial arrythmias such as atrial fibrillation.450 Similar to other diseases, imbalances to BA pool size and composition are likely to play key roles in the pathogenesis of cardiomyopathic diseases such as heart failure and atrial arrythmias.
It is still largely unknown how potent systemic FXR activity is in modulating atherosclerosis. Some evidence exists supporting that CDCA is a 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A (HMG-CoA) Reductase inhibitor.451 Double knockout FXR−/− ApoE−/− mice fed a HF diet were found to have increased atherosclerotic lesion sizes and abnormal plasma lipid profiles.452 Furthermore, FXR signaling within vascular smooth muscle was found to blunt myocyte migration and the inflammatory response associated with atherosclerotic plaque development.453 Mechanistically, this occurs due to the FXR-SHP axis mediated repression of inducible nitric oxide synthase and cyclooxygenase-2.454 Although it may seem that FXR agonism definitively improves atherosclerosis, somewhat contrasting work has been published showing opposite net effects. FXR−/− in LDLr−/− or ApoE−/− mice reduced atherogenic lesion size and produced unexplained plasma lipid differences.455,456 Similar results have been found in humans, in which systemic FXR agonists like OCA increase serum LDL cholesterol in a dose-responsive relationship.457
It is important to remember that FXR has time-, dose-, and tissue-dependent impacts on lipid physiology. Within humans, hypercholesterolemia is positively correlated with circulating FGF19/ceramide concentrations and inversely correlated with 7α-hydroxycholesterol concentrations. This indicates that a relationship exists between cholesterol burden and gut FXR agonism (Fig. 15). Gut FXR-mediated ceramide synthesis has negative impacts on the cardiovascular system in HC fed mice, directly inducing macrophage to foam cell differentiation and NLRP3 initiation of plaque inflammation, driving further plaque development. Furthermore, gut FXR agonism in HC diet mice via FGF15 secretion represses hepatic CYP7A1, LDLr, and ABCG8 expression, but paradoxically has no activity on inducing gut ABCG5/ABCG8 expression, further driving hypercholesterolemia. Gut FXR−/− mice exhibited significantly reduced ASCVD development and SMPD3-mediated ceramide synthesis, which was reversed in gut FXR−/− mice that overexpressed SMPD3 via a lentivirus vector.358,359 It is likely that increased hepatic cholesterol burden increases BA synthetic flux, providing more gut FXR agonism and FGF15-mediated CYP7A1 repression. Repression of CYP7A1 biases BA pool composition towards the potent FXR agonist CDCA, further agonizing gut FXR and forming a positive feedback loop. Persistent gut FXR agonism enhances hepatic cholesterol burden, while ceramide expression directly accelerates atherosclerotic plaque development.
TGR5 is not without cardiovascular concern, in which 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced mouse models of biliary fibrosis have developed cardiac hypertrophy due to overactive TGR5-Akt signaling.458 In dogs TGR5 agonists have been found to induce reflex tachycardia due to TGR5-induced activation of the K(Ca)1.1 potassium leak channel within vascular smooth muscle.459 Contrasting results have been shown in other studies, in which TGR5 is cardioprotective by reducing cardiac stress and by reducing atherosclerosis.279,460 In human patients it was found that LCA levels negatively correlated with the presence of atheroma, highlighting the potential pro-atherosclerotic phenotype of a low LCA content BA pool.461 Further research is needed to pinpoint the positive and negative roles of TGR5 on cardiovascular physiology.
BA-centric pathophysiology IBDs
Mucosal immune cells within the gut must defend against pathogens, retain an endogenous balance of commensal bacteria, and prevent disruption of the gut mucosal barrier.462 BA pool size and composition may directly modulate the gut micro-environment. Millimolar concentrations of conjugated primary BAs are antimicrobial by triggering FXR agonism-induced defensin responses, assisting to prevent bacterial overgrowth.463,464,465 In some cases, BA-mediated effects have been found to be protective against opportunistic pathogens such as Clostridium difficile.466,467
DSS and 2,4,6-trinitrobenzene sulfonic acid (TNBS) mouse models of colitis have found that the extent of systemic FXR agonism impacting monocytes, macrophages, dendritic cells, and gut epithelia is inversely proportional to mucosal inflammation.468,469 A similar phenotype was found in two different rat models of intestinal inflammation, bile duct ligated cholestasis and mesenteric artery clamp induced reperfusion injury.470,471 From such it is believed that FXR represses mucosal inflammation by modulating both the innate immune system and intestinal epithelia, likely by inhibiting the NF-κB/NLRP3 induced expression of TNFα, IL-1β, and IL-6. In humans it was found that patients with colitis had decreased intestinal expression of FXR, while in mice FXR activation reduced colitis disease severity.101,191,472 Collectively, low gut FXR activity may be pathogenic for IBD.
TGR5 agonism modulates the integrity and inflammatory status of intestinal mucosa in DSS/TNBS rodent models of colitis.473 TGR5 activation was able to directly suppress LPS-induced inflammatory cytokine production in macrophages derived from patients with Crohn’s disease.282 Treatment of mucosal macrophages derived from Crohn’s disease patient biopsies with a TGR5 agonist directly reduced pro-inflammatory cytokine expression, supporting that situations of low TGR5 agonist concentration within the BA pool may be pathogenic towards IBD.282 TGR5 agonism additionally biases the differentiation of human monocytes into tolerogenic dendritic cells, secreting low levels of TNFα and IL-12.474 Lastly, TGR5 induced IL-10 expression has been found to increase the recruitment of anti-inflammatory peripheral regulatory T cells (pTregs) to inflamed colonic tissue.284
BA metabolites modulate IBD pathogenesis by influencing pTreg/Th17 differentiation
Control of the immune response to bacteria is crucial for proper barrier function (Fig. 16a). Secondary BAs like Ruminococcus gnavus 3α/β-HSDH C3-epimerized DCA (isoDCA) and ωMCA antagonize FXR transcriptional activity within the dendritic cells of mice, inducing the differentiation of pTregs dampening immune responses.189,475 This effect remained without ligand administration within FXR−/− mice dendritic cells, pinpointing the outcome as a direct endpoint of FXR antagonism. Cluster of Differentiation 4 (CD4) positive (CD4+) T cells are directly differentiated into pTregs by expression of the Forkhead Box P3 (FOXP3) transcription factor. On its own, expression of the Retinoid Orphan Receptor Gamma T (RORγt) transcription factor induces the differentiation of CD4+ T cells into pro-inflammatory T Helper 17 (Th17) cells, while its activation after that of FOXP3 produces pTregs with enhanced immunosuppressive abilities.189,476,477,478
BA-centric pathogenic mechanisms for IBD, cancer, and antiviral innate immune overactivity. a Novel BA metabolites enhance pTreg differentiation and inhibit Th17 differentiation, reducing autoimmune IBD-associated inflammation and enhancing gut-liver axis homeostasis. b The FGF15/19-[FGFR4/βk] axis can predispose for neoplastic promotion by inducing mitogenesis. In addition, the FGF15/19-[FGFR4/βk] axis can predispose for chemotherapeutic resistance by preventing chemotherapy-associated ferroptosis. c The Type 1 Interferon response induces TGR5-mediated activation of the cGAS-STING signaling cascade, enhancing antiviral responses, and potentially enhancing cGAS-STING-associated inflammation. Gold border proteins represent kinases, while gold arrows represent phosphorylation. mt is an abbreviation for “mitochondrial”. This figure was created with BioRender.com
The administration of isoDCA does not only induce pTreg differentiation but increases the number of FOXP3+/RORγt+ co-expressed Tregs, indicating enhanced anti-inflammatory activity. In addition to the role of isoDCA in producing pTregs, secondary BA VDR agonists have been found to enhance the differentiation of FOXP3+/RORγt+ Tregs.479 Seemingly contradictory in nature, it is still mechanistically unknown why FXR antagonists like isoDCA, ωMCA, and not UDCA provide pTreg induction when it is FXR agonism that induces well-characterized dendritic cell anti-inflammatory phenotypes. As a caveat, these in vitro experiments were co-cultures of T cells and dendritic cells, so it is still unknown if FXR antagonism at dendritic cells at the in vivo scale is more important for IBD pathogenesis compared to well-known potent general gut anti-inflammatory FXR agonism.
Alongside the FXR-sensitive impact of isoDCA and ωMCA on dendritic cell-induced pTreg differentiation, other secondary BA metabolites play extensive roles in directly modifying CD4+ differentiation into Th17s/pTregs, such as 3-oxo-LCA and the C3/C5 epimer of LCA (isoalloLCA). LCA is metabolized by microbial 3α-HSDH to produce 3-oxo-LCA, which inhibits Th17 differentiation by directly binding to and inhibiting the transcription factor RORγt. Bacteroidete phylum 5α/β-reductase and 3β-HSDH activity produces isoalloLCA from 3-oxo-LCA, which when exposed to CD4+ T cells induces mitochondrial reactive oxygen species (ROS) generation. This ROS by unknown mechanisms relaxes the chromatin of FOXP3, allowing for Nuclear Receptor Subfamily 4 Group A Member 1 (NR4A1) to bind and enhance FOXP3 transcription, producing FOXP3 and differentiating CD4+ T cells into pTregs.480
To further the importance of BAs in gut immune regulation, 3-oxo-LCA, isoalloLCA, and the bacterial microbiome prevalence of their required metabolic enzymes are all found to be significantly deficient in patients with IBDs, in which their concentrations are inversely correlated with the expression of human Th17-associated genes.481 Alongside this, human metagenomic studies have found that patients with IBDs have lower quantities of secondary BAs.482 Cumulatively, this indicates that a lack of both secondary BA substrates and their required metabolic enzymes may provide a deficiency of 3-oxo-LCA- and isoalloLCA-mediated immunosuppression, being pathogenic for IBDs.483,484 From a structural perspective, these three ligands have either planar or β-orientation C3 functional groups, perhaps serving as a basis for new structure-activity relationships investigating BA-mechanistic immunosuppressive therapies.
A bidirectional inflammatory highway, the gut-liver axis
The gut-liver axis holds a central role in BA physiology, in which hepatic function is bidirectionally impacted by the gut in terms of both human tissue and the microbiome. If the liver does not produce a substantial enough BA pool of the correct composition, both gut inflammation and overall bacterial burden increase. This negative hepatic-induced gut phenotype decreases the integrity and increases permeability of the mucosal barrier, enhancing hepatic exposure to bacterial toxins such as LPS, which are now able in higher quantities to enter hepatic portal circulation and trigger hepatic inflammation.485,486 Hepatic inflammation may then induce phenotypes associated with NAFLD/NASH and further detriment BA-regulated gut health.487,488 To illustrate this point, a deficiency in PXR agonizing BAs has been shown to increase the prevalence of BSH containing Lactobacillus. Although this outcome may modulate FXR-induced defensin production, PXR agonism deficiency decreases mucosal barrier function by enhancing Toll-Like Receptor 4 (TLR4) mediated inflammatory signaling, likely enhancing LPS burden on the liver.489,490
BA-centric pathophysiology of cancer
Primary and secondary BAs have been widely associated with carcinogenesis and positive or negative impacts dependent on the concentration administered and the type of neoplasm.491 This heterogeneity in outcome is likely driven by the tissue-dependent extent and selection of BA receptor expression and the again tissue-dependent outcome of specific receptors. Nuclear BA receptors such as FXR, PXR, LXR, CAR, VDR, membrane receptors such as TGR5, SIPR2, CHRM2, and CHARM3, and BA-adjacent receptors such as FGFR4/βK influence many downstream pathways commonly associated with neoplastic development such as EGFR, MAPK, STAT3, NF-κB, TLR4, HNF4α, β-catenin, Suppressor of Cytokine Signaling 3 (SOCS3), and in the case of FGFR4/βK, directly FGFR4.492,493,494,495,496,497
The impact of BAs on carcinogenesis is concentration dependent, in which low concentrations are anti-cancerous and high concentrations are carcinogenic. This ambivalence is likely due to their amphipathic nature and from such polypharmacology, which at lower doses is likely inactive but at supraphysiological doses may act as a surfactant damaging membranes to trigger Phospholipase A1 (PLA2)-mediated inflammation and ROS, while PKC activation mediates p38-MAPK-p53-[NF-κB] signaling, apoptosis, and inflammation.466,498,499,500 The inherent cytotoxicity of high-dose BA exposure is supported by the numerous endogenous physiological mechanisms in place to detoxify high levels of lipophilic BAs.
Out of all BAs, UDCA appears to be the most fit as a tumor-suppressive therapy. Throughout various concentrations ranging from 100 μM to 800 μM, UDCA has been found to be tumor suppressive in many different cancers, such as hepatocellular carcinoma, gastric cancer, colon cancer, cholangiocarcinoma, prostate cancer, and glioblastoma.501,502,503,504,505,506,507,508,509,510 LCA from 0.3 μM to 200 μM, DCA from 10 μM to >500 μM, and CDCA from 10 μM to >500 μM have been found to exert pro-apoptotic and anti-proliferative signaling in multiple different cancers such as breast cancer, colorectal cancer, gastric cancer, hepatocellular carcinoma, ovarian cancer, and prostate cancer.497,511,512,513,514,515,516,517 At the same time, LCA, DCA, and CDCA have been found to be pro-carcinogenic in similar concentration ranges, enhancing cholangiocarcinoma, colorectal cancer, gastric cancer, hepatocellular carcinoma, Barett’s esophagus, along with many other cancers.303,493,518,519,520,521,522,523,524,525,526,527,528 This further establishes that finely-tuned research is needed to pinpoint tissue-dependent specific thresholds in which certain BAs switch from primarily anti-carcinogenic to pro-carcinogenic pharmacodynamic profiles.
FGFR4 overexpression is carcinogenic and provides chemotherapeutic resistance
The FGF15/19-[FGFR4/βK] axis is highly associated with both carcinogenesis and chemotherapeutic resistance (Fig. 16b). FGF15/19-[FGFR4/βK] axis activity directly activates mitogenic signaling pathways within hepatocytes, such as PI3K-Akt and Ras-Raf-MEK-ERK, being strongly associated with the promotion of human hepatocellular carcinoma.529,530 Alongside the direct promotion of hepatocellular carcinoma, FGFR4/βK prevents ferroptosis within human hepatocytes and breast tissue. Mechanistically, FGF15/19-[FGFR4/βK] axis signaling induces ERK-RSK phosphorylation and inhibition of GSK3β. Inhibition of GSK3β prevents its activity within the β-catenin destruction complex, preventing β-catenin phosphorylation/ubiquitination, and allowing for its accumulation. β-catenin upon displacing the protein Groucho from Transcription Factor 4 (TCF4) within the nucleus activates TCF4 and induces the expression of Solute Carrier Family 7 Member 11 (SLC7A11) and Ferroportin 1 (FPN1).531,532 Both of these proteins are anti-ferroptotic. FPN1 directly effluxes reactive ferrous iron while SLC7A11 exchanges extracellular cysteine for intracellular glutamate, maintaining intracellular glutathione stores and ensuring the activity of Glutathione Peroxidase 4 (GPX4)-mediated detoxification of iron-induced lipid peroxidation.533,534 If overactive this pathway may strongly repress the ability for cytotoxic agents to kill tumors, acting as a major mechanism of chemotherapeutic resistance for pan-kinase inhibitors such as Lenvatinib.535
Besides that of the FGF15/19-FGFR4/βK axis, FXR and TGR5 activity have been found to reduce carcinogenesis. FXR−/− mice have significantly higher rates of hepatic cancer.413,417 In addition, TGR5 by unknown mechanisms antagonizes the activity of Signal Transducer and Activator of Transcription 3 (STAT3) signaling pathways, resulting in suppressed Lipopolysaccharide (LPS) or IL-6 induced proliferation of liver, gastric, colorectal, and breast cancer.281,536
BA receptor-mediated contributions to the antiviral innate immune response
BA activity at TGR5 plays a significant role within the innate antiviral response (Fig. 16c). The type 1 interferon response associated with viral infections directly induces the expression of TGR5. From such, TGR5 agonism participates in a positive feedback loop, upregulating Akt/Interferon Regulatory Protein 3 (IRF3) signaling for further type 1 interferon synthesis and antiviral responses.537 In addition, TGR5 induced β-Arrestin activity has been found to activate Src, providing interplay between the FXR and TGR5 signaling cascades and in this case further upregulating antiviral responses of the innate immune system by phosphorylating Stimulator of Interferon Genes (STING), Tank-Binding Kinase 1 (TBK1), and IRF3, collectively upregulating the latter portion of the cGAS-STING pathway.538 This is especially interesting since recent research on the cGAS-STING pathway has indicated its overactivity in the pathogenesis of age-related inflammatory, neurodegenerative, and autoimmune diseases.539,540 Therefore, changes to the BA pool composition may directly enhance or repress overactive cGAS-STING pathway activity and its associated pathogenic effects.
Druggable targets, BA pharmacology
FXR modulators
Steroidal FXR modulators
Alongside endogenous BA FXR modulators, semisynthetic steroidal modulators of FXR have been synthesized, the most famous of which is OCA also known as INT-747 (Intercept Pharmaceuticals) (Fig. 17). OCA is structurally 6α-ethyl-CDCA and touts an impressive 100-fold increase in receptor potency compared to endogenous CDCA.541,542 A further modified derivative of OCA known as EDP-305 (Enanta Pharmaceuticals) is ~16-fold more potent than OCA and shows no activity at TGR5.543 Another derivative of OCA, TC-100, is C11β-OH OCA, a fully selective FXR agonist. TC-100 is roughly 16-fold more water soluble compared to OCA, limiting hydrophobic BA-induced toxicity, and ensuring rapid excretion from hepatocytes, likely exerting more gut-centric agonism.544 MFA-1 (Merck) was discovered as a steroidal FXR agonist but binds inverted in orientation to FXR compared to endogenous BAs. No transcriptional data for this has been published.545 Lastly, the betulinic acid derivative Compound F6 (Shanghai Institute of Materia Medica) was discovered as a potent gut-restricted FXR antagonist.546
Structures of key FXR and TGR5 modulators. Red circles are drawn around the following key structural motifs: non-steroidal FXR agonist isoxazole pharmacophore, the acrylic acid motif of fexaramine likely to provide gut-selectivity, various kinetophores allowing for TGR5 gut-selectivity, and an example of a metabolic soft spot again providing gut-selective TGR5 agonism. This figure was created with BioRender.com
Non-steroidal FXR modulators
The discovery and development of non-steroidal FXR agonists was a direct attempt to eliminate some of the adverse effect profile associated with steroidal FXR agonists, ideally eliminating pruritis and a pro-atherogenic serum profile.547 In general, drugs with a steroidal scaffold have the risk of binding to other steroid receptors (glucocorticoid, mineralocorticoid, androgen, estrogen, progesterone), from such deriving some of their adverse effects. Many believed that a FXR-selective non-steroidal scaffold could improve pruritis, since it was known that steroidal scaffolds could bind to TGR5 on dorsal root ganglionic neurons and release itch neuropeptides.548 There was less enthusiasm for a non-steroidal solution against the induction of a pro-atherogenic lipid profile, since it was mechanistically explainable as an FXR-intrinsic outcome.
Most non-steroidal FXR agonists are within the isoxazole “hammerhead” family, originally derived from GW4064 (GlaxoSmithKline).549 GW4064 has a low bioavailability, tropism for the Estrogen Receptor-related Receptor Alpha (ERRα), and has a short half-life.550 The short half-life and low bioavailability is general for the hammerhead class of FXR agonists, which are structurally similar enough to endogenous BAs to be hepatically taurine conjugated by BACS and BAT. Before conjugation, hammerhead family FXR agonists are ligands for ASBT-mediated gut absorption, but once conjugated are not, reducing their bioavailability by inhibiting enterohepatic circulation.551
Many compounds synthesized have been isoxazole-bearing derivatives of GW4064, such as the full agonists tropifexor (Novartis) and cilofexor (Gilead Sciences).552,553,554,555,556 Although abandoned, PX-102 (Gilead Sciences) was the structural intermediate between GW4064 and cilofexor (Gilead Sciences).551,557,558 Of this same class Novartis has the partial agonist nidufexor (also known as LMB763).559 TERN-101 (Terns Pharmaceuticals) was similarly discovered but instead as a partial agonist.560,561 Other isoxazole FXR agonists such as BMS-986318 (Bristol Myers Squibb) and the more recent BMS-986339 (Bristol Myers Squibb) have been discovered as high potency FXR agonists.562,563 The last major molecule of the isoxazole family is HPG1860 (Hepagene Therapeutics).564
Besides that of GW4064 derivative scaffolds, WAY-362450 (Wyeth Pharmaceuticals which was acquired by Pfizer) is a potent full FXR agonist but is horribly insoluble and has been tested only in mice.565,566 In 2003 fexaramine (Salk Institute) was discovered, which exhibited partial gut FXR agonism and no significant hepatic agonism. Fexaramine has very poor oral bioavailability, indicated by a single dose oral vs. intraperitoneal relative ~10% bioavailability in mice, establishing fexaramine as the prototype for gut-restricted FXR agonists.551,567,568 No clinical data has been published on fexaramine, however, the systemically active derivative of fexaramine MET409 (Metacrine) has been tested in humans against NASH and obtains serum concentrations high enough to trigger robust hepatic FXR agonism.569 Structure activity relationships have found that many of the general modifications performed on fexaramine to produce MET409 retain gut-restriction, however it is hypothesized that removing the Michael acceptor acrylic acid motif is what removes gut-restriction.551,570 This indicates that fexaramine may be gut-restricted due to covalent adduct formation within intestinal epithelia. This is corroborated by the recent discovery of the systemic FXR agonist LH-10 (Guangdong School of Pharmacy), which is another fexaramine derivative that is systemically active after removing the acrylic acid motif.571
Two recent developments have occurred in non-steroidal FXR modulator synthesis, namely the creation of a gut-restricted FXR antagonist and the identification of new natural product non-steroidal FXR agonist scaffolds. The 4-aminophenylacetamide scaffold V023-9340 (Guangxi Key Laboratory) was found to be potent gut-restricted FXR antagonist without significant hepatic suppression.572 Lastly, the sesquiterpenoid Compound 27 (Southwest University) and the glucoside flavonoid licraside (Lanzhou University) have been found to be novel scaffold non-steroidal FXR agonists.573,574
Key preclinical results—systemic FXR modulators
Systemic FXR agonists have emerged as potent but temperamental therapeutics. The endogenous weak FXR agonist CA was found to repress triglyceride levels in KK-Ay mice models, dampening hepatic steatosis and NAFLD development.150 OCA in Zucker fa/fa obese rats was found to reverse insulin resistance, correct obesity-induced lipid abnormalities, protect against weight gain, and was protective against hepatic steatosis.173 If treated with the FXR agonist OCA, the colons of DSS-induced colitis mice were found to have lower levels of the pro-inflammatory cytokines IL-1β and IL-6.469 In addition, OCA repressed TLR4-induced pro-inflammatory activity within intestinal epithelial cells, while reducing inflammatory cytokines expression in cultured human CD14+ monocytes and dendritic cells.191,469
GW4064, WAY-362450, PX-102, tropifexor, nidufexor, and cilofexor are also potent in treating cardiometabolic diseases. GW4064 was found to repress gluconeogenesis and lipogenesis within a db/db diabetic mice model.170 However, long-term dosing of GW4064 in obese insulin-resistant mice due to overactive FXR activity led to exacerbated weight gain and further worsened insulin resistance.391 GW4064 was also found to improve cholestatic hepatotoxicity in rats and reperfusion damage by upregulating SHP in Kupfer cells.575,576 WAY-362450 prevented fructose-induced hepatic steatosis in C57BL/6J mice and protected against NASH onset for mice fed a methionine and choline-deficient diet.577,578 WAY-362450 administration had a protective role against atherosclerotic lesion formation in ApoE−/− or LDLr−/− mice.190,579 Administration of tropifexor, nidufexor, and cilofexor have all been found to improve NASH symptoms in choline-deficient HF diet, chemical/dietary, and insulin-resistant obese NASH models in rodents.559,580,581 OCA, cilofexor, and a non-steroidal tool-compound named INT-2228 (Intercept Pharmaceuticals) had comparable impacts on serum LDL/HDL cholesterol levels in chimeric PXB-mice, reproducing the phenotype of heightened LDL cholesterol and lowered HDL cholesterol.582 BMS-986318 and BMS-986339 have both been found to strongly activate FXR-mediated antifibrotic effects in mice bile duct ligation NASH models.562,563 LH-10, licraside, and Compound 27 were all found to ameliorate α-napthylisothiocyanate (ANIT) mice model cholestasis.571,573,574 HPG1860 was found to prevent CCl4-induced NASH model inflammation, steatosis, ballooning, and fibrosis similar in effect to OCA.564
Key preclinical results—gut-restricted FXR modulators
Fexaramine in DIO mice reduced diet-induced weight gain, insulin resistance, fasting serum total cholesterol/triglycerides, serum TNFα/IL-1β, gluconeogenesis, and enhanced basal metabolic rate within adipose tissue. Mechanistically, fexaramine activates gut FXR-induced expression of FGF15/19, which represses CYP7A1, shunting cholesterol into the alternative pathway of synthesis.151,567,583,584 Enhanced BA pool composition of CDCA is metabolized by fexaramine-induced increases to BSH and bai operon containing bacteria. The net effect of this is a smaller total BA pool with a higher composition of LCA, enhancing TGR5 agonism and TGR5-induced GLP-1 release.568,585
In addition to fexaramine’s impact to BA pool composition, TGR5−/− mice models have found that the changes in weight gain, insulin sensitivity, and triglycerides may be due to FXR-dependent interplay with TGR5 signaling. Besides altering BA pool composition, this outcome is likely accomplished within adipose tissue and the liver by the action of the [FGF15/19]-[FGFR4/βK]-FXR axis on the FXRE found within the TGR5 promoter, increasing spare receptors and hence total BA pool TGR5 agonist potency.586 The TGR5-dependent ability of fexaramine to repress gluconeogenesis and lipogenesis hints that fexaramine may be able to induce ceramide metabolism. Fexaramine was able to induce DIO2 and PGC-1α expression in the same TGR5−/− model, highlighting unknown mechanisms in which FXR can mimic the phenotype typically seen with TGR5 agonists. This may be explainable by fexaramine-induced increases to BA pool LCA composition, likely triggering the effects of non-canonical BA receptors.568
Alongside the success of fexaramine, the direct administration of gut FXR-antagonistic BAs such as taurine/glycine conjugated βMCA and UDCA have been shown to prevent and reverse HF DIO, insulin resistance, hyperglycemia, and steatosis in mice. Three different experiments in mice have been performed using either gut-specific FXR−/− or taurine/glycine conjugated βMCA and have confirmed that the metabolic benefit is directly related to gut FXR inhibition of ceramide secretion.83,124,132 Conjugated UDCA administration through a ceramide-centric mechanism was found to strongly repress atherosclerotic plaque development in ApoE−/− mice.358 Intragastric administration of UDCA has been found to reduce NASH-associated hepatic inflammation and restore gut dysbiosis in a HF HC mice NASH model.587 V023-9340 and Compound F6 have both been found to improve metabolic outcomes in HF diet and Gubra-amylin mice NASH models.546,572 Compound F6 activity was directly tied to inhibiting intestinal ceramide synthesis.
Clinical results—FXR modulators
CA supplementation with 15 mg/kg was not found to reduce hepatic triglyceride content in humans.588 CDCA was found to accelerate colonic transit and improve bowel function in patients with constipation-predominant irritable bowel syndrome.589 EDP-305 was involved in two phase 2 studies, one for PBC and the other for NASH. For PBC EDP-305 was able to lower alkaline phosphatase, while for NASH it reduced serum alanine transaminase levels and hepatic lipid content.543,590 In addition, EDP-305 increased serum LDL cholesterol and caused pruritis. As a proof of concept, PX-102 was found in small human trials to repress BA synthesis and increase FGF19 secretion.557 Two phase 1 trials have been conducted for single and multiple ascending doses, however the results of such have not been published as the compound has been abandoned.591 WAY-362450 was involved in two phase 1 trials, one of which was terminated due to pharmacokinetic issues, while the other completed, both of which had no published results.592,593
Tropifexor was primarily designed as an anti-NASH therapeutic and has completed a phase I safety trial.552,594 Recently a phase IIa/b trial FLIGHT-FXR was completed in which decreases in alanine transaminase levels, decreased hepatic fat fraction, increased weight loss, and improved surrogate markers for NASH improvement but not actual histological disease improvement were observed.594,595 In addition, tropifexor was found to increase serum LDL cholesterol and cause pruritis. Secondary trials have found that tropifexor was able to improve cholestatic biomarkers and potentially serve as a drug for PBC.596 Nidufexor was designed as an anti-NASH drug but has been studied specifically in patients with NASH and diabetic nephropathy.559 The recently completed phase 2 trial found 24-week drug-dependent decreases in both 24-h urine albumin quantities and urinary albumin to creatinine ratios.594 Cilofexor was analyzed in phase 2 trials as an anti-NASH drug and was found to at 24 weeks reduce hepatic steatosis, improve liver biochemistry, and reduce serum BA concentrations.553,594 Two human trials have been conducted for HPG1860, a phase 1 pharmacokinetics study and a very recent phase 2a pharmacodynamic study in NASH patients.597,598 Although no results have been formally published, press releases from Hepagene indicate that phase 1 dosing saw no increases to LDL cholesterol and biomarker evidence of FXR-mediated suppression of BA synthesis. Press releases from the 12-week phase 2a trial discuss significantly decreased liver fat content, reduced serum alanine transaminase, a low incidence of pruritis, and no evidence of LDL cholesterol elevation.
TERN-101 has had two phase 2 trials initiated, LIFT for monotherapy against non-cirrhotic NASH, and DUET for TERN-101 and TERN-501 thyroid receptor beta agonist (TRβ) combination therapy. Within LIFT, TERN-101 monotherapy was non-superior at all doses compared to placebo for reducing alanine transaminase, although this may be an effect of the low study sample size.599 As of writing the DUET trial has no recorded results.600
A phase 1b trial for MET409 was performed in NASH patients in which it reduced hepatic adiposity, induced pruritis, and increased serum LDL cholesterol.569 A phase 2a trial was started for MET409 alone or in combination with empagliflozin for T2DM/NASH patients for which no results have been published.601 A phase 2 study on ileo-colonic delivery of UDCA had no major physiological effects, while two phase 3 trials are currently being recruited to test the effect of UDCA on statin-induced glucose intolerance and intestinal inflammation, respectively.602,603,604 Oral administration of 20 mg/kg/day of UDCA to morbidly obese patients was found to reproduce what is expected from preclinical studies, namely that gut-FXR inhibition increases cholesterol clearance as BAs and increases triglyceride synthesis.605
OCA—clinical outcomes and adverse effect profiles
The original OCA trials although somewhat effective were not without fault. In the phase 3 POISE trial evaluating 5–10 mg/day of OCA for PBC, OCA did reduce biomarkers of disease but significantly increased the risk of severe adverse events by ~3-fold in the 10 mg group compared to placebo.606 In addition, new-onset diabetes, dyslipidemia, and pruritis were dose-sensitive with OCA administration. Similar results were found against primary sclerosing cholangitis (PSC) with significant dose-dependent pruritis in the phase 2 AESOP trial, which included doses from 1–10 mg/day.607
Strong evidence of dose-dependent pharmacodynamics was identified within a phase 2 trial aimed at insulin resistance in NAFLD, in which OCA was tested at 25–50 mg/day within T2DM patients, most of which obese. The primary outcome of the trial was a two-step hyperinsulinemic-euglycemic clamp. Low-exposure insulin resistance was improved in the 25 mg/day group by 28.0%, while the high dose 50 mg/day group was non-significant with a point estimate of 20.1% improvement. High-exposure insulin resistance was improved by 18.3% in the 25 mg/day group, while the high dose 50 mg/day group was non-significant with a point estimate of 10.8% improvement.608,609 This lack of significance may be due to the small sample size of ~20 patients per group, but if interpreted as is, the trial reveals that OCA-mediated FXR agonism provides dose-dependent outcomes, where overshooting target agonist efficacy gives less favorable or potentially negative outcomes. Further illustrating such, increases to LDL cholesterol were found at all doses while decreased HDL cholesterol was only found at high doses.
Shifting focus to NASH indications, the phase 2 FLINT trial found that over 72 weeks 25 mg/day of OCA improved NASH histology. This was accompanied by increased VLDL cholesterol, LDL cholesterol, and decreased HDL cholesterol, replicating the FXR-associated preclinical adverse effect profile.457,610 Soon after FLINT, the phase 2 CONTROL trial found that 20 mg/day of atorvastatin was without surprise able to ameliorate FXR-induced LDL cholesterol induction by OCA dosed at 5–25 mg/day.611
What went wrong? OCA and the REGENERATE trial
Although OCA had somewhat positive results for PBC, PSC, NAFLD, and NASH, a common thread of adverse events persisted. The REGENERATE trial was the final nail in the coffin, whose original goal was to test the ability of OCA to treat pre-cirrhotic fibrosis secondary to NASH.612 Using results from the REGENERATE trial, the FDA rejected the NDA for the NASH indication of OCA, primarily due to its inability to at 10–25 mg/day meet the primary endpoint of improvement of NASH by more than one stage within 18 months. In addition, the FDA was cautious of the concerning benefit-to-risk profile of OCA due to its plentiful adverse effects, many of which were due to overtly strong systemic FXR agonism and repression of BA synthesis: drug-induced liver injury, excess risk of cholecystitis and bile stones, risk of new-onset dyslipidemia needing statin treatment, excessive risk for new-onset prediabetes/diabetes, acute kidney injury, and severe pruritis requiring discontinuation.7
In subsequent analyses of REGENERATE, OCA was able to obtain the primary endpoint but only by changing the method of pathological analysis from one pathologist to a consensus between three pathologists, in which OCA showed a dose-response relationship with the incidence of primary endpoint completion.
Let us now analyze one-by-one some of the most significant and near-significant adverse effects of the REGENERATE trial and try to mechanistically rationalize their occurrence. Dyslipidemia is explainable by our high total FXR model of lipid metabolism, high LDL cholesterol, low triglycerides, low VLDL cholesterol, and low HDL cholesterol. Short-term cardiovascular events may be explainable by the cardiomyotoxic or arrhythmogenic role of systemic FXR agonism, while longer-term results are explainable by a gut FXR-induced pro-atherogenic serum lipid profile. Gallbladder disease and related complications are directly explainable by FXR-mediated repression of hepatic BA synthesis, allowing for a more hydrophobic BA pool and cholesterol rich biliary fluid. A similar mechanism may explain drug-induced liver injury, in which a more hydrophobic and cytotoxic BA pool triggers hepatocyte damage. Hyperglycemia and new-onset diabetes is explainable by multiple factors: inability to allow for proper hepatic glycogen cycling due to overt GSK3β inhibition, reductions to systemic TGR5 agonism due to BA pool contraction, and reductions to hepatic and gut glycolysis, decreasing net GLP-1 secretion.
One last question remains, why do OCA and other FXR agonists cause pruritis? Human trials for OCA, tropifexor, cilofexor, EDP-305, and MET409 all identified pruritis.555 Therefore, pruritis is not induced by a steroidal scaffold or driven by ligand promiscuity at TGR5, but is instead FXR-intrinsic. Recently published research has found that FXR agonists such as OCA and cilofexor induce hepatic expression and secretion of the pruritogenic cytokine Interleukin-31 (IL-31), a large player in atopic dermatitis. Mechanistically, in PXB-chimeric mice it was found that serum IL-31 increased roughly 13-fold after OCA administration, supported by increasing hepatic and plasma IL-31 mRNA. Validation of this mechanism was found in humans, in which a dose-response relationship was found between IL-31 expression and cilofexor dosing in NASH patients. This dose-response relationship was not found for other competing causes, such as autotaxin-mediated lysophosphatidic acid synthesis. Pruritic severity in these patients was significantly correlated with serum IL-31 concentrations, establishing that FXR-induced IL-31 expression is the most plausible cause for OCA-induced pruritis.613
An old friend in disguise—is INT-787 aka TC-100 a FRESH take on FXR?
INT-787, a new name for TC-100 was recently announced by Intercept Therapeutics as their “next-generation” FXR agonist, primarily targeted at gut diseases and severe alcohol-associated hepatitis.614 Within an experiment in which HF diet ob/ob NASH model mice were given both INT-787 and OCA, only INT-787 lowered serum alanine transaminase, IL-1β, and TGFβ. Both OCA and INT-787 were able to restore the expression of matrix metalloprotease (MMP)-inhibitory Reversion-Inducing-Cysteine-Rich Protein with Kazul Motifs (RECK) proteins. OCA reduced MMP-2 and MMP-9 induction, while INT-787 only reduced MMP-2. Both OCA and INT-787 reduced A Disintegrin and Metalloproteinase Domain-Containing Protein 10 (ADAM10), 17 (ADAM17), and reduced the number of cytokeratin expressing hepatocytes. Lastly, INT-787 provided a stronger suppression of hepatic stellate cell activity compared to OCA.615
Although these initial results seem hopeful, more data is needed for a definitive conclusion. As previously discussed, the hydrophilic nature of TC-100 limits its hepatic exposure and provides a more gut-centric activity.544 Press briefings on the unpublished INT-787 phase 1 trial show evidence of outstandingly low non-dose-dependent pruritic risk and dose-dependent exposure, indicating that INT-787 may have a more favorable benefit-to-risk ratio compared to OCA.616 A human phase 2 trial on patients with severe alcohol-associated hepatitis named FRESH is currently recruiting with estimated trial results early to mid-2025.617
The next step forward for FXR—selective FXR modulators (SFXRMs)
IL-31 mediated pruritis, the induction of a pro-atherogenic serum lipid profile, and the potential risk of FGF19-[FGFR4/βK]-mediated carcinogenicity compel many to wonder if it is possible to selectively induce certain FXR-mediated downstream effects and prevent others? FXR transcriptional regulation of different genes is controlled by FXR isoform, tissue-specific coactivator/corepressor availability, and post-translational modification. From such, the next step forward in FXR modulator design should be towards the development of Selective FXR Modulators (SFXRMs). These modulators would conformationally support certain downstream effects, while weakly or absently supporting others, perhaps even producing tissue-dependent effects.618 We highlight three potential techniques to accomplish such. The first of which is to develop isoform-specific FXR modulators, biasing which FXRE-containing genes are expressed due to isoform-specific differences in FXRE motif preference.98,619 The second of which is to target orthosteric binding modes or allosteric sites that compete with or change the conformation of FXR domains responsible for tissue-dependent coactivator/corepressor recruitment.620,621,622 The last of which is to allosterically compete against FXR-modulator binding domains to prevent post-translational modification (acetylation/SUMOylation).623
Two molecules exhibit properties useful for future SFXRM development, one of which recruits a FXR corepressor while the other binds to an allosteric site and selectively modulates FXR activity.
The first of which is the marine sponge derived molecule theonellasterol (University of Perugia), which protects against cholestatic injury in mice.624 This FXR antagonist recruits Nuclear Receptor Corepressor 1 (NCOR1) to FXR and prevents its natural induction of OSTα, BSEP, and SHP. It also prevents FXR from repressing expression of MRP4, enhancing hepatic BA efflux in bile duct ligation mice models and preventing BA-overload induced toxicity.
The second of which is guggulsterone (Pharmacia Corp), which allosterically binds to FXR to prevent FXR association with coactivators, prevents unliganded-FXR release from corepressors, and prevents orthosteric ligand binding. In HepG2 cells, dose-titrations of guggulsterone enhances BSEP and represses SHP expression in the presence of CDCA, while in Caco2 cells, represses I-BABP expression in the presence of CDCA. In Huh-7 cells the effect on SHP was reversed, highlighting the tissue-dependence of coactivator recruitment. In rats, guggulsterone selectively induced BSEP and SHP expression without any FXR-mediated CYP7A1/CYP8B1 repression, lowered triglycerides, and raised HDL cholesterol, potentially providing a solution for the FXR-plagued pro-atherogenic serum lipid profile.625,626,627 Although guggulsterone showed no effect on lipid profiles within a small human study, it serves as a crucial tool compound to motivate the future development of SFXRMs.628
TGR5 modulators
Systemic TGR5 modulators
TGR5 agonists in recent years have gained popularity for their ability to stimulate GLP-1 secretion (Fig. 17). The most well-known potent and selective TGR5 agonist was originally discovered from structure-activity relationship studies on OCA, in which a single methyl group introduced to a suspected selectivity pocket provided strong TGR5 activity and removed most FXR activity.8 23-(S)-Methyl-OCA, also known as INT-777 (Intercept Pharmaceuticals) was found to have micromolar potency and exhibit 166% of LCA’s documented effect of inducing cAMP synthesis.629 A further modified version of INT-777 is also pharmacologically active as a TGR5 agonist and is known as BAR501 (BAR Pharmaceuticals).630 Other semisynthetic TGR5 agonists have been discovered such as 7-Methoxy-CDCA (University of Minnesota), which along with recent discovery of TGR5 allostery inspired the design of the positive allosteric modulators 7-Methoxy-CA and 12-Oxo-7-Methoxy-CA, also known as compounds B1 and A1, respectively (Changzhou University).631,632,633 Alongside semisynthetic steroidal scaffolds, natural steroidal TGR5 agonists have been discovered from plant extracts with hypoglycemic effects. These plant extracts were refined to discover the triterpenoids betulinic acid, oleanolic acid, and ursolic acid, all of which exhibit potent and selective TGR5 agonism (Louis Pasteur University).634,635
The first major non-steroidal TGR5 agonists were the 3-aryl-4-isoxazolecarboxamide scaffolds (GlaxoSmithKline), which exhibited decent pharmacological effects if given via an intrajejunal infusion to dogs but suffered from very poor oral bioavailability.636,637 Following from such the 1,4-bis(sulfonyl)-1,4-diazepane scaffold SB-756050 was discovered, which touted micromolar potency and full specificity for TGR5 (GlaxoSmithKline). When orally administered to humans in a phase 1 trial, SB-756050 exhibited horrible human non-linear pharmacokinetics and highly patient-dependent pharmacodynamic effects.638 Besides this trial, no notable in human TGR5 modulator trials have been reported. In response to this, TGR5 agonist development branched into diverse chemical space in an attempt to produce an orally bioavailable scaffold. Non-steroidal scaffolds such as imidazo[1,2-a]pyrimidine (Torrent Pharmaceuticals), 2-aryl-3-aminomethylquinoline (Kalypsys), triazole (Pfizer), tetrahydropyrido[4,3-d]pyrimidine (Pfizer), (pyrimidin-2-yl)azetidine (Novartis), nicotinamide (Shanghai institute of Materia Medica), isonicotinamide (Novartis), 3-phenoxypyrazine-2-carboxamide (Henan University), dihydropyridone (Pasteur institute), benzothiazole (Eli Lilly), pyridine (Roche/Takeda), and 2-thio-imidazole (Zydus Pharmaceuticals) have been reported.289,639,640,641,642,643,644,645,646,647,648
Preclinical results—systemic TGR5 modulators
In C57BL/6J mice INT-777 has been found to strongly induce GLP-1 secretion, enhance insulin-induced glucose uptake into skeletal muscle, protect against HF DIO mice via DIO2/UCP1 induction, protect against steatosis by repressing hepatic triglyceride synthesis, and inhibit gluconeogenesis by inhibition of FOXO1 and repression of G6Pase/PEPCK.380,649 The positive allosteric modulators B1 and A1 have been found to increase the intrinsic efficacy of CDCA agonism at TGR5, but not increase total maximal cAMP induction.632
Multiple non-steroidal scaffolds have been able to either induce GLP-1 secretion and/or reduce oral glucose tolerance test (OGTT) Area Under the Curve (AUC) in either C57BL/6J mice or human NCI-H716 cells.639,640,641,642,643,645,646,647 In HF high fructose fed mice, the orally administered steroidal TGR5 agonist BAR501 was able to reverse insulin resistance, reverse histological markers of NASH, and increase BADT energy expenditure by inducing the expression of UCP1/PGC-1α.650 The imidazo[1,2-a]pyrimidine scaffold TRC210258 was found to lower triglyceride levels, LDL cholesterol levels, increase HDL cholesterol, and improve glycemic control within DIO mice and hamsters.644 Viewing cardiometabolic health, INT-777 administration inhibited ASCVD development in HC fed LDLr−/− mice. This was mechanistically explained to be a downstream effect of elevated cAMP levels, which repressed NF-κB activation.279 This result was replicated when dual knockout ApoE−/− and LDLr−/− mice were treated with the dual FXR/TGR5 agonists, but was lost once the model was expanded to a ApoE−/− LDLr−/− TGR5−/− triple knockout.651,652
The isonicotinamide TGR5 agonist scaffold was able to successfully inhibit TNFα and IL-12 synthesis both in human monocytes and mice models, additionally stabilizing macrophages within the M2 anti-inflammatory phenotype. This effect was reversed in TGR5−/− mice.289 Similarly, BAR501 administration was shown to similarly reinforce M2 macrophage phenotypes during mice models of colitis, along with suppressed expression of TNFα, IL-1β, IL-7, and IFNγ.284 Both examples highlight how TGR5 agonists may serve as immunomodulators in chronic inflammation.
What’s wrong with systemic TGR5 modulators?
Analogous to the adverse effect profile of systemic FXR agonists, systemic TGR5 agonists have their burden of adverse effects. Alongside their general positive physiological role, preclinical doses high enough to induce GLP-1 expression have also been found to negatively impact gallbladder function, producing a gallbladder that is overfilled and unresponsive to CCK-induced contraction. Mechanistically, cAMP-induced CFTR agonism enhances biliary flow to fill the gallbladder, while cAMP-activated PKA phosphorylates myosin light chain kinase, inhibiting its ability to produce smooth muscle contraction.269,653 The gallbladder effects of systemic TGR5 agonists are very similar to pruritis for systemic FXR agonists, in that both are intrinsically caused by receptor activation and are not due to ligand ambiguity.647,648,653
Leveraging size, polarity, and metabolic stability to save the gallbladder
To remove TGR5-mediated side effects at the gallbladder many have attempted to produce gut-restricted TGR5 agonists, providing GLP-1 release without significant exposure to the gallbladder. Three major strategies have been implemented to obtain gut-restricted activity: increasing molecular weight, introducing a very polar motif known as a kinetophore to reduce membrane permeability, or to introduce a metabolic soft spot.654 Each of these approaches has its own intrinsic challenge. TGR5 is expressed basolaterally within enterocytes, meaning that gut-restricted agonists must retain low systemic permeability but enough permeability to reach the basolateral enterocyte surface in meaningful quantities. Endogenous BAs are able to bypass this requirement and reach the basolateral surface in small quantities via passive diffusion or substantial quantities by using ASBT/I-BABP/(OSTα/β-MRP3)-mediated transport.655,656
Preclinical results—gut-restricted TGR5 modulators
The first attempts at a gut-restricted TGR5 agonist employed the usage of a large molecular weight compound. Compound 15c (Shanghai Institute of Materia Medica) was designed as a large ~1400 Da, ~230 Å2 polar surface area, low permeability, and pseudosymmetric molecule, based upon the previously discovered nicotinamide scaffold (Compound 2.1 - Shanghai Institute of Materia Medica).647 Non-trivially this large molecule appears systemically with nanomolar concentrations within the bile and serum. The Caco-2 efflux ratio of 61 indicates that in normal enterocytes reverse efflux may limit basolateral exposure, while within enteroendocrine L-cells it may successfully accumulate on the basolateral face.657 This molecule exhibited enhanced GLP-1 secretion in human and mice in vitro models, along with a significantly reduced effect on gallbladder filling relative to compound 2.1 within mice models.658
Using the kinetophore approach the same group built upon this design further by including quaternary ammonium groups to mimic that of the BA sequestrant cholestyramine to generate compound 26a (Shanghai Institute of Materia Medica). Compound 26a was found to have even lower intestinal permeability compared to 15c and was able to produce long-lasting hypoglycemia in ob/ob mice by inducing GLP-1 secretion, however, had complicated long-term effects on gallbladder filling.659 Compound 15c was modified by thiophene replacement to a thiazolidine and the replacement of the quaternary ammonium groups to a polyol-bearing motif to produce compound 12 (Ardelyx Therapeutics). Compound 12 had more predictable gallbladder effects and sustained mice GLP-1 secretion.660 Other approaches have been used within this same kinetophore approach, such as the addition of sulfonate motifs (Compound 24 - Pasteur Institute/Compound 22-Na - Shanghai Institute of Materia Medica), carboxylic acid motifs (Compound 19.1 - Shanghai Institute of Materia Medica), polyol motifs (Compound 30-Na - Shanghai Institute of Materia Medica), and PEGylated motifs (PEG-Compound 24 - Pasteur Institute), many of which exhibiting drastically reduced TGR5-mediated effects on gallbladder filling.654,657,661,662
Compound 19.1 was further modified by the implementation of an ethoxymethyl ester motif to produce compound 19.2 (Shanghai Institute of Materia Medica). The design of compound 19.2 improved from that of compound 19.1 and all those before it by the incorporation of a plasma metabolic soft spot. If the compound was able to partition into the systemic circulation, it would be hydrolyzed by plasma esterases and become inactive as a TGR5 agonist. This allowed for compound 19.2 to exert strong TGR5 agonism and exhibit good gut-permeability without worrying about systemic exposure. As a result, compound 19.2 exhibited multiple-fold increases in GLP-1 secretion in human and mice in vitro models, reduced AUC for oral glucose tolerance tests in mice, had near-zero detection in the plasma or gallbladder in mice, and displayed non-significant increases to gallbladder weight.663
Can gut-restricted TGR5 agonists become the next Ozempic?
Current pharmacological techniques to manipulate GLP-1 receptor agonism are based on peptidic injectable formulations or peptidic oral formulations co-formulated with salcaprozate sodium to protect against gastric acid-catalyzed hydrolysis.664 The discovery and synthesis of gut-restricted TGR5 agonists with minimal systemic exposure provides an exciting, oral, non-peptidic, and paradigm-shifting opportunity for future GLP-1 relevant drug discovery. Although this new modality for treatment seems promising, no human trials have been conducted yet.
Novel BA-centric targets against disease
Preclinical FXR/TGR5 dual agonists treat metabolic disease and the TNFα-[NF-κB] axis
Alongside mutually exclusive FXR and TGR5 modulators, developments have been made to discover novel BA-centric therapeutic approaches (Table 2). INT-767 (Intercept Pharmaceuticals) is a dual FXR/TGR5 agonist that displays properties consistent with the pharmacology of both FXR and TGR5. Mimicking a pure FXR agonist, INT-767 represses CYP7A1/CYP8B1 transcription within the classical pathway of BA synthesis and by unknown mechanisms induces the expression of alternative pathway genes. Similar to that of a pure TGR5 agonist, INT-767 has been found to induce cAMP levels, raise intracellular calcium levels, and enhance GLP-1 secretion, improving insulin resistance, lipid tolerance, and mitochondrial function within DIO mice.208,665 Additional studies have found that INT-767 has a unique role in reversing HF DIO by normalizing glucose metabolism, NAFLD by repressing both lipogenesis and inflammation, and NASH by reducing histological disease severity and TNFα-induced NF-κB activity in mice.290,666,667
Besides INT-767, similar results have been found when analyzing the phytochemical FXR/TGR5 dual agonist deoxyschizandrin (Longhua Hospital). Deoxyschizandrin was found to counter HF DIO in mice by promoting energy expenditure, anorexia, and leptin sensitivity. Within both HF DIO and methionine and choline-deficient L-amino acid diet mice, deoxyschizandrin was found to effectively reduce NAFLD hepatic phenotypes.668
Interplay between FXR/TGR5 dual agonists and the TNFα-[NF-κB]-YY1-FXR axis
The TNFα-[NF-κB] axis directly induces the expression of the FXR repressor YY1, another unique target for NAFLD/NASH drug discovery.669 If one wishes to enhance the expression and activity of FXR in NAFLD or NASH, it is then natural to wish to inhibit the activity of or repress the expression of YY1. FXR/TGR5 dual agonists such as INT-767 directly inhibit the TNFα-[NF-κB] axis, analogous to known techniques already employed to inhibit the expression of YY1.670 Besides upstream regulation, YY1 targeted therapy is likely to provide stronger and more relevant impacts for therapeutic modulation of NAFLD/NASH. Many approaches have already been attempted to inhibit YY1. Besides upstream NF-κB inhibition, others have used YY1 targeted siRNA or the administration of nitric oxide (NO) donors such as Diethylenetriamine (DETA) NONOate, which S-nitrosylate and inhibit the YY1 DNA binding domain.670,671,672,673,674,675,676,677 No small molecule competitive antagonist for YY1 has been discovered, opening a wealth of opportunity in drug discovery, perhaps being a prime target for PROTAC development.
Preclinical FXR/URAT1, FXR/FABP1, TGR5/CysLT1R, and TGR5/RORγt modulators
In contrast to INT-767 and deoxyschizandrin, other dual receptor modulators have been found that impact both a BA-centric receptor and another non-BA-centric disease specific receptor. The first of which was compound 4 (Guangdong University), which was found to function as a FXR agonist and a Human Urate Transporter 1 (URAT1) inhibitor. Mechanistically it is thought that FXR-mediated inhibition of the NLRP3 inflammasome will decrease gout-associated inflammation, while URAT1 inhibition will function as a uricosuric, enhancing uric acid excretion in the urine.678
The dual modulator ZLY28 (Guangdong Pharmaceutical University) was discovered to both be a gut-restricted FXR agonist and Fatty Acid Binding Protein 1 (FABP1) antagonist. FABP1 is essential for lipid chaperoning and the gut-mediated absorption of fatty acids. FABP1 antagonism and FXR agonism therefore is synergistic to treat NASH. Within CCl4 treated mice this compound exhibited full FXR agonism within the gut evidenced by FGF19-mediated effects hepatically, but no systemic FXR agonism. Alongside this, reduced hepatic steatosis, inflammation, and ballooning was observed with significantly lower hepatic triglyceride accumulation compared to OCA, indicating synergistic efficacy.679
The dual modulator compound 2.2 (University of Perugia) was found to be a TGR5 agonist and a Cysteinyl Leukotriene Receptor 1 (CysLT1R) antagonist. Compound 2.2 was designed as an anti-NASH drug, which was found to inhibit hepatic steatosis, the hepatic expression of inflammatory biomarkers, and prevent DIO in HF HC mice.680
The novel RORγt inverse agonist and TGR5 agonist compound 7 (University of Naples) has been shown to provide robust anti-inflammatory activity in mice models of TNBS-induced colitis, reducing TNFα, IL-1β, and IL-6 expression while increasing IL-10 and TGFβ expression. This compound provides immense potential as a therapeutic against IBD by inhibiting both NF-κB/NLRP3 signaling and the differentiation of Th17 cells.681
Preclinical FXR/LXR, FXR/PXR, and FXR/PPARγ modulators
Two dual modulators have been discovered that impact both BA-canonical and non-canonical receptors. The first of which is the phytochemical withaferin A (JSS Medical College), which was found to be a LXR/FXR dual agonist. Withaferin A was found to potently suppress steatosis, fibrosis, and TGFβ expression in HF diet NAFLD mice, in addition to preventing lipid droplet accumulation in HepG2 and Huh7 cells.682
The second canonical/non-canonical dual modulators are compounds 5/11 (University of Naples), found to be FXR antagonists and PXR agonists. These compounds were able to repress FXR-mediated activity, suppress IL-8, and suppress IL-1β expression in HepG2 cells. Repressed FXR agonism may enhance biliary flow and help cholestatic diseases, while PXR-induced IL-8/IL-1β suppression may provide benefit in IBDs.683,684
The last canonical/non-canonical dual modulator discussed is compound 18 (Hiroshima International University), a novel dual partial agonist of FXR and PPARγ providing strong mechanistic synergy for T2DM-induced NAFLD. Within Huh7 cells compound 18 was found to slightly increase BSEP/OSTα expression relative to an inactive control, while markedly inducing SHP expression and repressing SREBP-1C expression. Phosphorylation of PPARγ at S273 has been associated with higher levels of insulin resistance. Administration of compound 18 to 3T3-L1 cells was found to suppress PPARγ S273 phosphorylation as potent as the FDA-approved PPARγ agonist rosiglitazone.685 Cumulatively PPARγ-mediated insulin sensitization in conjunction with FXR-mediated hepatic effects may produce a strong clinical candidate. Although it remains to be seen if the PPARγ agonism produces the same side effects that make currently available PPARγ agonists undesirable, such as weight gain, increased fluid retention, and a higher risk for the development of congestive heart failure.686
Biasing BA pool composition, can we inhibit CYP8B1?
Due to the association of 12α-OH BAs in T2DM, obesity, and NAFLD/NASH, immense interest has been generated in the drug discovery of CYP8B1 inhibitors. In perfect timing the enzymatic characterization and X-ray crystal structure of CYP8B1 has been published within the last year, allowing for structure-based drug design.687 CYP8B1 inhibition may serve as an excellent target for pharmacological inhibition, since shunting BA synthesis into the alternative pathway will produce a similar effect to that of fexaramine, producing a more TGR5 agonistic BA pool.
Preclinical and clinical benefits of FGF19 analogs
In the same nature of modifying endogenous BA physiology to impact disease, recent efforts have focused on the synthesis and characterization of FGF15/19 analogs. Subcutaneous FGF19 administration was found to be anti-diabetic and anti-obesogenic by inhibiting AgRP/NPY neurons within the hypothalamic hunger center of mice.126,127 This results in an overall reduction in caloric intake leading to reduced weight gain and improved insulin sensitivity. The subcutaneous administration of the FGF19 analog Aldafermin, also known as NGM282 (NGMBio), has been shown to provide a rapid, sustained, and robust decrease in hepatic lipid content along with improvements to NASH histopathology in both animal models and human clinical trials.688,689,690
Potential solutions against FGFR4-mediated carcinogenic impacts
Although the FGF15/19-[FGFR4/βK] axis provides benefits like those provided by GLP-1 agonists, overactive FGF15/19-[FGFR4/βK] axis activity as previously discussed can be carcinogenic. From such, many pharmaceutical companies have developed treatments that may target cancer cells that use FGFR4/βK overexpression as a mechanism for chemotherapeutic resistance, developing pan-FGFR inhibitors and selective FGFR4 inhibitors.691,692 Similar to other cases of chemotherapeutic resistance, mutations have been found in FGFR4 such as FGFR4-V550L or FGFR4-V550M which limit the design and applicability of current FGFR4 antagonists. In line with parallel research on FGFR2, PROTAC-mediated FGFR4 degradation may allow for one to target regions of the receptor not intrinsically associated with inhibition, providing more opportunities against mutation.693,694,695
BA sequestrants increase TGR5 agonism and GLP-1 release
Besides centrally acting targets, effort has been put into finding gut-restricted non-BA-receptor targets. The first of which is a repurposing of the already FDA-approved BA sequestrant anionic exchange resins: colesevelam, cholestyramine, and colestipol. BA sequestrants prevent the enteric reabsorption of BAs, increasing the luminal concentration of BAs present in the distal gastrointestinal tract. BA sequestrants increase the fecal excretion of BAs, lowering systemic LDL cholesterol by shunting it into BA synthesis. This leads to increased BA exposure and agonism of enteroendocrine L-cell TGR5, enhancing systemic GLP-1 secretion in mice.696 The enhanced GLP-1 secretion was correlated with improved insulin sensitivity and suppressed hepatic glycogenolysis in a DIO rat model.697,698 Alongside DIO rat models, this GLP-1 secretory outcome was found and validated in a systemic review of phase II human trials, serving as evidence for the potential usage of BA sequestrants not just for hypercholesterolemia but as an insulin-sensitizing and anorexic therapeutic.699,700,701
ASBT inhibitors increase BA synthesis and reduce cholestatic risk
Analogous to the role of BA sequestrants, ASBT inhibitors prevent the ileal reuptake of BAs. Decreased ileal reuptake of BAs reduces hepatic FXR agonism and enhances hepatic BA synthesis and flux through the biliary tree, lowering serum cholesterol and cholestatic risk.702 Preliminary results in MRP2−/− mice models have indicated that ASBT inhibitors have a functional role in the treatment of PSC, inhibiting inflammatory/profibrotic gene expression.703 The ASBT inhibitor A4250 also known as Odevixibat (Alibero Pharma) was already FDA-approved as a first-in-class drug used to treat familial intrahepatic cholestasis, while the second compound SC-435 (University of Connecticut) has been used purely as a test compound.704,705,706 Alongside these current compounds, two new ASBT inhibitors are in human clinical trials, volixibat (Mirium Pharmaceuticals) and maralixibat (Mirium Pharmaceuticals). Maralixibat is currently approved for Alagille syndrome and recently completed the phase 2b/3 MARCH trial for progressive familial intrahepatic cholestasis, showing positive safety and efficacy data.707 Volixibat is currently in in two phase 2b/3b trials, VISTAS for PSC, and VANTAGE for PBC.708,709 Both of which are likely to be approved by 2027-2028.
Microbial modification changes BA pool composition
The last gut-restricted non-BA-receptor target is that of the gut microbiome. Modification of the gut microbiome directly impacts both the primary to secondary and conjugated to unconjugated ratios within the BA pool.710,711 Current efforts are to either pharmacologically reduce BSH-containing bacterial or administer probiotic/prebiotic mixes to enrich for BSH-containing bacteria.712 An example of the first technique is exemplified with metformin, which by unknown mechanisms decreases Bacteroides fragilis, which in mice increased gut UDCA levels and provided the positive benefit of reducing ceramide synthesis.713 The alternative approach is highlighted by the probiotic supplement VSL#3 (VSL3 Pharma), which has been shown to induce BA deconjugation and maturation into secondary BAs, induce BA synthesis, enhance insulin sensitivity by driving BA pool composition to favor TGR5 agonism, and protect mice from NASH.714,715 Alongside BA pool composition changes it is theorized that a higher prevalence of BSH-containing bacteria reduces the surfactant efficacy of gut BAs, reducing systemic LDL cholesterol by inhibiting cholesterol absorption and increasing cholesterol excretion as BAs.54
Conclusions and future perspectives
Continuing with BAs—recommendations for future success
It is essential to acknowledge the pharmacological complexity of BA receptor modulators, exemplified by OCA’s capacity to impact numerous physiological circuits and induce severe side effects. Messy pharmacology, low bioavailability, dose-dependent cytotoxicity, and non-linear pharmacokinetics have plagued BA-centric drug discovery. However, we believe that dismissing BA research prematurely is unwarranted, since significant progress has been made addressing these concerns and unlocking new potential for BA receptor modulators.
Messy receptor pharmacology has been substantially improved by developing tissue-specific drugs. Many of the original side effects of OCA have been directly remedied, or at least mechanistically understood to pave the way for future innovation. For instance, hepatic FXR-mediated IL-31 pruritis has been strongly mitigated by the usage of gut-restricted FXR agonists. Although challenges such as a pro-atherogenic serum lipid profile persist, emerging strategies such as SFXRMs, the discovery and usage of new non-steroidal scaffolds, and scaffold-specific structure activity relationships have been found to meaningfully reduce adverse effect incidence and severity. Similarly, positive developments have been seen in the case of TGR5 agonists, in which the initial alarming concern about gallbladder non-contractility has been solved by the implementation of gut-restricted TGR5 agonists. Despite the intrinsic complications of modulating FXR and TGR5, tremendous preclinical experimentation and clinical validation has given us the tools to remove less favorable outcomes, maximizing the chance of therapeutic success (Table 3).
Improving low oral bioavailability, dose-dependent cytotoxicity, and non-linear pharmacokinetics have been the strongest forces behind non-steroidal scaffold research. Besides the negative outcomes of WAY-362450 for FXR and SB-756050 for TGR5, most if not all non-steroidal scaffolds have shown dramatically improved pharmacokinetic parameters. Diverging from endogenous BAs, non-steroidal or heavily modified steroidal scaffolds provide a refined approach for clinical development, removing most of the amphipathic surfactant-like effects that trigger dose-dependent cytotoxicity.
Recent advancements in both tissue-targeted and structurally diverse approaches have provided ample opportunities to wrangle the originally untamable beast of BA physiology. Combining this with the incredibly important role of BA physiology in the pathogenesis of cardiometabolic, inflammatory, and neoplastic diseases, there is immense clinical potential and a high likelihood for the future success of BA-centric therapeutics.
How may BA therapeutics be better or worse than the existing therapeutic paradigm?
The current landscape of T2DM treatment predominantly revolves around generating insulin analogs or potentiating endogenous insulin signaling. Besides recent developments in GLP-1 receptor agonists and Sodium-Glucose Cotransporter-2 (SGLT2) inhibitors, existing therapies either require injections or have negative side effects such as weight gain. Progress has been made against obesity, but approved therapeutics only focus on hunger repression or inhibiting the dietary uptake of lipids. While the potential approval of a Thyroid Receptor Beta (THβ) agonist such as resmetirom (Madrigal Pharmaceuticals) may offer promise as a first NAFLD/NASH drug, there are currently no FDA-approved drugs for these conditions.716 ASCVD lacks a cure, relying on slowing the rate of the inevitable by pharmacologically lowering LDL cholesterol. In the realm of IBD, there is no curative treatment, only the usage of aminosalicylate, corticosteroid, or biologic therapy to reduce intestinal inflammation. Hepatocellular carcinoma chemotherapy resistance employs small molecule antagonists against FGFR4, but most of these approaches are strongly impacted by patient-specific mutations.
Contrasting with the status-quo, BA-centric approaches offer some distinct advantages over the traditional approaches. BA therapeutics offer significant benefits for T2DM, obesity, and NAFLD/NASH treatment relative to existing therapeutics, inhibiting ceramide synthesis, strongly modulating hepatic metabolism, and mirroring the existing therapeutic viability of GLP-1 receptor pharmacology. While BA therapeutics may not surpass the immense benefit of statin therapy, their ability to prevent ceramide synthesis is likely to strongly impact the inflammatory aspects of ASCVD disease progression. IBD management with BA therapeutics has a high benefit compared to the traditional approach directly targeting the Th17 and Treg centric pathway that drives disease pathogenesis. Lastly, a more advanced BA therapeutic approach such as PROTAC development may provide more profound FGFR4 suppression, better outcomes, and a lower likelihood for patient-specific resistance.
Navigating preclinical to clinical translational challenges
BA research encounters many challenges that prevent the direct translation of preclinical findings into clinical applications. There are four major translational challenges seen between preclinical research and clinical outcomes that must be minimized to ensure translational success. The first challenge arises from the substantial differences in lipid metabolism between rodents and humans, potentially biasing both cholesterol availability for BA synthesis and interpretability of lipid-centric outcomes. Rodents, unlike humans, predominantly store cholesterol within HDL, intrinsically having a lower risk for ASCVD before humanization techniques are applied.717 Addressing this, known solutions involve the development of rodent genetic variants or human hepatocyte xenotransplantation models that express lipid profiles very similar to that of humans.
Similarly, the second translational challenge stems from species-specific differences between the BA pools of rodents and humans. For example, rodents produce large quantities of FXR-antagonistic MCAs while humans do not. Although currently unresolved, genetic ablation of CYP2C70 with or without CYP2A12 has been found to produce a BA pool more akin to that of humans.22,25,718,719,720 Further genetic and biochemical research is crucial for refining the BA pools of rodents to more similarly match that of humans.
The third translational challenge revolves around species-specific differences in the coding sequence of BA receptors. Beyond the impacts of varying the composition of a single BA within the BA pool, small differences in the coding sequence of BA receptors strongly modulate the potency and efficacy of all known receptor agonists/antagonists. A prime example of such are MCBAs, whose modulatory activity on canonical BA scaffold receptor pharmacology in some cases is completely different species-to-species. To further highlight the issue, many reports have shown that species-specific differences to the BA receptor coding sequence can cause considerable shifts up to a half-order in magnitude in assay EC50/IC50 results when comparing human and rodent models.654,721 To address this challenge, the most straightforward solution may be to humanize rodent BA receptor coding sequences to match those of humans.
The final translational challenge in BA research relates to intrinsic species-specific differences in signaling cascades, in which post-translational protein modifications, transcription factor cis-elements, and their biological outcomes may differ from species to species.722 While altering the substrate selectivity and enzymatic activity of an entire signaling cascade proves challenging due to the multitude of targets, adding, removing, or changing the identity of cis-element sequences emerges as a viable option.
Future directions for BA-centric research
BA-centric research has developed tremendously since its inception, but as more questions are answered many new questions are asked. From the perspective of biochemistry many questions need to be answered pertaining to BA synthesis and maturation. It is crucial to reveal what factors drive BA synthesis between the classical and alternative pathway of synthesis, highlighted in importance by the abnormal 12α-OH BA levels seen in T2DM, obese, and NAFLD/NASH patients. From such, more research needs to be done on the mechanisms connecting TGR5 and CYP8B1, especially how this may be modulated during insulin resistance.
From the perspective of physiology, perhaps the most imperative question to further investigate is the effect of gut FXR-induced ceramides on disease pathogenesis. Alongside such, more work is needed to pinpoint human organ-specific BA pool compositions, which in tandem with a better understanding of the gut/liver FGF15/19-[FGFR4/βK]-FXR cascades would prove incredibly important. The impacts of FXR and TGR5 within mucosal immunity also warrant further investigation, in which there needs to be a better consensus on how these two signaling pathways regulate Treg and Th17 differentiation. The discovery of non-canonical BA receptors opens many questions, importantly opening the door for new modulatory points within the FXR/TGR5 signaling cascades and BA physiology as a whole. Lastly, more research is needed to investigate the physiological purpose and functions of MCBAs.
BA pharmacology is evolving rapidly. Gut restricted receptor modulators allow for potent pharmacological effects without systemic side effects that almost discarded the therapeutic field as a whole. Further research is needed to develop additional ways to circumvent the traditional pitfalls of BA receptor modulators. SFXRMs should be thoroughly investigated, alongside new design strategies for gut-restriction, non-steroidal scaffolds, tissue-specific formulations, and strongly said it is imperative to see if the potent preclinical effects of gut-restricted FXR agonists/antagonists and TGR5 agonists translate to humans.
Don’t give up on BAs
If developed correctly, systemic and gut-restricted FXR/TGR5 modulators, upstream regulators of BA signaling cascades, proteins that control BA pool size/composition, and microbiome-targeted therapies provide very attractive pharmacological targets for drug discovery against cardiometabolic, inflammatory, and neoplastic diseases. BAs are widespread physiological regulators, modulating carbohydrate, lipid, protein, inflammatory, and basal metabolic pathways. Abnormal BA pool size and/or composition can physiologically predispose for cardiometabolic disease, notably by upregulating the synthesis of ceramides. Abnormal BA pool size or composition additionally influences the ensemble biochemical properties of the BA pool, such as surfactant efficacy and relative agonism between FXR/TGR5.
There is a dire need for new innovations in drug discovery to more effectively treat cardiometabolic, inflammatory, and neoplastic diseases. In this review, we provided a bottom-up approach on BAs, explaining their biochemistry, physiology, and pharmacology at canonical and non-canonical receptors (Fig. 18). Using such, we thoroughly dissected how abnormal BA physiology directly induces disease pathogenesis and in the case of cardiometabolic disease is highly driven by gut-synthesized ceramides. Lastly, we rationalized novel targets for further translational drug discovery and provided future perspectives. Cardiometabolic, inflammatory, and neoplastic diseases are hard to drug due to complex biochemistry, the lack of deterministic models for disease pathogenesis, and have no well-defined curative treatments, leaving a large gap in the market for new therapies to ease the burden and treat unmet needs. BAs and their associated signaling cascades are crucial for proper health and homeostasis. Although BA therapeutics had a tumultuous start, they have now been refined to reveal a high therapeutic potential, simply said, don’t give up on BAs.
Summary—BA signaling in health and disease. BAs and their associated signaling pathways are central regulators of health. Their biochemistry, physiology, and pharmacology can protect against or if aberrant induce the pathogenesis of disease. BA therapeutics offer immense clinical potential, which if further developed may provide novel treatments for multiple high morbidity/mortality conditions. This figure was created with BioRender.com
References
Xu, J. et al. National Vital Statistics Reports, Volume 70, Number 8 July 26, 2016. Deaths: final data 2019. Natl. Vital Stat. Rep. 70, (2021).
Hirode, G. & Wong, R. J. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA 323, 2526–2528 (2020).
Liang, X. et al. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–18. Postgrad. Med. J. 99, 985–992 (2023).
Choudhuri, S. & Klaassen, C. D. Molecular regulation of bile acid homeostasis. Drug Metab. Dispos. 50, 425–455 (2022).
Chiang, J. Y. L. Bile acid metabolism and signaling. Compr. Physiol. 3, 1191–1212 (2013).
Yang, Y. & Zhang, J. Bile acid metabolism and circadian rhythms. Am. J. Physiol. Gastrointest. Liver Physiol. 319, G549–G563 (2020).
May 19, 2023: Meeting of the Gastrointestinal Drugs Advisory Committee Meeting Announcement - 05/19/2023. https://www.fda.gov/advisory-committees/committees-and-meeting-materials/may-19-2023-meeting-gastrointestinal-drugs-advisory-committee-meeting-announcement-05192023#event-materials (FDA, 2023).
Thomas, C. et al. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Perino, A., Demagny, H., Velazquez-Villegas, L. & Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 101, 683–731 (2021).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Yetti, H. et al. Bile acid detoxifying enzymes limit susceptibility to liver fibrosis in female SHRSP5/Dmcr rats fed with a high-fat-cholesterol diet. PLOS One. 13, e0192863 (2018).
Lake, A. D. et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 268, 132–140 (2013).
Worthmann, A. et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 23, 839–849 (2017).
Ishibashi, S. et al. Disruption of cholesterol 7α-hydroxylase gene in mice. J. Biol. Chem. 271, 18017–18023 (1996).
Ferdinandusse, S. & Houten, S. M. Peroxisomes and bile acid biosynthesis. Biochim. Biophys. Acta BBA Mol. Cell Res. 1763, 1427–1440 (2006).
Li, J. & Dawson, P. A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 895–911 (2019).
Alnouti, Y. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol. Sci. 108, 225–246 (2009).
Trottier, J. et al. Profiling serum bile acid glucuronides in humans: gender divergences, genetic determinants and response to fenofibrate. Clin. Pharmacol. Ther. 94, 533–543 (2013).
Chiang, J. Y. Recent advances in understanding bile acid homeostasis. F1000Research. 6, 2029 (2017).
Botham, K. M. & Boyd, G. S. The metabolism of chenodeoxycholic acid to beta-muricholic acid in rat liver. Eur. J. Biochem. 134, 191–196 (1983).
Sacquet, E. et al. Intestinal absorption, excretion, and biotransformation of hyodeoxycholic acid in man. J. Lipid Res. 24, 604–613 (1983).
de Boer, J. F. et al. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice [S]. J. Lipid Res. 61, 291–305 (2020).
Xie, Y. et al. Gamma-muricholic acid inhibits nonalcoholic steatohepatitis: abolishment of steatosis-dependent peroxidative impairment by FXR/SHP/LXRα/FASN signaling. Nutrients 15, 1255 (2023).
Di Ciaula, A. et al. Bile acid physiology. Ann. Hepatol. 16, S4–S14 (2017).
Honda, A. et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 61, 54–69 (2020).
Wahlström, A., Sayin, S. I., Marschall, H.-U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Tanaka, H., Hashiba, H., Kok, J. & Mierau, I. Bile salt hydrolase of Bifidobacterium longum-biochemical and genetic characterization. Appl. Environ. Microbiol. 66, 2502–2512 (2000).
Guzior, D. V. & Quinn, R. A. Review: microbial transformations of human bile acids. Microbiome 9, 140 (2021).
Ridlon, J. M., Kang, D.-J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Foley, M. H. et al. Bile salt hydrolases shape the bile acid landscape and restrict Clostridioides difficile growth in the murine gut. Nat. Microbiol. 8, 611–628 (2023).
Kim, G.-B., Yi, S.-H. & Lee, B. H. Purification and characterization of three different types of bile salt hydrolases from Bifidobacterium strains. J. Dairy Sci. 87, 258–266 (2004).
Elkins, C. A., Moser, S. A. & Savage, D. C. Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology 147, 3403–3412 (2001).
Corzo, G. & Gilliland, S. E. Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J. Dairy Sci. 82, 472–480 (1999).
Coleman, J. P. & Hudson, L. L. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl. Environ. Microbiol. 61, 2514–2520 (1995).
Wijaya, A. et al. Cloning of the bile salt hydrolase (bsh) gene from Enterococcus faecium FAIR-E 345 and chromosomal location of bsh genes in food enterococci. J. Food Prot. 67, 2772–2778 (2004).
Dussurget, O. et al. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 45, 1095–1106 (2002).
Dean, M. et al. Characterization of cholylglycine hydrolase from a bile-adapted strain of Xanthomonas maltophilia and its application for quantitative hydrolysis of conjugated bile salts. Appl. Environ. Microbiol. 68, 3126–3128 (2002).
Kawamoto, K., Horibe, I. & Uchida, K. Purification and characterization of a new hydrolase for conjugated bile acids, chenodeoxycholyltaurine hydrolase, from Bacteroides vulgatus. J. Biochem. 106, 1049–1053 (1989).
Delpino, M. V. et al. A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infect. Immun. 75, 299–305 (2007).
Joyce, S. A. & Gahan, C. G. M. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Dig. Dis. Basel Switz. 35, 169–177 (2017).
Doden, H. et al. Metabolism of oxo-bile acids and characterization of recombinant 12α-hydroxysteroid dehydrogenases from bile acid 7α-dehydroxylating human gut bacteria. Appl. Environ. Microbiol. 84, e00235–18 (2018).
Quinn, R. A. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579, 123–129 (2020).
Guzior, D. V. et al. Bile salt hydrolase acyltransferase activity expands bile acid diversity. Nature 626, 852–858 (2024).
Rimal, B. et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature 626, 859–863 (2024).
van de Waterbeemd, H., Karajiannis, H. & El Tayar, N. Lipophilicity of amino acids. Amino Acids 7, 129–145 (1994).
Ridlon, J. M. & Hylemon, P. B. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium. J. Lipid Res. 53, 66–76 (2012).
Mallonee, D. H., Adams, J. L. & Hylemon, P. B. The bile acid-inducible baiB gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme A ligase. J. Bacteriol. 174, 2065–2071 (1992).
Dawson, J. A., Mallonee, D. H., Björkhem, I. & Hylemon, P. B. Expression and characterization of a C24 bile acid 7 alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J. Lipid Res. 37, 1258–1267 (1996).
Coleman, J. P., White, W. B. & Hylemon, P. B. Molecular cloning of bile acid 7-dehydroxylase from Eubacterium sp. strain VPI 12708. J. Bacteriol. 169, 1516–1521 (1987).
Kang, D.-J. et al. Clostridium scindens baiCD and baiH genes encode stereo-specific 7alpha/7beta-hydroxy-3-oxo-delta4-cholenoic acid oxidoreductases. Biochim. Biophys. Acta 1781, 16–25 (2008).
Mallonee, D. H. & Hylemon, P. B. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 178, 7053–7058 (1996).
Heinken, A. et al. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 7, 75 (2019).
Bhowmik, S. et al. Structure and functional characterization of a bile acid 7α dehydratase BaiE in secondary bile acid synthesis. Proteins 84, 316–331 (2016).
Ridlon, J. M. et al. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39 (2016).
Funabashi, M. et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582, 566–570 (2020).
Harris, S. C. et al. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243T. Gut Microbes 9, 523–539 (2018).
Hirano, S. & Masuda, N. Epimerization of the 7-hydroxy group of bile acids by the combination of two kinds of microorganisms with 7 alpha- and 7 beta-hydroxysteroid dehydrogenase activity, respectively. J. Lipid Res. 22, 1060–1068 (1981).
Eggert, T., Bakonyi, D. & Hummel, W. Enzymatic routes for the synthesis of ursodeoxycholic acid. J. Biotechnol. 191, 11–21 (2014).
Giovannini, P. P. et al. 7α- and 12α-Hydroxysteroid dehydrogenases from Acinetobacter calcoaceticus lwoffii: a new integrated chemo-enzymatic route to ursodeoxycholic acid. Steroids 73, 1385–1390 (2008).
Wegner, K. et al. Rapid analysis of bile acids in different biological matrices using LC-ESI-MS/MS for the investigation of bile acid transformation by mammalian gut bacteria. Anal. Bioanal. Chem. 409, 1231–1245 (2017).
Nouioui, I. et al. Genome-based taxonomic classification of the phylum actinobacteria. Front. Microbiol. 9, (2018).
Mythen, S. M. et al. Targeted synthesis and characterization of a gene cluster encoding NAD(P)H-dependent 3α-, 3β-, and 12α-hydroxysteroid dehydrogenases from Eggerthella CAG:298, a gut metagenomic sequence. Appl. Environ. Microbiol. 84, e02475–17 (2018).
Lepercq, P. et al. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol. Lett. 235, 65–72 (2004).
Pedrini, P. et al. Xanthomonas maltophilia CBS 897.97 as a source of new 7beta- and 7alpha-hydroxysteroid dehydrogenases and cholylglycine hydrolase: improved biotransformations of bile acids. Steroids 71, 189–198 (2006).
Lee, J.-Y. et al. Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J. Lipid Res. 54, 3062–3069 (2013).
Ferrandi, E. E. et al. In search of sustainable chemical processes: cloning, recombinant expression, and functional characterization of the 7α- and 7β-hydroxysteroid dehydrogenases from Clostridium absonum. Appl. Microbiol. Biotechnol. 95, 1221–1233 (2012).
Liu, L., Aigner, A. & Schmid, R. D. Identification, cloning, heterologous expression, and characterization of a NADPH-dependent 7β-hydroxysteroid dehydrogenase from Collinsella aerofaciens. Appl. Microbiol. Biotechnol. 90, 127–135 (2011).
Macdonald, I. A., Jellett, J. F., Mahony, D. E. & Holdeman, L. V. Bile salt 3α- and 12α-hydroxysteroid dehydrogenases from eubacterium lentum and related organisms. Appl. Environ. Microbiol. 37, 992–1000 (1979).
Edenharder, R. & Schneider, J. 12 beta-dehydrogenation of bile acids by Clostridium paraputrificum, C. tertium, and C. difficile and epimerization at carbon-12 of deoxycholic acid by cocultivation with 12 alpha-dehydrogenating Eubacterium lentum. Appl. Environ. Microbiol. 49, 964–968 (1985).
Heuman, D. M., Hylemon, P. B. & Vlahcevic, Z. R. Regulation of bile acid synthesis. III. Correlation between biliary bile salt hydrophobicity index and the activities of enzymes regulating cholesterol and bile acid synthesis in the rat. J. Lipid Res. 30, 1161–1171 (1989).
Chiang, J. Y. L. & Ferrell, J. M. Bile acid metabolism in liver pathobiology. Gene Expr. 18, 71–87 (2018).
Thakare, R. et al. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 38, 1323–1335 (2018).
Setchell, K. D. R. et al. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology 144, 945–955.e6 (2013).
van de Peppel, I. P., Bodewes, F. A. J. A., Verkade, H. J. & Jonker, J. W. Bile acid homeostasis in gastrointestinal and metabolic complications of cystic fibrosis. J. Cyst. Fibros. 18, 313–320 (2019).
Suga, T. et al. Preference of conjugated bile acids over unconjugated bile acids as substrates for OATP1B1 and OATP1B3. PLoS ONE 12, e0169719 (2017).
Kullak-Ublick, G. A., Stieger, B., Hagenbuch, B. & Meier, P. J. Hepatic transport of bile salts. Semin. Liver Dis. 20, 273–292 (2000).
Wang, D. Q.-H., Tazuma, S., Cohen, D. E. & Carey, M. C. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G494–G502 (2003).
Hofmann, A. F., Hagey, L. R. & Krasowski, M. D. Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 51, 226–246 (2010).
Higuchi, S. The physiological importance of bile acid structure and composition on glucose homeostasis. Curr. Diab. Rep. 20, 42 (2020).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Wang, H. et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3, 543–553 (1999).
Lew, J.-L. et al. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 279, 8856–8861 (2004).
Li, F. et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4, 2384 (2013).
Maruyama, T. et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719 (2002).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
Chevre, R. et al. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. FASEB J. 32, 3792–3802 (2018).
Mukhopadhyay, S. & Maitra, U. Chemistry and biology of bile acids. Curr. Sci. 87, 1666–1683 (2004).
Halilbasic, E., Claudel, T. & Trauner, M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 58, 155–168 (2013).
Boyer, J. L. In Comprehensive Physiology (ed. Terjung, R.) 1035–1078 (Wiley, 2013).
Dawson, P. A., Lan, T. & Rao, A. Bile acid transporters. J. Lipid Res. 50, 2340–2357 (2009).
Shneider, B. L. et al. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Invest. 95, 745–754 (1995).
Houten, S. & Auwerx, J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann. Med. 36, 482–491 (2004).
Hofmann, A. F. & Hagey, L. R. Bile Acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 65, 2461–2483 (2008).
Dawson, P. A. et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 278, 33920–33927 (2003).
Rao, A. et al. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl Acad. Sci. USA 105, 3891–3896 (2008).
Kemper, J. K. Regulation of FXR transcriptional activity in health and disease: emerging roles of FXR cofactors and post-translational modifications. Biochim. Biophys. Acta 1812, 842–850 (2011).
Jiang, L. et al. Farnesoid X receptor (FXR): structures and ligands. Comput. Struct. Biotechnol. J. 19, 2148–2159 (2021).
Ramos Pittol, J. M. et al. FXR isoforms control different metabolic functions in liver cells via binding to specific DNA motifs. Gastroenterology 159, 1853–1865.e10 (2020).
Cunningham, F. et al. Ensembl 2022. Nucleic Acids Res. 50, D988–D995 (2022).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Torres, J. et al. Farnesoid X receptor expression is decreased in colonic mucosa of patients with primary sclerosing cholangitis and colitis-associated neoplasia. Inflamm. Bowel Dis. 19, 275–282 (2013).
Bailey, A. M. et al. FXR silencing in human colon cancer by DNA methylation and KRAS signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G48–G58 (2014).
Selmin, O. I. et al. Inactivation of adenomatous polyposis coli reduces bile acid/farnesoid X receptor expression through Fxr gene CpG methylation in mouse colon tumors and human colon cancer cells. J. Nutr. 146, 236–242 (2016).
Cabrerizo, R. et al. Promoter DNA methylation of farnesoid X receptor and pregnane X receptor modulates the intrahepatic cholestasis of pregnancy phenotype. PLoS ONE 9, e87697 (2014).
Wan, Y.-J. Y. & Sheng, L. Regulation of bile acid receptor activity. Liver Res. 2, 180–185 (2018).
Kemper, J. K. et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 10, 392–404 (2009).
Purushotham, A. et al. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1α/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol. Cell. Biol. 32, 1226–1236 (2012).
Yang, F., Hu, Y., Liu, H.-X. & Wan, Y.-J. Y. MiR-22-silenced cyclin A expression in colon and liver cancer cells is regulated by bile acid receptor. J. Biol. Chem. 290, 6507–6515 (2015).
Xu, D. et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 193, 409–424 (2011).
Choi, S.-E. et al. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12, 1062–1072 (2013).
Balasubramaniyan, N., Luo, Y., Sun, A.-Q. & Suchy, F. J. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J. Biol. Chem. 288, 13850–13862 (2013).
Zhang, Y., Hagedorn, C. H. & Wang, L. Role of nuclear receptor SHP in metabolism and cancer. Biochim. Biophys. Acta 1812, 893–908 (2011).
Miao, J. et al. Functional specificities of Brm and Brg-1 Swi/Snf ATPases in the feedback regulation of hepatic bile acid biosynthesis. Mol. Cell. Biol. 29, 6170–6181 (2009).
Kadam, S. & Emerson, B. M. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell. 11, 377–389 (2003).
Strobeck, M. W. et al. Compensation of BRG-1 function by Brm: insight into the role of the core SWI-SNF subunits in retinoblastoma tumor suppressor signaling. J. Biol. Chem. 277, 4782–4789 (2002).
Smith, Z., Ryerson, D. & Kemper, J. K. Epigenomic regulation of bile acid metabolism: emerging role of transcriptional cofactors. Mol. Cell. Endocrinol. 368, 59–70 (2013).
Fang, S. et al. Coordinated recruitment of histone methyltransferase G9a and other chromatin-modifying enzymes in SHP-mediated regulation of hepatic bile acid metabolism. Mol. Cell. Biol. 27, 1407–1424 (2007).
Goo, Y.-H. et al. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol. Cell. Biol. 23, 140–149 (2003).
Lee, J. et al. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc. Natl Acad. Sci. USA 106, 8513–8518 (2009).
Lee, S., Roeder, R. G. & Lee, J. W. Roles of histone H3-lysine 4 methyltransferase complexes in NR-mediated gene transcription. Prog. Mol. Biol. Transl. Sci. 87, 343–382 (2009).
Kim, D.-H., Lee, J., Lee, B. & Lee, J. W. ASCOM controls farnesoid X receptor transactivation through its associated histone H3 lysine 4 methyltransferase activity. Mol. Endocrinol. 23, 1556–1562 (2009).
Kim, D.-H., Kim, J. & Lee, J. W. Requirement for MLL3 in p53 regulation of hepatic expression of small heterodimer partner and bile acid homeostasis. Mol. Endocrinol. 25, 2076–2083 (2011).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Zhang, J. H. et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G940–G948 (2013).
Marcelin, G. et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 3, 19–28 (2013).
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Byun, S. et al. Postprandial FGF19-induced phosphorylation by Src is critical for FXR function in bile acid homeostasis. Nat. Commun. 9, 2590 (2018).
Zhang, S. Q. et al. Shp2 regulates Src family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell. 13, 341–355 (2004).
Li, S. et al. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 20, 320–332 (2014).
Belov, A. A. & Mohammadi, M. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci. Signal. 5, pe49–pe49 (2012).
Xie, C. et al. An intestinal farnesoid X receptor–ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017).
Miao, J. et al. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin–proteasomal degradation. Genes Dev. 23, 986–996 (2009).
Somm, E. & Jornayvaz, F. R. Fibroblast growth factor 15/19: from basic functions to therapeutic perspectives. Endocr. Rev. 39, 960–989 (2018).
Goodwin, B. et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 6, 517–526 (2000).
Stroup, D. & Chiang, J. Y. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1). J. Lipid Res. 41, 1–11 (2000).
Zhang, M. & Chiang, J. Y. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J. Biol. Chem. 276, 41690–41699 (2001).
Yang, Y., Zhang, M., Eggertsen, G. & Chiang, J. Y. L. On the mechanism of bile acid inhibition of rat sterol 12alpha-hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4alpha. Biochim. Biophys. Acta 1583, 63–73 (2002).
Yoshida, E. et al. Functional association between CBP and HNF4 in trans-activation. Biochem. Biophys. Res. Commun. 241, 664–669 (1997).
Cooney, A. J., Tsai, S. Y., O’Malley, B. W. & Tsai, M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell. Biol. 12, 4153–4163 (1992).
Ge, M., Shao, R. & He, H. Advances in understanding the regulatory mechanism of cholesterol 7α-hydroxylase. Biochem. Pharmacol. 164, 152–164 (2019).
Wang, Y. et al. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat. Commun. 11, 3612 (2020).
Wan, Z. Y. et al. Mechanistic target of rapamycin complex 1 is an essential mediator of metabolic and mitogenic effects of fibroblast growth factor 19 in hepatoma cells. Hepatology 64, 1289–1301 (2016).
Kerr, T. A. et al. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev. Cell. 2, 713–720 (2002).
Kir, S. et al. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 287, 41334–41341 (2012).
Kim, I. et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48, 2664–2672 (2007).
Rizzo, G. et al. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr. Drug Targets Immune Endocr. Metab. Disord. 5, 289–303 (2005).
Eloranta, J. J. & Kullak-Ublick, G. A. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch. Biochem. Biophys. 433, 397–412 (2005).
Zollner, G., Marschall, H.-U., Wagner, M. & Trauner, M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol. Pharm. 3, 231–251 (2006).
Watanabe, M. et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 (2004).
Yoshikawa, T. et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 21, 2991–3000 (2001).
Kim, Y.-C. et al. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat. Commun. 11, 5969 (2020).
Wang, Y., Viscarra, J., Kim, S.-J. & Sul, H. S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 16, 678–689 (2015).
Kast, H. R. et al. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 15, 1720–1728 (2001).
Claudel, T. et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology 125, 544–555 (2003).
Hayhurst, G. P. et al. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 (2001).
Pineda Torra, I. et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol. Endocrinol. 17, 259–272 (2003).
Savkur, R. S., Bramlett, K. S., Michael, L. F. & Burris, T. P. Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem. Biophys. Res. Commun. 329, 391–396 (2005).
Hirokane, H. et al. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J. Biol. Chem. 279, 45685–45692 (2004).
Claudel, T. et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109, 961–971 (2002).
Mak, P. A., Kast-Woelbern, H. R., Anisfeld, A. M. & Edwards, P. A. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J. Lipid Res. 43, 2037–2041 (2002).
Dong, B. et al. Activation of FXR by obeticholic acid induces hepatic gene expression of SR-BI through a novel mechanism of transcriptional synergy with the nuclear receptor LXR. Int. J. Mol. Med. 43, 1927–1938 (2019).
Zhang, Y. et al. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J. Biol. Chem. 285, 3035–3043 (2010).
Xu, Y. et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 64, 1072–1085 (2016).
Jakulj, L. et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 24, 783–794 (2016).
Yu, L. et al. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 280, 8742–8747 (2005).
Al-Dury, S. et al. Obeticholic acid may increase the risk of gallstone formation in susceptible patients. J. Hepatol. 71, 986–991 (2019).
Langhi, C. et al. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 582, 949–955 (2008).
Ghosh Laskar, M., Eriksson, M., Rudling, M. & Angelin, B. Treatment with the natural FXR agonist chenodeoxycholic acid reduces clearance of plasma LDL whilst decreasing circulating PCSK9, lipoprotein(a) and apolipoprotein C-III. J. Intern. Med. 281, 575–585 (2017).
Zhang, Y. et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006).
Ma, K., Saha, P. K., Chan, L. & Moore, D. D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 116, 1102–1109 (2006).
Cariou, B. et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 281, 11039–11049 (2006).
Cipriani, S., Mencarelli, A., Palladino, G. & Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 51, 771–784 (2010).
Caron, S. et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell. Biol. 33, 2202–2211 (2013).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Yamagata, K. et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 279, 23158–23165 (2004).
Wattanavanitchakorn, S. et al. CCAAT-enhancer binding protein-α (C/EBPα) and hepatocyte nuclear factor 4α (HNF4α) regulate expression of the human fructose-1,6-bisphosphatase 1 (FBP1) gene in human hepatocellular carcinoma HepG2 cells. PLoS ONE 13, e0194252 (2018).
Zhang, X., Yang, S., Chen, J. & Su, Z. Unraveling the regulation of hepatic gluconeogenesis. Front. Endocrinol. 9, 802 (2019).
Trabelsi, M.-S. et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 6, 7629 (2015).
Wondisford, A. R. et al. Control of Foxo1 gene expression by co-activator P300. J. Biol. Chem. 289, 4326–4333 (2014).
Potthoff, M. J. et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 13, 729–738 (2011).
Lin, J. et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119, 121–135 (2004).
Koo, S.-H. et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. 10, 530–534 (2004).
Massafra, V. et al. Farnesoid X receptor activation promotes hepatic amino acid catabolism and ammonium clearance in mice. Gastroenterology 152, 1462–1476.e10 (2017).
Renga, B. et al. The nuclear receptor FXR regulates hepatic transport and metabolism of glutamine and glutamate. Biochim. Biophys. Acta 1812, 1522–1531 (2011).
Gingras, A.-C., Raught, B. & Sonenberg, N. eIF4 Initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68, 913–963 (1999).
Schote, A. B., Turner, J. D., Schiltz, J. & Muller, C. P. Nuclear receptors in human immune cells: expression and correlations. Mol. Immunol. 44, 1436–1445 (2007).
Yao, J. et al. FXR agonist GW4064 alleviates endotoxin-induced hepatic inflammation by repressing macrophage activation. World J. Gastroenterol. 20, 14430–14441 (2014).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. -Heart Circ. Physiol. 296, H272–H281 (2009).
Vavassori, P. et al. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 (2009).
Hao, H. et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 25, 856–867.e5 (2017).
Wang, Y.-D. et al. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48, 1632–1643 (2008).
Malhi, H., Guicciardi, M. E. & Gores, G. J. Hepatocyte death: a clear and present danger. Physiol. Rev. 90, 1165–1194 (2010).
Wang, H. et al. Noncanonical farnesoid X receptor signaling inhibits apoptosis and impedes liver fibrosis. EBioMedicine 37, 322–333 (2018).
Gai, Z. et al. Farnesoid X receptor activation induces the degradation of hepatotoxic 1-deoxysphingolipids in non-alcoholic fatty liver disease. Liver Int. 40, 844–859 (2020).
Bhogal, H. K. & Sanyal, A. J. The molecular pathogenesis of cholestasis in sepsis. Front. Biosci. -Elite 5, 87–96 (2013).
Adachi, T. et al. The involvement of endoplasmic reticulum stress in bile acid-induced hepatocellular injury. J. Clin. Biochem. Nutr. 54, 129–135 (2014).
Panzitt, K. & Wagner, M. FXR in liver physiology: multiple faces to regulate liver metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166133 (2021).
Yin, Z., Pascual, C. & Klionsky, D. J. Autophagy: machinery and regulation. Microb. Cell. 3, 588–596 (2016).
Seok, S. et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516, 108–111 (2014).
Lee, J. M. et al. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516, 112–115 (2014).
Ferré, P. & Foufelle, F. A new role for a metabolic star: AMP-activated protein kinase stimulates fat absorption. Cell Metab. 13, 1–2 (2011).
Lien, F. et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J. Clin. Invest. 124, 1037–1051 (2014).
Guo, C., Chen, W.-D. & Wang, Y.-D. TGR5, not only a metabolic regulator. Front. Physiol. 7, 646 (2016).
Gao, S. et al. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 predicts prognosis of acute-on-chronic hepatitis B liver failure. J. Viral Hepat. 22, 112–119 (2015).
Han, L.-Y. et al. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) is a potential biomarker for hepatitis B Virus associated hepatocellular carcinoma. Int. J. Med. Sci. 11, 164–171 (2014).
Pathak, P. et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 292, 11055–11069 (2017).
Wang, X. X. et al. G Protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378 (2016).
Rajagopal, S. et al. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G527–G535 (2013).
Qi, Y.-C. et al. Taurochenodeoxycholic acid mediates cAMP-PKA-CREB signaling pathway. Chin. J. Nat. Med. 18, 898–906 (2020).
Yang, W. et al. TGR5 agonist inhibits intestinal epithelial cell apoptosis via cAMP/PKA/c-FLIP/JNK signaling pathway and ameliorates dextran sulfate sodium-induced ulcerative colitis. Acta Pharmacol. Sin. 44, 1649–1664 (2023).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Zhao, L.-J. & Zhang, S.-F. Activation of TGR5 promotes mitochondrial biogenesis in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 500, 952–957 (2018).
Donepudi, A. C., Boehme, S., Li, F. & Chiang, J. Y. L. G protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis. Hepatology 65, 813–827 (2017).
McGavigan, A. K. et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66, 226–234 (2017).
Holter, M. M., Chirikjian, M. K., Govani, V. N. & Cummings, B. P. TGR5 signaling in hepatic metabolic health. Nutrients 12, 2598 (2020).
Yan, Y. et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat. Commun. 13, 6408 (2022).
Lammel Lindemann, J. A. et al. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol. Cell. Endocrinol. 388, 32–40 (2014).
Bianco, A. C., Sheng, X. Y. & Silva, J. E. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J. Biol. Chem. 263, 18168–18175 (1988).
de Jesus, L. A. et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Invest. 108, 1379–1385 (2001).
Giudicelli, Y. Thyroid-hormone modulation of the number of beta-adrenergic receptors in rat fat-cell membranes. Biochem. J. 176, 1007–1010 (1978).
Volke, L. & Krause, K. Effect of thyroid hormones on adipose tissue flexibility. Eur. Thyroid J. 10, 1–9 (2021).
Lee, J.-Y. et al. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol. -Cell Physiol. 302, C463–C472 (2012).
Periasamy, M. et al. Role of SERCA pump in muscle thermogenesis and metabolism. Compr. Physiol. 7, 879–890 (2017).
Periasamy, M., Herrera, J. L. & Reis, F. C. G. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab. J. 41, 327–336 (2017).
Zekri, Y., Flamant, F. & Gauthier, K. Central vs. peripheral action of thyroid hormone in adaptive thermogenesis: a burning topic. Cells 10, 1327 (2021).
Brent, G. A. Mechanisms of thyroid hormone action. J. Clin. Invest. 122, 3035–3043 (2012).
Ventura-Clapier, R., Garnier, A. & Veksler, V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1. Cardiovasc. Res. 79, 208–217 (2008).
Schmid, A. et al. Evidence of functional bile acid signaling pathways in adipocytes. Mol. Cell. Endocrinol. 483, 1–10 (2019).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Evans, M. J. & Scarpulla, R. C. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 4, 1023–1034 (1990).
Virbasius, J. V. & Scarpulla, R. C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl Acad. Sci. 91, 1309–1313 (1994).
Gleyzer, N., Vercauteren, K. & Scarpulla, R. C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 25, 1354–1366 (2005).
Chen, L. et al. PGC-1α-mediated mitochondrial quality control: molecular mechanisms and implications for heart failure. Front. Cell Dev. Biol. 10, 871357 (2022).
Iwaki, M. et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 (2003).
Berg, A. H. et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001).
Combs, T. P. et al. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875–1881 (2001).
Hada, Y. et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem. Biophys. Res. Commun. 356, 487–493 (2007).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Yamauchi, T. et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 (2007).
Yamauchi, T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003).
Sharma, A. X. & Holland, W. L. Adiponectin and its hydrolase-activated receptors. J. Nat. Sci. 3, e396 (2017).
Kim, Y. et al. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell Death Dis. 13, 1–20 (2022).
Botta, A. et al. An adiponectin-S1P axis protects against lipid induced insulin resistance and cardiomyocyte cell death via reduction of oxidative stress. Nutr. Metab. 16, 14 (2019).
Lancaster, G. I. & Febbraio, M. A. Adiponectin sphings into action. Nat. Med. 17, 37–38 (2011).
Li, Y., Talbot, C. L. & Chaurasia, B. Ceramides in adipose tissue. Front. Endocrinol. 11, 407 (2020).
Kumar, D. P. et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem. Biophys. Res. Commun. 427, 600–605 (2012).
Aktories, K. et al. cAMP guided his way: a life for G protein-mediated signal transduction and molecular pharmacology—tribute to Karl H. Jakobs. Naunyn. Schmiedebergs Arch. Pharmacol. 392, 887–911 (2019).
Hamilton, A. et al. Adrenaline stimulates glucagon secretion by Tpc2-dependent Ca2+ mobilization from acidic stores in pancreatic α-cells. Diabetes 67, 1128–1139 (2018).
Fujimoto, K. et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J. Biol. Chem. 277, 50497–50502 (2002).
Schmidt, M., Dekker, F. J. & Maarsingh, H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 65, 670–709 (2013).
Maczewsky, J. et al. TGR5 activation promotes stimulus-secretion coupling of pancreatic β-cells via a PKA-dependent pathway. Diabetes 68, 324–336 (2018).
Light, P. E., Manning Fox, J. E., Riedel, M. J. & Wheeler, M. B. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol. Endocrinol. 16, 2135–2144 (2002).
Nakazaki, M. et al. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 51, 3440–3449 (2002).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Vettorazzi, J. F. et al. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 65, 54–63 (2016).
Katsuma, S., Hirasawa, A. & Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329, 386–390 (2005).
Higuchi, S. et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice. Gut 69, 1620–1628 (2020).
Roberts, R. E. et al. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin. Endocrinol. 74, 67–72 (2011).
Kuhre, R. E. et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 11, 84–95 (2018).
Bala, V. et al. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 5, 420 (2014).
Gündüz, D. et al. Role of PI3K/Akt and MEK/ERK signalling in cAMP/Epac-mediated endothelial barrier stabilisation. Front. Physiol 10, 1387 (2019).
Fu, W. & Hall, M. N. Regulation of mTORC2 Signaling. Genes 11, 1045 (2020).
Cunningham, R. P., Sheldon, R. D. & Rector, R. S. The emerging role of hepatocellular eNOS in non-alcoholic fatty liver disease development. Front. Physiol. 11, 767 (2020).
Kida, T. et al. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33, 1663–1669 (2013).
García-Morales, V., Luaces-Regueira, M. & Campos-Toimil, M. The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochem. Pharmacol. 145, 94–101 (2017).
Keitel, V. et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 50, 861–870 (2009).
Flass, T. & Narkewicz, M. R. Cirrhosis and other liver disease in cystic fibrosis. J. Cyst. Fibros. 12, 116–124 (2013).
Deutschmann, K. et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 1864, 1319–1325 (2018).
Beuers, U. et al. The biliary HCO3− umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 52, 1489–1496 (2010).
Hohenester, S. et al. A biliary HCO3− umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55, 173–183 (2012).
Perino, A. & Schoonjans, K. TGR5 and immunometabolism: insights from physiology and pharmacology. Trends Pharmacol. Sci. 36, 847–857 (2015).
Descombes, P. & Schibler, U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67, 569–579 (1991).
Sunilkumar, S., Kimball, S. R. & Dennis, M. D. Glucagon transiently stimulates mTORC1 by activation of an EPAC/Rap1 signaling axis. Cell. Signal. 84, 110010 (2021).
Chen, H. et al. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 22, 4038–4043 (2012).
Perino, A. et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J. Clin. Invest. 124, 5424–5436 (2014).
Pols, T. W. H. et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14, 747–757 (2011).
Wang, Y.-D. et al. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54, 1421–1432 (2011).
Guo, C. et al. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell proliferation and migration through antagonizing STAT3 signaling pathway. Oncotarget 6, 34402–34413 (2015).
Yoneno, K. et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 139, 19–29 (2013).
Koga, K. et al. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity 30, 372–383 (2009).
Biagioli, M. et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J. Immunol. 199, 718–733 (2017).
Wang, P. et al. p-CREB-1 promotes hepatic fibrosis through the transactivation of transforming growth factor-β1 expression in rats. Int. J. Mol. Med. 38, 521–528 (2016).
Dempsey, L. A. Bile acids block NLRP3. Nat. Immunol. 17, 1243–1243 (2016).
Shi, Y. et al. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation. Front. Immunol. 11, 609060 (2021).
Liang, H. et al. TGR5 activation attenuates neuroinflammation via Pellino3 inhibition of caspase-8/NLRP3 after middle cerebral artery occlusion in rats. J. Neuroinflammation 18, 40 (2021).
Högenauer, K. et al. G-Protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J. Med. Chem. 57, 10343–10354 (2014).
McMahan, R. H. et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 288, 11761–11770 (2013).
Wammers, M. et al. Reprogramming of pro-inflammatory human macrophages to an anti-inflammatory phenotype by bile acids. Sci. Rep. 8, 255 (2018).
Liu, R. et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 60, 908–918 (2014).
Liu, R. et al. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J. Biol. Chem. 290, 30988–31002 (2015).
Studer, E. et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55, 267–276 (2012).
Burg, N., Salmon, J. E. & Hla, T. Sphingosine 1-phosphate receptor-targeted therapeutics in rheumatic diseases. Nat. Rev. Rheumatol. 18, 335–351 (2022).
Nagahashi, M. et al. Conjugated bile acid activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 61, 1216–1226 (2015).
Nagahashi, M. et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J. Lipid Res. 57, 1636–1643 (2016).
Yang, J. et al. Sphingosine 1-phosphate (S1P)/S1P receptor2/3 axis promotes inflammatory M1 polarization of bone marrow-derived monocyte/macrophage via G(α)i/o/PI3K/JNK pathway. Cell. Physiol. Biochem. 49, 1677–1693 (2018).
Karimian, G. et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochim. Biophys. Acta 1832, 1922–1929 (2013).
Wang, Y. et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 65, 2005–2018 (2017).
Wang, X. et al. S1PR2/RhoA/ROCK1 pathway promotes inflammatory bowel disease by inducing intestinal vascular endothelial barrier damage and M1 macrophage polarization. Biochem. Pharmacol. 201, 115077 (2022).
Schledwitz, A. et al. Differential actions of muscarinic receptor subtypes in gastric, pancreatic, and colon cancer. Int. J. Mol. Sci. 22, 13153 (2021).
Cheng, K. et al. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim. Biophys. Acta 1588, 48–55 (2002).
Xie, G. et al. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G521–G529 (2005).
Zhang, L. et al. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. OncoTargets Ther. 9, 6719–6726 (2016).
Von Rosenvinge, E. C. & Raufman, J.-P. Muscarinic receptor signaling in colon cancer. Cancers 3, 971–981 (2011).
Amonyingcharoen, S. et al. Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway. Int. J. Oncol. 46, 2317–2326 (2015).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Li, Z. et al. Nuclear receptor atlas of female mouse liver parenchymal, endothelial, and Kupffer cells. Physiol. Genom. 45, 268–275 (2013).
Norman, A. W. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542–5548 (2006).
Nehring, J. A., Zierold, C. & DeLuca, H. F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl Acad. Sci. USA 104, 10006–10009 (2007).
Han, S. & Chiang, J. Y. L. Mechanism of vitamin D receptor inhibition of cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. Drug Metab. Dispos. Biol. Fate Chem. 37, 469–478 (2009).
Jurutka, P. W. et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D(3) and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J. Cell. Biochem. 94, 917–943 (2005).
Chen, X. et al. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol. Pharmacol. 69, 1913–1923 (2006).
McCarthy, T. C., Li, X. & Sinal, C. J. Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J. Biol. Chem. 280, 23232–23242 (2005).
Chatterjee, B., Echchgadda, I. & Song, C. S. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 400, 165–191 (2005).
Honjo, Y. et al. 1,25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. J. Endocrinol. 188, 635–643 (2006).
Chow, E. C. Y. et al. Vitamin D receptor activation down-regulates the small heterodimer partner and increases CYP7A1 to lower cholesterol. Gastroenterology 146, 1048–1059.e7 (2014).
Ogura, M. et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J. Pharmacol. Exp. Ther. 328, 564–570 (2009).
Wang, J. et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48, 1396–1406 (2016).
Zhang, J., Zhang, Y., Xia, Y. & Sun, J. Imbalance of the intestinal virome and altered viral-bacterial interactions caused by a conditional deletion of the vitamin D receptor. Gut Microbes. 13, 1957408 (2021).
Lamba, V. et al. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol. 199, 251–265 (2004).
Wang, Y.-M., Ong, S. S., Chai, S. C. & Chen, T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin. Drug Metab. Toxicol. 8, 803–817 (2012).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
Wistuba, W. et al. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J. Gastroenterol. 13, 4230–4235 (2007).
Xie, W. et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl Acad. Sci. USA 98, 3375–3380 (2001).
Bhalla, S. et al. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J. Biol. Chem. 279, 45139–45147 (2004).
Li, T. & Chiang, J. Y. L. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G74–G84 (2005).
Zhou, J. et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281, 15013–15020 (2006).
Nakamura, K., Moore, R., Negishi, M. & Sueyoshi, T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J. Biol. Chem. 282, 9768–9776 (2007).
Kodama, S., Moore, R., Yamamoto, Y. & Negishi, M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem. J. 407, 373–381 (2007).
Choi, H. S. et al. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J. Biol. Chem. 272, 23565–23571 (1997).
Baes, M. et al. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol. Cell. Biol. 14, 1544–1552 (1994).
Kovács, P. et al. Lithocholic acid, a metabolite of the microbiome, increases oxidative stress in breast cancer. Cancers 11, 1255 (2019).
Wagner, M. et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42, 420–430 (2005).
Janowski, B. A. et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383, 728–731 (1996).
Svensson, S. et al. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 22, 4625–4633 (2003).
Chuu, C.-P., Kokontis, J. M., Hiipakka, R. A. & Liao, S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J. Biomed. Sci. 14, 543–553 (2007).
Venkateswaran, A. et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl Acad. Sci. USA 97, 12097–12102 (2000).
Bideyan, L. et al. Integrative analysis reveals multiple modes of LXR transcriptional regulation in liver. Proc. Natl Acad. Sci. USA 119, e2122683119 (2022).
Li, S. et al. Adeno-associated virus-based caveolin-1 delivery via different routes for the prevention of cholesterol gallstone formation. Lipids Health Dis. 21, 109 (2022).
Remaley, A. T. et al. Comparative genome analysis of potential regulatory elements in the ABCG5–ABCG8 gene cluster. Biochem. Biophys. Res. Commun. 295, 276–282 (2002).
Goodwin, B. et al. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol. Endocrinol. 17, 386–394 (2003).
De Marino, S. et al. Hyodeoxycholic acid derivatives as liver X receptor α and G-protein-coupled bile acid receptor agonists. Sci. Rep. 7, 43290 (2017).
Pawlak, M., Lefebvre, P. & Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 62, 720–733 (2015).
Brocker, C. N. et al. Extrahepatic PPARα modulates fatty acid oxidation and attenuates fasting-induced hepatosteatosis in mice. J. Lipid Res. 59, 2140–2152 (2018).
Zhong, J. et al. Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Nat. Commun. 14, 5451 (2023).
Xie, C. et al. Hepatocyte peroxisome proliferator-activated receptor α regulates bile acid synthesis and transport. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 1396–1411 (2019).
Zhang, Y., Lickteig, A. J., Csanaky, I. L. & Klaassen, C. D. Editor’s Highlight: clofibrate decreases bile acids in livers of male mice by increasing biliary bile acid excretion in a PPARα-dependent manner. Toxicol. Sci. 160, 351–360 (2017).
Brocker, C. N. et al. Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat. Commun. 11, 5847 (2020).
Xiong, X. et al. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J. Hepatol. 60, 847–854 (2014).
Li, M., Makkinje, A. & Damuni, Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 271, 11059–11062 (1996).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019).
Bharath, L. P. et al. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 64, 3914–3926 (2015).
Stratford, S., Dewald, D. B. & Summers, S. A. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 354, 359–368 (2001).
Powell, D. J. et al. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem. J. 382, 619–629 (2004).
Powell, D. J., Hajduch, E., Kular, G. & Hundal, H. S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol. Cell. Biol. 23, 7794–7808 (2003).
Wu, Q. et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Invest. 131, e142865 (2021).
Grebe, A., Hoss, F. & Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122, 1722–1740 (2018).
Vandanmagsar, B. et al. The NALP3/NLRP3 inflammasome instigates obesity-induced autoinflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Chen, C.-L. et al. Anti-dengue virus nonstructural protein 1 antibodies cause NO-mediated endothelial cell apoptosis via ceramide-regulated glycogen synthase kinase-3β and NF-κB activation. J. Immunol. 191, 1744–1752 (2013).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 919 (2014).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Hammerschmidt, P. et al. CerS6-derived sphingolipids interact with mff and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552.e23 (2019).
Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165838 (2020).
Chávez-Talavera, O., Tailleux, A., Lefebvre, P. & Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694.e3 (2017).
Molinaro, A., Wahlström, A. & Marschall, H.-U. Role of bile acids in metabolic control. Trends Endocrinol. Metab. 29, 31–41 (2018).
Sonne, D. P. et al. Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 101, 3002–3009 (2016).
Cariou, B. et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr. Metab. 8, 48 (2011).
Li, T. et al. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J. Biol. Chem. 287, 1861–1873 (2012).
Haeusler, R. A. et al. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 15, 65–74 (2012).
Duane, W. C. & Javitt, N. B. 27-Hydroxycholesterol: production rates in normal human subjects. J. Lipid Res. 40, 1194–1199 (1999).
Haeusler, R. A. et al. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 62, 4184–4191 (2013).
Kaur, A. et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes 64, 1168–1179 (2015).
Puigserver, P. et al. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature 423, 550–555 (2003).
Langlet, F. et al. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell 171, 824–835.e18 (2017).
Mráz, M. et al. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-α agonist treatment. Physiol. Res. 60, 627–636 (2011).
Schreuder, T. C. M. A. et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G440–G445 (2010).
Kim, H. & Fang, S. Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis. Lab. Anim. Res. 34, 140–146 (2018).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Guo, C. et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45, 802–816 (2016).
Meng, Z. et al. Insufficient bile acid signaling impairs liver repair in CYP27−/− mice. J. Hepatol. 55, 885–895 (2011).
Zheng, X. et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat. Commun. 12, 1487 (2021).
Zheng, X. et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 33, 791–803.e7 (2021).
Prinz, P. et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front. Neurosci. 9, 199 (2015).
Biemann, R. et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: results of a randomized controlled trial. Sci. Rep. 6, 30173 (2016).
Li, M. et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 13, 2060 (2022).
Li, R. et al. Low production of 12α-hydroxylated bile acids prevents hepatic steatosis in Cyp2c70−/− mice by reducing fat absorption. J. Lipid Res. 62, 100134 (2021).
Lee, J.-Y. et al. 12α-Hydroxylated bile acid induces hepatic steatosis with dysbiosis in rats. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 1865, 158811 (2020).
Haeusler, R. A. et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J. Clin. Endocrinol. Metab. 101, 1935–1944 (2016).
Watanabe, M. et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 286, 26913–26920 (2011).
Prawitt, J. et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60, 1861–1871 (2011).
Zhang, Y. et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol. Endocrinol. 26, 272–280 (2012).
Jiao, T. et al. Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease. Acta Pharmacol. Sin. 43, 1103–1119 (2022).
Gonzalez, F. J., Jiang, C., Xie, C. & Patterson, A. D. Intestinal farnesoid X receptor signaling modulates metabolic disease. Dig. Dis. 35, 178–184 (2017).
Lambert, G. et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278, 2563–2570 (2003).
Parséus, A. et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437 (2017).
Schmitt, J. et al. Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal. Liver Int. 35, 1133–1144 (2015).
Velazquez-Villegas, L. A. et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 9, 245 (2018).
Castellanos-Jankiewicz, A. et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 33, 1483–1492.e10 (2021).
Somm, E. et al. β-Klotho deficiency protects against obesity through a crosstalk between liver, microbiota, and brown adipose tissue. JCI Insight 2, e91809 (2017). 91809.
Somm, E. et al. β-Klotho deficiency shifts the gut-liver bile acid axis and induces hepatic alterations in mice. Am. J. Physiol. Endocrinol. Metab. 315, E833–E847 (2018).
Wei, M. et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. eBioMedicine. 55, 102766 (2020).
Teodoro, J. S. et al. Chenodeoxycholic acid has non-thermogenic, mitodynamic anti-obesity effects in an in vitro CRISPR/Cas9 model of bile acid receptor TGR5 knockdown. Int. J. Mol. Sci. 22, 11738 (2021).
Arab, J. P. et al. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65, 350–362 (2017).
Bechmann, L. P. et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57, 1394–1406 (2013).
Jahnel, J. et al. Serum bile acid levels in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 61, 85 (2015).
Puri, P. et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67, 534–548 (2018).
Min, H.-K. et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15, 665–674 (2012).
Jiao, N. et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67, 1881–1891 (2018).
Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Degirolamo, C. et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 61, 161–170 (2015).
Yang, F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67, 863–867 (2007).
Wang, Y. et al. Pharmacological therapy of metabolic dysfunction-associated steatotic liver disease-driven hepatocellular carcinoma. Front. Pharmacol. 14, 1336216 (2024).
Yang, Z.-X., Shen, W. & Sun, H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol. Int. 4, 741–748 (2010).
Miyata, M. et al. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol. Pharm. Bull. 34, 1885–1889 (2011).
Kim, I. et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28, 940–946 (2007).
Kuang, J. et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 35, 1752–1766.e8 (2023).
Mouzaki, M. et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE 11, e0151829 (2016).
Zisser, A., Ipsen, D. H. & Tveden-Nyborg, P. Hepatic stellate cell activation and inactivation in NASH-fibrosis—roles as putative treatment targets? Biomedicines 9, 365 (2021).
Wang, M. et al. Roles of hepatic stellate cells in NAFLD: from the perspective of inflammation and fibrosis. Front. Pharmacol. 13, 958428 (2022).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Carino, A. et al. Disruption of TFGβ-SMAD3 pathway by the nuclear receptor SHP mediates the antifibrotic activities of BAR704, a novel highly selective FXR ligand. Pharmacol. Res. 131, 17–31 (2018).
Fiorucci, S. et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127, 1497–1512 (2004).
Fiorucci, S. et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor γ contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J. Pharmacol. Exp. Ther. 315, 58–68 (2005).
Renga, B. et al. SHP-dependent and -independent induction of peroxisome proliferator-activated receptor-γ by the bile acid sensor farnesoid X receptor counter-regulates the pro-inflammatory phenotype of liver myofibroblasts. Inflamm. Res. 60, 577–587 (2011).
Zhou, J. et al. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat. Commun. 11, 240 (2020).
Mencarelli, A. et al. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J. Immunol. 183, 6657–6666 (2009).
Zhou, M. et al. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol. Commun. 1, 1024–1042 (2017).
Zhou, M. et al. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology 63, 914–929 (2016).
Keitel, V. et al. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 372, 78–84 (2008).
Xie, G. et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. eBioMedicine. 66, 103290 (2021).
Finn, P. D. et al. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G412–G424 (2019).
Khairnar, R., Islam, M. A., Fleishman, J. & Kumar, S. Shedding light on non-alcoholic fatty liver disease: pathogenesis, molecular mechanisms, models, and emerging therapeutics. Life Sci. 312, 121185 (2023).
Dowman, J. K., Tomlinson, J. W. & Newsome, P. N. Pathogenesis of non-alcoholic fatty liver disease. QJM Int. J. Med. 103, 71–83 (2010).
Peng, C. et al. Non-alcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front. Pharmacol. 11, 603926 (2020).
Jiao, Y., Lu, Y. & Li, X. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 36, 44–50 (2015).
Legry, V. et al. Yin Yang 1 and farnesoid X receptor: a balancing act in non-alcoholic fatty liver disease? Gut 63, 1–2 (2014).
Cunningham, J. T. et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450, 736–740 (2007).
Blättler, S. M. et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 15, 505–517 (2012).
Gordon, S., Akopyan, G., Garban, H. & Bonavida, B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene 25, 1125–1142 (2006).
Mu, N. et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci. Rep. 6, 20059 (2016).
Stovall, D. B., Sui, G., Stovall, D. B. & Sui, G. In Advances in Prostate Cancer (IntechOpen, 2013).
Van Quickelberghe, E. et al. A protein-protein interaction map of the TNF-induced NF-κB signal transduction pathway. Sci. Data 5, 180289 (2018).
Ceccarelli, S. et al. LPS-induced TNF-α factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget 6, 41434–41452 (2015).
Sethi, J. K. & Hotamisligil, G. S. Metabolic Messengers: tumour necrosis factor. Nat. Metab. 3, 1302–1312 (2021).
Lu, Y. et al. Yin Yang 1 promotes hepatic steatosis through repression of farnesoid X receptor in obese mice. Gut 63, 170–178 (2014).
Vasavan, T. et al. Heart and bile acids—clinical consequences of altered bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1345–1355 (2018).
Pu, J. et al. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur. Heart J. 34, 1834–1845 (2013).
Rainer, P. P. et al. Bile acids induce arrhythmias in human atrial myocardium–implications for altered serum bile acid composition in patients with atrial fibrillation. Heart Br. Card. Soc. 99, 1685–1692 (2013).
Nilsson, L.-M. et al. Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem. Biophys. Res. Commun. 357, 707–711 (2007).
Hanniman, E. A., Lambert, G., McCarthy, T. C. & Sinal, C. J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 46, 2595–2604 (2005).
Bishop-Bailey, D., Walsh, D. T. & Warner, T. D. Expression and activation of the farnesoid X receptor in the vasculature. Proc. Natl Acad. Sci. USA 101, 3668–3673 (2004).
Li, Y. T. Y. et al. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 27, 2606–2611 (2007).
Zhang, Y. et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 26, 2316–2321 (2006).
Guo, G. L. et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta 1761, 1401–1409 (2006).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Desai, M. S. et al. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology 51, 2097–2107 (2010).
Fryer, R. M. et al. G protein-coupled bile acid receptor 1 stimulation mediates arterial vasodilation through a K(Ca)1.1 (BK(Ca))-dependent mechanism. J. Pharmacol. Exp. Ther. 348, 421–431 (2014).
Eblimit, Z. et al. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc. Ther. 36, e12462 (2018).
Duboc, H. et al. Crosstalk between the hepatologist and the cardiologist: a future place for the lithocholic acid as a coronary atheroma risk factor? Hepatology 56, 2426 (2012).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA 103, 3920–3925 (2006).
Begley, M., Gahan, C. G. M. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
Úbeda, M. et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 64, 1049–1057 (2016).
Bernstein, H., Bernstein, C., Payne, C. M. & Dvorak, K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 15, 3329–3340 (2009).
Buffie, C. G. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015).
Massafra, V. et al. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim. Biophys. Acta 1862, 166–173 (2016).
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Ceulemans, L. J. et al. Farnesoid X receptor activation attenuates intestinal ischemia reperfusion injury in rats. PLoS ONE 12, e0169331 (2017).
Verbeke, L. et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am. J. Pathol. 185, 409–419 (2015).
Nijmeijer, R. M. et al. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory Bowel disease. PLoS ONE 6, e23745 (2011).
Cipriani, S. et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE 6, e25637 (2011).
Ichikawa, R. et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology 136, 153–162 (2012).
Devlin, A. S. & Fischbach, M. A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 11, 685–690 (2015).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Chang, D. et al. The conserved non-coding sequences CNS6 and CNS9 control cytokine-induced rorc transcription during T helper 17 cell differentiation. Immunity 53, 614–626.e4 (2020).
Yang, B.-H. et al. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 9, 444–457 (2016).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Paik, D. et al. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 603, 907–912 (2022).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Sinha, S. R. et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 27, 659–670.e5 (2020).
Das, P., Marcišauskas, S., Ji, B. & Nielsen, J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics 20, 517 (2019).
Hsu, C. L. & Schnabl, B. The gut–liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733 (2023).
Yan, M. et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Signal Transduct. Target. Ther. 8, 1–26 (2023).
Fukunishi, S. et al. Lipopolysaccharides accelerate hepatic steatosis in the development of nonalcoholic fatty liver disease in Zucker rats. J. Clin. Biochem. Nutr. 54, 39–44 (2014).
Alonso-Peña, M. et al. Impact of liver inflammation on bile acid side chain shortening and amidation. Cells 11, 3983 (2022).
Venkatesh, M. et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 41, 296–310 (2014).
Little, M. et al. Understanding the physiological functions of the host xenobiotic-sensing nuclear receptors PXR and CAR on the gut microbiome using genetically modified mice. Acta Pharm. Sin. B. 12, 801–820 (2022).
Phelan, J. P. et al. Rethinking the bile acid/gut microbiome axis in cancer. Oncotarget 8, 115736–115747 (2017).
Yasuda, H. et al. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem. Biophys. Res. Commun. 354, 154–159 (2007).
Nagathihalli, N. S. et al. Novel mechanistic insights into ectodomain shedding of EGFR Ligands Amphiregulin and TGF-α: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 74, 2062–2072 (2014).
Wu, J., Gong, J., Geng, J. & Song, Y. Deoxycholic acid induces the overexpression of intestinal mucin, MUC2, via NF-kB signaling pathway in human esophageal adenocarcinoma cells. BMC Cancer 8, 333 (2008).
Ward, J. B. J. et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G550–G558 (2017).
Ni, Z. et al. TGR5-HNF4α axis contributes to bile acid-induced gastric intestinal metaplasia markers expression. Cell Death Discov. 6, 56 (2020).
Xu, Z. et al. FXR ligands protect against hepatocellular inflammation via SOCS3 induction. Cell. Signal. 24, 1658–1664 (2012).
Anwer, M. S. Intracellular signaling by bile acids. J. Bio Sci. 20, 1–23 (2012).
Bernstein, H. et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589, 47–65 (2005).
Jia, W., Xie, G. & Jia, W. Bile acid–microbiota cross-talk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15, 111–128 (2018).
Yao, Z. et al. Ursodeoxycholic acid inhibits glioblastoma progression via endoplasmic reticulum stress related apoptosis and synergizes with the proteasome inhibitor bortezomib. ACS Chem. Neurosci. 11, 1337–1346 (2020).
Lee, S. et al. Synergistic effect of ursodeoxycholic acid on the antitumor activity of sorafenib in hepatocellular carcinoma cells via modulation of STAT3 and ERK. Int. J. Mol. Med. 42, 2551–2559 (2018).
Liu, H. et al. Mechanism of apoptotic effects induced selectively by ursodeoxycholic acid on human hepatoma cell lines. World J. Gastroenterol. 13, 1652–1658 (2007).
Lim, S.-C., Choi, J. E., Kang, H. S. & Han, S. I. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int. J. Cancer 126, 1582–1595 (2010).
Wu, Y.-C., Chiu, C.-F., Hsueh, C.-T. & Hsueh, C.-T. The role of bile acids in cellular invasiveness of gastric cancer. Cancer Cell Int. 18, 75 (2018).
Lim, S.-C. et al. Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis 32, 723–731 (2011).
Im, E. & Martinez, J. D. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J. Nutr. 134, 483–486 (2004).
Im, E., Akare, S., Powell, A. & Martinez, J. D. Ursodeoxycholic acid can suppress deoxycholic acid-induced apoptosis by stimulating Akt/PKB-dependent survival signaling. Nutr. Cancer 51, 110–116 (2005).
Saeki, T. et al. Ursodeoxycholic acid protects colon cancer HCT116 cells from deoxycholic acid-induced apoptosis by inhibiting apoptosome formation. Nutr. Cancer 64, 617–626 (2012).
Alpini, G. et al. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G973–G982 (2004).
Mikó, E. et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim. Biophys. Acta Bioenerg. 1859, 958–974 (2018).
Goldberg, A. A., Titorenko, V. I., Beach, A. & Sanderson, J. T. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 1, e122 (2013).
Jin, Q. et al. Bile acids upregulate BRCA1 and downregulate estrogen receptor 1 gene expression in ovarian cancer cells. Eur. J. Cancer Prev. 27, 553–556 (2018).
Song, W. et al. Apoptosis of human gastric carcinoma SGC-7901 induced by deoxycholic acid via the mitochondrial-dependent pathway. Appl. Biochem. Biotechnol. 171, 1061–1071 (2013).
Sun, J. et al. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J. Steroid Biochem. Mol. Biol. 111, 37–40 (2008).
Vogel, S. M. et al. Lithocholic acid is an endogenous inhibitor of MDM4 and MDM2. Proc. Natl Acad. Sci. USA 109, 16906–16910 (2012).
Luu, T. H. et al. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell. Oncol. Dordr. 41, 13–24 (2018).
Fukase, K. et al. Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci. 99, 1785–1792 (2008).
Yoon, J.-H. et al. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology 122, 985–993 (2002).
Debruyne, P. R. et al. Bile acids stimulate invasion and haptotaxis in human colorectal cancer cells through activation of multiple oncogenic signaling pathways. Oncogene 21, 6740–6750 (2002).
Yu, J.-H. et al. Bile acids promote gastric intestinal metaplasia by upregulating CDX2 and MUC2 expression via the FXR/NF-κB signalling pathway. Int. J. Oncol. 54, 879–892 (2019).
Carino, A. et al. The bile acid receptor GPBAR1 (TGR5) is expressed in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines. Oncotarget 7, 61021–61035 (2016).
Jang, E. S. et al. Sodium taurocholate cotransporting polypeptide mediates dual actions of deoxycholic acid in human hepatocellular carcinoma cells: enhanced apoptosis versus growth stimulation. J. Cancer Res. Clin. Oncol. 140, 133–144 (2014).
Yen, C.-J. et al. Bile acid exposure up-regulates tuberous sclerosis complex 1/mammalian target of rapamycin pathway in Barrett’s-associated esophageal adenocarcinoma. Cancer Res. 68, 2632–2640 (2008).
Prichard, D. O. et al. Deoxycholic acid promotes development of gastroesophageal reflux disease and Barrett’s oesophagus by modulating integrin-αv trafficking. J. Cell. Mol. Med. 21, 3612–3625 (2017).
Joshi, S. et al. Bile acids-mediated overexpression of MUC4 via FAK-dependent c-Jun activation in pancreatic cancer. Mol. Oncol. 10, 1063–1077 (2016).
Liu, X. et al. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 412, 194–207 (2018).
Krishnamurthy, K., Wang, G., Rokhfeld, D. & Bieberich, E. Deoxycholate promotes survival of breast cancer cells by reducing the level of pro-apoptotic ceramide. Breast Cancer Res. BCR 10, R106 (2008).
Chen, Z. et al. The role of fibroblast growth factor 19 in hepatocellular carcinoma. Am. J. Pathol. 191, 1180–1192 (2021).
Liu, Y. et al. Dissecting the role of the FGF19-FGFR4 signaling pathway in cancer development and progression. Front. Cell Dev. Biol. 8, 95 (2020).
Zou, Y. et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat. Commun. 13, 2672 (2022).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 7, 1–23 (2022).
Wang, Y. et al. Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases. Signal Transduct. Target. Ther. 8, 1–45 (2023).
Lewerenz, J. et al. The cystine/glutamate antiporter system xc− in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 18, 522–555 (2013).
Iseda, N. et al. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor‐4 inhibition in hepatocellular carcinoma. Cancer Sci. 113, 2272–2287 (2022).
Qi, Y. et al. The bile acid membrane receptor TGR5 in cancer: friend or foe? Molecules 27, 5292 (2022).
Xiong, Q. et al. Metabolite-sensing G protein coupled receptor TGR5 protects host from viral infection through amplifying type I interferon responses. Front. Immunol. 9, 2289 (2018).
Hu, M.-M. et al. Virus-induced accumulation of intracellular bile acids activates the TGR5-β-arrestin-SRC axis to enable innate antiviral immunity. Cell Res. 29, 193–205 (2019).
Gulen, M. F. et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374–380 (2023).
Ou, L., Zhang, A., Cheng, Y. & Chen, Y. The cGAS-STING pathway: a promising immunotherapy target. Front. Immunol. 12, 795048 (2021).
Zhang, Y. et al. Obeticholic acid, a selective farnesoid X receptor agonist, regulates bile acid homeostasis in sandwich-cultured human hepatocytes. Pharmacol. Res. Perspect. 5, e00329 (2017).
Zhang, Y. et al. Comparative potency of obeticholic acid and natural bile acids on FXR in hepatic and intestinal in vitro cell models. Pharmacol. Res. Perspect. 5, e00368 (2017).
Ratziu, Y. et al. EDP-305 in patients with NASH: a phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 76, 506–517 (2022).
Pellicciari, R. et al. Discovery of 3α,7α,11β-trihydroxy-6α-ethyl-5β-cholan-24-oic acid (TC-100), a novel bile acid as potent and highly selective FXR agonist for enterohepatic disorders. J. Med. Chem. 59, 9201–9214 (2016).
Soisson, S. M. et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc. Natl Acad. Sci. USA 105, 5337–5342 (2008).
Zhang, C. et al. Discovery of betulinic acid derivatives as potent intestinal farnesoid X receptor antagonists to ameliorate nonalcoholic steatohepatitis. J. Med. Chem. 65, 13452–13472 (2022).
Panzitt, K., Zollner, G., Marschall, H.-U. & Wagner, M. Recent advances on FXR-targeting therapeutics. Mol. Cell. Endocrinol. 552, 111678 (2022).
Alemi, F. et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Invest. 123, 1513–1530 (2013).
Gege, C. et al. Knocking on FXR’s Door: The. Curr. Top. Med. Chem. 14, 2143–2158 (2014).
Dwivedi, S. K. D. et al. Bile acid receptor agonist GW4064 regulates PPARγ COactivator-1α Expression through Estrogen Receptor-related Receptor α. Mol. Endocrinol. 25, 922–932 (2011).
Gege, C. et al. In Bile Acids and Their Receptors (eds. Fiorucci, S. & Distrutti, E.) 167–205 (Springer International Publishing, 2019).
Tully, D. C. et al. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J. Med. Chem. 60, 9960–9973 (2017).
Patel, K. et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 72, 58–71 (2020).
Jiang, L. et al. Structural insight into the molecular mechanism of cilofexor binding to the farnesoid X receptor. Biochem. Biophys. Res. Commun. 595, 1–6 (2022).
Kremoser, C. FXR agonists for NASH: how are they different and what difference do they make? J. Hepatol. 75, 12–15 (2021).
Trauner, M. et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS‐9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 70, 788 (2019).
Al-Khaifi, A., Rudling, M. & Angelin, B. An FXR agonist reduces bile acid synthesis independently of increases in FGF19 in healthy volunteers. Gastroenterology 155, 1012–1016 (2018).
Traussnigg, S. et al. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien. Klin. Wochenschr. 133, 441–451 (2021).
Chianelli, D. et al. Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 63, 3868–3880 (2020).
Wang, Y. et al. Safety, pharmacokinetics, pharmacodynamics, and formulation of liver‐distributed farnesoid X‐receptor agonist TERN‐101 in healthy volunteers. Clin Pharmacol Drug Dev 10, 1198–1208 (2021).
Genin, M. J. et al. Discovery of 6-(4-{[5-Cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl]methoxy}piperidin-1-yl)-1-methyl-1H-indole-3-carboxylic acid: a novel FXR agonist for the treatment of dyslipidemia. J. Med. Chem. 58, 9768–9772 (2015).
Carpenter, J. et al. Discovery of BMS-986318, a potent nonbile acid FXR agonist for the treatment of nonalcoholic steatohepatitis. ACS Med. Chem. Lett. 12, 1413–1420 (2021).
Nara, S. J. et al. Discovery of BMS-986339, a pharmacologically differentiated farnesoid X receptor agonist for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 65, 8948–8960 (2022).
Mo, C. et al. Discovery of HPG1860, a structurally novel nonbile acid FXR agonist currently in clinical development for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 66, 9363–9375 (2023).
Flatt, B. et al. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J. Med. Chem. 52, 904–907 (2009).
Evans, M. J. et al. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G543–G552 (2009).
Downes, M. et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol. Cell. 11, 1079–1092 (2003).
Fang, S. et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 21, 159–165 (2015).
Harrison, S. A. et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J. Hepatol. 75, 25–33 (2021).
Wang, H. et al. A novel intestinal-restricted FXR agonist. Bioorg. Med. Chem. Lett. 27, 3386–3390 (2017).
Huang, W. et al. Discovery of LH10, a novel fexaramine-based FXR agonist for the treatment of liver disease. Bioorg. Chem. 143, 107071 (2024).
Chen, C. et al. Discovery of 4-aminophenylacetamide derivatives as intestine-specific farnesoid X receptor antagonists for the potential treatment of nonalcoholic steatohepatitis. Eur. J. Med. Chem. 264, 115992 (2024).
Xi, L. et al. Licraside as novel potent FXR agonist for relieving cholestasis: structure-based drug discovery and biological evaluation studies. Front. Pharmacol. 14, 1197856 (2023).
Yao, Z. et al. The discovery of a new potent FXR agonist based on natural product screening. Bioorg. Chem. 143, 106979 (2024).
Liu, Y. et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 112, 1678–1687 (2003).
Jin, D. et al. Farnesoid X receptor activation protects liver from ischemia/reperfusion injury by up-regulating small heterodimer partner in Kupffer cells. Hepatol. Commun. 4, 540–554 (2020).
Zhang, S., Wang, J., Liu, Q. & Harnish, D. C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51, 380–388 (2009).
Liu, X. et al. Activation of farnesoid X receptor (FXR) protects against fructose-induced liver steatosis via inflammatory inhibition and ADRP reduction. Biochem. Biophys. Res. Commun. 450, 117–123 (2014).
Hartman, H. B. et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 50, 1090–1100 (2009).
Hernandez, E. D. et al. Tropifexor‐mediated abrogation of steatohepatitis and fibrosis is associated with the antioxidative gene expression profile in rodents. Hepatol. Commun. 3, 1085–1097 (2019).
Schwabl, P. et al. The non-steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines 9, 60 (2021).
Papazyan, R. et al. FXR activation by obeticholic acid or nonsteroidal agonists induces a human-like lipoprotein cholesterol change in mice with humanized chimeric liver. J. Lipid Res. 59, 982–993 (2018).
Miyata, S. et al. Discovery, optimization, and evaluation of non-bile acid FXR/TGR5 dual agonists. Sci. Rep. 11, 9196 (2021).
Nemetchek, M. D. et al. A structural mechanism of nuclear receptor biased agonism. Proc. Natl Acad. Sci. USA 119, e2215333119 (2022).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G‐protein bile acid receptor‐1 signaling to improve metabolism. Hepatology 68, 1574 (2018).
Zhou, W., Akinrotimi, O., Dadlani, N. & Anakk, S. FXR regulates adipose tissue remodeling during obesity. FASEB J. 35, S1 (2021).
Li, H. et al. Ursodeoxycholic acid treatment restores gut microbiota and alleviates liver inflammation in non-alcoholic steatohepatitic mouse model. Front. Pharmacol. 12, 788558 (2021).
Ahmad, Z. et al. Cholic acid for hepatic steatosis in patients with lipodystrophy: a randomized, controlled trial. Eur. J. Endocrinol. 168, 771–778 (2013).
Rao, A. S. et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 139, 1549–1558 (2010).
Enanta Pharmaceuticals, Inc. A Phase 2 Dose Ranging, Randomized, Double Blind, Placebo-Controlled Study Evaluating the Safety, Tolerability, Pharmacokinetics and Efficacy of EDP-305 in Subjects With Primary Biliary Cholangitis (PBC) With or Without an Inadequate Response to Ursodeoxycholic Acid (UDCA) (2021).
US National Library of Medicine. ClinicalTrials.gov (NCT01998659) (2014).
US National Library of Medicine. ClinicalTrials.gov (NCT00499629) https://clinicaltrials.gov/study/NCT00499629 (2008).
US National Library of Medicine. ClinicalTrials.gov (NCT00509756) https://clinicaltrials.gov/study/NCT00509756 (2010) .
US National Library of Medicine. ClinicalTrials.gov (NCT03890120) (2023).
Sanyal, A. J. et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat. Med. 29, 392–400 (2023).
Schramm, C. et al. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomised trial in patients with primary biliary cholangitis. JHEP Rep. Innov. Hepatol. 4, 100544 (2022).
Hepagene (Shanghai) Co., Ltd. A Randomized, Double-Blind, Placebo Controlled, Single and Multiple Ascending Doses (SAD/MAD) Study Following Oral Administration in Healthy Subjects to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of HPG1860 (2022).
Hepagene (Shanghai) Co., Ltd. A Randomized, Double-blind, Placebo-controlled Parallel Group Phase 2a Study to Evaluate the Efficacy and Safety of HPG1860 in Subjects With Nonalcoholic Steatohepatitis (2022).
Terns, Inc. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 2a Clinical Trial to Evaluate the Safety, Tolerability, Efficacy, and Pharmacokinetics of Orally Administered TERN-101 Tablets in Adult Patients With Presumed Non-Cirrhotic Non-Alcoholic Steatohepatitis (NASH) (2022).
Terns, Inc. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 2a Clinical Study to Evaluate the Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Orally Administered TERN-501 as Monotherapy as Well as in Combination With TERN-101 in Noncirrhotic Adults With Presumed Non-Alcoholic Steatohepatitis (NASH) (2023).
Metacrine, Inc. A Phase 2A Study to Evaluate MET409 Alone or in Combination With Empagliflozin in Patients With Type 2 Diabetes Mellitus (T2DM) and Nonalcoholic Steatohepatitis (NASH) (2021).
Calderon, G. et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine 55, 102759 (2020).
Sinha, S. R. The Role of Secondary Bile Acids in Intestinal Inflammation (2023).
First Affiliated Hospital Xi’an Jiaotong University. Effect of UDCA on the New Onset Diabetes and Glucose Intolerance Induced by Statin-A Multicenter, Prospective, Random Controlled Trial (2022).
Mueller, M. et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 62, 1398–1404 (2015).
Nevens, F. et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375, 631–643 (2016).
Kowdley, K. V. et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 73, 94–101 (2020).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1 (2013).
US National Library of Medicine. ClinicalTrials.gov (NCT00501592) (2012).
Siddiqui, M. S. et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J. Hepatol. 72, 25–33 (2020).
Pockros, P. J. et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 39, 2082–2093 (2019).
Sanyal, A. J. et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J. Hepatol. 79, 1110–1120 (2023).
Xu, J. et al. IL‐31 levels correlate with pruritus in patients with cholestatic and metabolic liver diseases and is farnesoid X receptor responsive in NASH. Hepatology 77, 20 (2023).
Intercept Pharmaceuticals, Inc. 2023 Q2 - Results - Earnings Call Presentation (NASDAQ:ICPT) | Seeking Alpha (2023).
Di Pasqua, L. G. et al. FXR agonists INT-787 and OCA increase RECK and inhibit liver steatosis and inflammation in diet-induced ob/ob mouse model of NASH. Liver Int. 44, 214–227 (2024).
Intercept Pharmaceuticals. Intercept Announces Development Program for Next-Generation FXR Agonist INT-787 in Severe Alcohol-Associated Hepatitis. https://www.interceptpharma.com/about-us/news/?id=2550372(2022).
Intercept Pharmaceuticals. A Phase 2a, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Dose-escalation, Proof-of-Concept Study Evaluating the Safety, Tolerability, Efficacy and Pharmacokinetics of INT-787 in Subjects With Severe Alcohol Associated Hepatitis (2024).
Burris, T. P. et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 65, 710–778 (2013).
Thomas, A. M. et al. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 51, 1410–1419 (2010).
Massafra, V., Pellicciari, R., Gioiello, A. & van Mil, S. W. C. Progress and challenges of selective farnesoid X receptor modulation. Pharmacol. Ther. 191, 162–177 (2018).
Pellicciari, R. et al. Back door modulation of the farnesoid X receptor: design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J. Med. Chem. 49, 4208–4215 (2006).
Meijer, F. A., Leijten-van de Gevel, I. A., de Vries, R. M. J. M. & Brunsveld, L. Allosteric small molecule modulators of nuclear receptors. Mol. Cell. Endocrinol. 485, 20–34 (2019).
Kim, D. et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 34, 184–199 (2015).
Renga, B. et al. Discovery that theonellasterol a marine sponge sterol is a highly selective FXR antagonist that protects against liver injury in cholestasis. PLoS ONE 7, e30443 (2012).
Urizar, N. L. et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296, 1703–1706 (2002).
Wu, J. et al. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol. Endocrinol. 16, 1590–1597 (2002).
Cui, J. et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 278, 10214–10220 (2003).
Szapary, P. O. et al. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA 290, 765–772 (2003).
Pellicciari, R. et al. Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52, 7958–7961 (2009).
Renga, B. et al. Reversal of endothelial dysfunction by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1 dependent regulation of H2S generation and endothelin-1. PLoS ONE 10, e0141082 (2015).
Yang, F. et al. Structural basis of GPBAR activation and bile acid recognition. Nature 587, 499–504 (2020).
Li, Y. et al. Discovery and biological evaluation of cholic acid derivatives as potent TGR5 positive allosteric modulators. Bioorg. Med. Chem. 92, 117418 (2023).
Nakhi, A. et al. 7-Methylation of chenodeoxycholic acid derivatives yields a substantial increase in TGR5 receptor potency. J. Med. Chem. 62, 6824–6830 (2019).
Sato, H. et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 362, 793–798 (2007).
Genet, C. et al. Structure−activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J. Med. Chem. 53, 178–190 (2010).
Budzik, B. W. et al. Synthesis and structure–activity relationships of a series of 3-aryl-4-isoxazolecarboxamides as a new class of TGR5 agonists. Bioorg. Med. Chem. Lett. 20, 1363–1367 (2010).
Evans, K. A. et al. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J. Med. Chem. 52, 7962–7965 (2009).
Hodge, R. J. et al. Safety, pharmacokinetics, and pharmacodynamic effects of a selective TGR5 agonist, SB-756050, in betes. Clin. Pharmacol. Drug Dev. 2, 213–222 (2013).
Herbert, M. R. et al. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg. Med. Chem. Lett. 20, 5718–5721 (2010).
Futatsugi, K. et al. Optimization of triazole-based TGR5 agonists towards orally available agents. MedChemComm 4, 205–210 (2012).
Piotrowski, D. W. et al. Identification of tetrahydropyrido[4,3-d]pyrimidine amides as a new class of orally bioavailable TGR5 agonists. ACS Med. Chem. Lett. 4, 63–68 (2012).
Agarwal, S. et al. Discovery of a potent and orally efficacious TGR5 receptor agonist. ACS Med. Chem. Lett. 7, 51–55 (2015).
Phillips, D. P. et al. Discovery of trifluoromethyl(pyrimidin-2-yl)azetidine-2-carboxamides as potent, orally bioavailable TGR5 (GPBAR1) agonists: structure–activity relationships, lead optimization, and chronic in vivo efficacy. J. Med. Chem. 57, 3263–3282 (2014).
Zambad, S. P. et al. TRC210258, a novel TGR5 agonist, reduces glycemic and dyslipidemic cardiovascular risk in animal models of diabesity. Diabetes Metab. Syndr. Obes. Targets Ther. 7, 1–14 (2013).
Zhao, S. et al. Design, synthesis and evaluation of 3-phenoxypyrazine-2-carboxamide derivatives as potent TGR5 agonists. RSC Adv. 12, 3618–3629 (2022).
Picon, S. et al. Discovery, structure–activity relationships, and in vivo activity of dihydropyridone agonists of the bile acid receptor TGR5. J. Med. Chem. 66, 11732–11760 (2023).
Duan, H. et al. Design, synthesis, and antidiabetic activity of 4-phenoxynicotinamide and 4-phenoxypyrimidine-5-carboxamide derivatives as potent and orally efficacious TGR5 agonists. J. Med. Chem. 55, 10475–10489 (2012).
Briere, D. A. et al. Novel small molecule agonist of TGR5 possesses anti-diabetic effects but causes gallbladder filling in mice. PLoS ONE 10, e0136873 (2015).
de Oliveira, M. C. et al. Bile acid receptor agonists INT747 and INT777 decrease oestrogen deficiency-related postmenopausal obesity and hepatic steatosis in mice. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1862, 2054–2062 (2016).
Carino, A. et al. Gpbar1 agonism promotes a Pgc-1α-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci. Rep. 7, 13689 (2017).
Miyazaki-Anzai, S. et al. Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation. J. Lipid Res. 59, 1709–1713 (2018).
Miyazaki-Anzai, S. et al. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE 9, e108270 (2014).
Li, T. et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol. 25, 1066–1071 (2011).
Zhuo, N. et al. Discovery of betulinic acid derivatives as gut-restricted TGR5 agonists: Balancing the potency and physicochemical properties. Bioorg. Chem. 144, 107132 (2024).
Brighton, C. A. et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology 156, 3961–3970 (2015).
Tough, I. R., Schwartz, T. W. & Cox, H. M. Synthetic G protein-coupled bile acid receptor agonists and bile acids act via basolateral receptors in ileal and colonic mucosa. Neurogastroenterol. Motil. 32, e13943 (2020).
Lasalle, M. et al. Topical intestinal aminoimidazole agonists of G-protein-coupled bile acid receptor 1 promote glucagon like peptide-1 secretion and improve glucose tolerance. J. Med. Chem. 60, 4185–4211 (2017).
Duan, H. et al. Discovery of intestinal targeted TGR5 agonists for the treatment of type 2 diabetes. J. Med. Chem. 58, 3315–3328 (2015).
Cao, H. et al. Intestinally-targeted TGR5 agonists equipped with quaternary ammonium have an improved hypoglycemic effect and reduced gallbladder filling effect. Sci. Rep. 6, 28676 (2016).
Chen, T. et al. Design of gut-restricted thiazolidine agonists of G protein-coupled bile acid receptor 1 (GPBAR1, TGR5). J. Med. Chem. 61, 7589–7613 (2018).
Hoguet, V. et al. Beyond the rule of 5: impact of PEGylation with various polymer sizes on pharmacokinetic properties, structure–properties relationships of mPEGylated small agonists of TGR5 receptor. J. Med. Chem. 64, 1593–1610 (2021).
Zou, Q. et al. 4-Benzofuranyloxynicotinamide derivatives are novel potent and orally available TGR5 agonists. Eur. J. Med. Chem. 82, 1–15 (2014).
Han, F. et al. Design of G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) soft drugs with reduced gallbladder-filling effects. Eur. J. Med. Chem. 203, 112619 (2020).
Kim, H. S. & Jung, C. H. Oral semaglutide, the first ingestible glucagon-like peptide-1 receptor agonist: could it be a magic bullet for type 2 diabetes? Int. J. Mol. Sci. 22, 9936 (2021).
Comeglio, P. et al. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J. Endocrinol. 238, 107–127 (2018).
Jadhav, K. et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 9, 131–140 (2018).
Hu, Y.-B., Liu, X.-Y. & Zhan, W. Farnesoid X receptor agonist INT-767 attenuates liver steatosis and inflammation in rat model of nonalcoholic steatohepatitis. Drug Des. Devel. Ther. 12, 2213–2221 (2018).
Gu, M. et al. Deoxyschizandrin ameliorates obesity and non-alcoholic fatty liver disease: Involvement of dual Farnesyl X receptor/G protein-coupled bile acid receptor 1 activation and leptin sensitization. Phytother. Res. 37, 2771–2786 (2023).
Sarvagalla, S., Kolapalli, S. P. & Vallabhapurapu, S. The two sides of YY1 in cancer: a friend and a foe. Front. Oncol. 9, 1230 (2019).
Vega, M. I., Jazirehi, A. R., Huerta-Yepez, S. & Bonavida, B. Rituximab-induced inhibition of YY1 and Bcl-xL expression in ramos non-Hodgkin’s lymphoma cell line via inhibition of NF-κB activity: role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectively. J. Immunol. 175, 2174–2183 (2005).
Garbán, H. J. & Bonavida, B. Nitric oxide inhibits the transcription repressor yin-yang 1 binding activity at the silencer region of the fas promoter: a pivotal role for nitric oxide in the up-regulation of fas gene expression in human tumor cells. J. Immunol. 167, 75–81 (2001).
Bonavida, B. & Baritaki, S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide 24, 1–7 (2011).
Zhang, N. et al. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene 32, 5078–5088 (2013).
Huang, T. et al. MiR-186 inhibits proliferation, migration, and invasion of non-small cell lung cancer cells by downregulating Yin Yang 1. Cancer Biomark. 21, 221–228 (2018).
Zhang, Y. et al. miR‑29a suppresses IL‑13‑induced cell invasion by inhibiting YY1 in the AKT pathway in lung adenocarcinoma A549 cells. Oncol. Rep. 39, 2613–2623 (2018).
Zhou, W.-Y. et al. MicroRNA-181 targets Yin Yang 1 expression and inhibits cervical cancer progression. Mol. Med. Rep. 11, 4541–4546 (2015).
Gao, Y. et al. miR‑218 inhibits the proliferation of human glioma cells through downregulation of Yin Yang 1. Mol. Med. Rep. 17, 1926–1932 (2018).
Huang, W. et al. Design, synthesis, and biological studies of dual URAT1 inhibitor and FXR agonist based on benzbromarone. Bioorg. Med. Chem. 75, 117073 (2022).
Ren, Q. et al. Discovery of the first-in-class intestinal restricted FXR and FABP1 dual modulator ZLY28 for the treatment of nonalcoholic fatty liver disease. J. Med. Chem. 66, 6082–6104 (2023).
Fiorucci, S. et al. Discovery of a potent and orally active dual GPBAR1/CysLT1R modulator for the treatment of metabolic fatty liver disease. Front. Pharmacol. 13, 858137 (2022).
Fiorillo, B. et al. Discovery of a novel class of dual GPBAR1 agonists–RORγt inverse agonists for the treatment of IL-17-mediated disorders. ACS Omega 8, 5983–5994 (2023).
Shiragannavar, V. D. et al. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 14, 1135952 (2023).
Festa, C. et al. Investigation around the oxadiazole core in the discovery of a new chemotype of potent and selective FXR antagonists. ACS Med. Chem. Lett. 10, 504–510 (2019).
Finamore, C. et al. Expanding the library of 1,2,4-oxadiazole derivatives: discovery of new farnesoid X receptor (FXR) antagonists/pregnane X receptor (PXR) agonists. Molecules 28, 2840 (2023).
Yamashita, Y. et al. Discovery of FXR/PPARγ dual partial agonist. Bioorg. Med. Chem. 85, 117238 (2023).
Lebovitz, H. E. Thiazolidinediones: the forgotten diabetes medications. Curr. Diab. Rep. 19, 151 (2019).
Liu, J., Carlson, H. A. & Scott, E. E. The structure and characterization of human cytochrome P450 8B1 supports future drug design for nonalcoholic fatty liver disease and diabetes. J. Biol. Chem. 298, 102344 (2022).
DePaoli, A. M. et al. FGF19 analog as a surgical factor mimetic that contributes to metabolic effects beyond glucose homeostasis. Diabetes 68, 1315–1328 (2019).
Harrison, S. A. et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 71, 1198–1212 (2020).
Harrison, S. A. et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 391, 1174–1185 (2018).
Liu, Y. et al. Novel regulatory factors and small-molecule inhibitors of FGFR4 in cancer. Front. Pharmacol. 12, 633453 (2021).
Lang, L. & Teng, Y. Fibroblast growth factor receptor 4 targeting in cancer: new insights into mechanisms and therapeutic strategies. Cells 8, 31 (2019).
Chen, X. et al. Insight into the design of FGFR4 selective inhibitors in cancer therapy: prospects and challenges. Eur. J. Med. Chem. 263, 115947 (2024).
Zhao, H.-Y. et al. Discovery of potent PROTACs targeting EGFR mutants through the optimization of covalent EGFR ligands. J. Med. Chem. 65, 4709–4726 (2022).
Ma, L. et al. Discovery of a selective and orally bioavailable FGFR2 degrader for treating gastric cancer. J. Med. Chem. 66, 7438–7453 (2023).
Harach, T. et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci. Rep. 2, 430 (2012).
Shang, Q. et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G419–G424 (2010).
Nwose, O. M. & Jones, M. R. Atypical mechanism of glucose modulation by colesevelam in patients with type 2 diabetes. Clin. Med. Insights Endocrinol. Diabetes 6, 75–79 (2013).
Brufau, G. et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 52, 1455–1464 (2010).
Hansen, M. et al. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: a systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complications. 31, 918–927 (2017).
Potthoff, M. J. et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G371–G380 (2013).
West, K. L. et al. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis 171, 201–210 (2003).
Miethke, A. G. et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 63, 512–523 (2016).
West, K. L. et al. 1-[4-[4[(4R,5R)-3,3-Dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl]phenoxy]butyl]-4-aza-1-azoniabicyclo[2.2.2]octane methanesulfonate (SC-435), an ileal apical sodium-codependent bile acid transporter inhibitor alters hepatic cholesterol metabolism and lowers plasma low-density lipoprotein-cholesterol concentrations in guinea pigs. J. Pharmacol. Exp. Ther. 303, 293–299 (2002).
Al-Dury, S. et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci. Rep. 8, 6658 (2018).
Baghdasaryan, A. et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J. Hepatol. 64, 674–681 (2016).
Mirum Pharmaceuticals, Inc. MRX-502: Randomized Double-blind Placebo-controlled Phase 3 Study to Evaluate the Efficacy and Safety of Maralixibat in the Treatment of Subjects With Progressive Familial Intrahepatic Cholestasis (PFIC) - MARCH-PFIC (2023).
Mirum Pharmaceuticals, Inc. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients With Primary Biliary Cholangitis (2024).
Mirum Pharmaceuticals, Inc. A Randomized Double-Blind Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients With Primary Sclerosing Cholangitis (2024).
Li, L. et al. Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease. Front. Immunol. 13, 974305 (2022).
Devlin, S. Bacterial modification of bile acids alters host physiology. FASEB J. 33, 93.2–93.2 (2019).
de Bruijn, V. et al. Antibiotic-induced changes in microbiome-related metabolites and bile acids in rat plasma. Metabolites 10, 242 (2020).
Sun, L. et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 24, 1919–1929 (2018).
Degirolamo, C. et al. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 7, 12–18 (2014).
Mencarelli, A. et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS ONE 7, e45425 (2012).
Harrison, S. A. et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 29, 2919–2928 (2023).
Sari, G. et al. A mouse model of humanized liver shows a human-like lipid profile, but does not form atherosclerotic plaque after western type diet. Biochem. Biophys. Res. Commun. 524, 510–515 (2020).
Guo, G. L. & Chiang, J. Y. L. Is CYP2C70 the key to new mouse models to understand bile acids in humans? J. Lipid Res. 61, 269–271 (2020).
Hasan, M. N. et al. Glycine-β-muricholic acid improves liver fibrosis and gut barrier function by reducing bile acid pool size and hydrophobicity in male Cyp2c70 knockout mice. Cells 12, 1371 (2023).
Takahashi, S. et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 57, 2130–2137 (2016).
Gardès, C. et al. Differential regulation of bile acid and cholesterol metabolism by the farnesoid X receptor in Ldlr−/− mice versus hamsters [S]. J. Lipid Res. 54, 1283–1299 (2013).
Song, X. et al. Mechanistic insights into isoform-dependent and species-specific regulation of bile salt export pump by farnesoid X receptor. J. Lipid Res. 54, 3030–3044 (2013).
Honda, A. et al. Increased bile acid concentration in liver tissue with cholesterol gallstone disease. J. Gastroenterol. 30, 61–66 (1995).
Gómez, C. et al. Development and validation of a highly sensitive LC-MS/MS method for the analysis of bile acids in serum, plasma, and liver tissue samples. Metabolites 10, 282 (2020).
James, S. C. et al. Concentrations of fecal bile acids in participants with functional gut disorders and healthy controls. Metabolites 11, 612 (2021).
Jäntti, S. E. et al. Quantitative profiling of bile acids in blood, adipose tissue, intestine, and gall bladder samples using ultra high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 406, 7799–7815 (2014).
Straniero, S. et al. Of mice and men: murine bile acids explain species differences in the regulation of bile acid and cholesterol metabolism. J. Lipid Res. 61, 480–491 (2020).
Zheng, W. et al. Structural insights into the heterodimeric complex of the nuclear receptors FXR and RXR. J. Biol. Chem. 293, 12535–12541 (2018).
Acknowledgements
The authors would like to thank St. John’s University for its continuous support in addition to thanking BioRender.com for figure creation. This work is supported by a seed grant from St. John’s University College of Pharmacy & Health Sciences.
Author information
Authors and Affiliations
Contributions
Joshua S. Fleishman and Sunil Kumar confirm being the sole contributors of this manuscript, have read and approved it for publication. J.S.F. and S.K. conceived and organized the original outline for the manuscript. J.S.F. solely collected the references, researched, wrote, and created figures for the manuscript. J.S.F. and S.K. jointly edited and reviewed the manuscript. S.K. advised manuscript production and provided feedback for further development. All authors have read and approved the review article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This is a review article; data is not subject to ethical approval.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fleishman, J.S., Kumar, S. Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Sig Transduct Target Ther 9, 97 (2024). https://doi.org/10.1038/s41392-024-01811-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01811-6