Abstract
Autoimmune diseases are a diverse group of conditions characterized by aberrant B cell and T cell reactivity to normal constituents of the host. These diseases occur widely and affect individuals of all ages, especially women. Among these diseases, the most prominent immunological manifestation is the production of autoantibodies, which provide valuable biomarkers for diagnosis, classification and disease activity. Although T cells have a key role in pathogenesis, they are technically more difficult to assay. In general, autoimmune disease results from an interplay between a genetic predisposition and environmental factors. Genetic predisposition to autoimmunity is complex and can involve multiple genes that regulate the function of immune cell populations. Less frequently, autoimmunity can result from single-gene mutations that affect key regulatory pathways. Infection seems to be a common trigger for autoimmune disease, although the microbiota can also influence pathogenesis. As shown in seminal studies, patients may express autoantibodies many years before the appearance of clinical or laboratory signs of disease — a period called pre-clinical autoimmunity. Monitoring autoantibody expression in at-risk populations may therefore enable early detection and the initiation of therapy to prevent or attenuate tissue damage. Autoimmunity may not be static, however, and remission can be achieved by some patients treated with current agents.
Key points
-
Autoimmune diseases lead to diverse patterns of inflammation and organ dysfunction.
-
Autoantibodies are valuable markers for diagnosis, classification and of disease activity.
-
Although T cells play a key part in disease, their assessment is challenging.
-
Autoimmune disease reflects the interplay of genetic and environmental factors.
-
Pre-clinical autoimmune disease provides a window of time for early or preventive treatment.
Similar content being viewed by others
Introduction
Autoimmune diseases are a diverse group of conditions characterized by immune disturbances that cause aberrant B cell and T cell reactivity to normal constituents of the host. These diseases can involve essentially any organ system and affect individuals of any age, with a much greater prevalence among women. Although certain mechanisms unite these conditions into a single category, the clinical manifestations of autoimmune disease are highly varied. These manifestations range from acute, life-threatening organ failure to subtle laboratory abnormalities that can easily escape notice. Clinically, autoimmune diseases can be restricted in the pattern of organ involvement (organ-specific) or be generalized (systemic or non-organ-specific)1.
The diversity of autoimmune disease is striking and challenges the entire medical enterprise. For the clinician, the challenge is to reach a diagnosis for a patient who presents with concerning signs and symptoms that could arise from a variety of aetiologies, each of which may require a distinct and sometimes divergent approach to management. For the investigator, the challenge is to explicate the role of autoreactivity in a clinical syndrome and determine whether autoreactivity is crucial or merely incidental. For the healthcare system, the challenge is to develop cost-effective strategies for early diagnosis and treatment and, optimally, prevention.
This Review provides a conceptual framework for autoimmune disease, with an emphasis on autoantibodies given that these are the mainstay of diagnosis and classification and provide valuable biomarkers with which to explore the mechanisms that underlie autoimmune disease and the consequences for the patient.
Criteria for autoimmune disease
Since autoimmune diseases are clinically so heterogeneous, a concerted search for aberrant B and T cell autoreactivity is necessary to identify those conditions in which autoimmune mechanisms drive pathogenesis and determine outcome. A set of clinical and laboratory criteria provides a framework to guide these investigations.
Clinical and laboratory findings
Autoimmunity can occur in the absence of signs and symptoms of disease; only some individuals who display aberrant B and/or T cell autoreactivity will develop clinical manifestations of any kind. Elucidation of autoimmune disease therefore requires an understanding not only of the processes underlying the development of autoimmunity (that is, defined by the presence of B and/or T cell autoreactivity) but also of the transition to actual disease with characteristic clinical findings2. Although the terms ‘autoimmunity’ and ‘autoimmune disease’ are often used interchangeably, the meanings are distinct — an important issue in both research and clinical care.
As indicated by the diversity of diseases that are categorized as autoimmune, no simple or uniform set of signs and symptoms signifies an autoimmune aetiology; correspondingly, an autoimmune disease may present with manifestations that can result from many different pathological processes. In the realm of nephrology, glomerulonephritides arise from diverse processes including autoimmunity and infection3. The clinical manifestations as well as findings on kidney biopsy can be similar regardless of aetiology. Determination of underlying autoimmunity therefore requires adjunctive laboratory testing, with measurement of autoantibodies the most common and informative modality currently available. Although T cells are critical in many autoimmune diseases, assays of T cell autoreactivity are challenging and not routinely performed, leading to the emphasis of autoantibodies in this setting.
Laboratory testing for autoimmune disease uses well validated assays to establish the presence of autoantibodies. Although many kidney diseases have long been thought to be autoimmune in origin, in some cases, the identification of the target of autoreactivity has happened only recently. In membranous nephropathy, autoantibodies to the M-type phospholipase A2 receptor 1 (PLA2R) were first described in 2009 (ref. 4). Antibodies to thrombospondin type 1 domain containing 7a (THSD7A) were described in 2014 and additional antigens were identified in subsequent years5. For some conditions, the identification of target antigens is just beginning, as illustrated by the demonstration of autoantibodies to nephrin in a subset of patients with minimal change disease6.
The determination that a disease is autoimmune involves criteria that are similar to Koch’s postulates for infectious diseases and are sometimes denoted as the Witebsky’s postulates7. Specifically, such determination requires the presence of autoreactive B cells or T cells in patient blood; induction of the disease by transfer of cells or antibodies to experimental animals; transplacental passage of the disease; demonstration of characteristic pathophysiology in an in vitro or animal model; and demonstration of autoreactivity (for example, the presence of immune complexes) at a site of damage. Not surprisingly, many diseases that are considered autoimmune do not fully meet these criteria.
In the absence of characteristic serological findings, classification of a disease in terms of its aetiology can be uncertain and lead to nonspecific terminology, such as ‘inflammatory disease’, ‘immune-mediated disease’ or ‘seronegative autoimmunity’. Once autoantibodies are found, the disease can be re-classified and the aetiology better established. The classification of a disease as autoimmune, however, does not mean that the autoantibodies mediate the clinical manifestations. Additional studies are needed to show that the autoantibody induces pathology. Furthermore, autoantibodies may be only one element in the pathophysiology because T cells, neutrophils and macrophages can also mediate inflammation and tissue damage.
Autoantibodies as markers
Despite the fact that autoantibodies are valuable biomarkers for diagnosis and classification, there is often no obvious link between autoantibody specificity and the resulting immunopathology; this situation is especially true when the autoantigen is an intracellular molecule that is widely expressed in cells. The association between autoantibodies and tissue pathology tends to be more obvious in the context of organ-specific autoimmune diseases than in systemic autoimmune diseases. The reason that certain autoantibodies track with clinical findings remains unknown despite the strength of these associations.
Within a diagnostic category, serological findings can be quite diverse. In systemic sclerosis, for example, antibodies to the protein Scl-70 (DNA topoisomerase) are associated with diffuse skin involvement whereas antibodies to centromere proteins are associated with more limited skin disease8. These autoantibodies react with ubiquitous nuclear antigens, making the relationship between the antibody specificity and the pattern of cutaneous fibrosis difficult to understand. Similarly, in inflammatory myopathy, antibodies to transfer RNA synthetase enzymes are associated with interstitial lung disease; interestingly, patients with this condition usually express antibodies to just one of these enzymes9.
For some conditions, the pathophysiology points to processes that might be affected by an autoantibody. In myasthaenia gravis, for example, antibodies to the acetylcholine receptor can bind at the neuromuscular junction and, in model systems, induce the neurophysiological changes associated with clinical weakness10. The finding of an autoantibody to one molecule at the neuromuscular junction has led to a more directed search for other autoantibodies, leading to the identification of antibodies to MuSK (muscle-specific tyrosine kinase).
For clinical conditions in which a target antigen is well defined, the role of autoimmunity can be assessed in immunization models. Classic examples of this type of model include experimental allergic encephalomyelitis (a model of multiple sclerosis induced by immunization with myelin basic protein) and collagen-induced arthritis (a model of rheumatoid arthritis induced by immunization with collagen). The role of autoimmunity can also be explored in rodent models of spontaneous disease. These mice (for example, the New Zealand black–white hybrid mouse model of systemic lupus erythematosus (SLE) and the nonobese diabetic mouse model of type I diabetes) were discovered fortuitously during breeding programmes and incidentally found to have features of autoimmunity, such as the production of anti-DNA autoantibodies and glomerulonephritis in so-called ‘lupus mice’11.
The utility of serological testing extends beyond diagnosis to encompass prognosis and assessment of disease activity and treatment response, and depends on the availability of robust assays for routine testing; quantitative assays can be especially informative. Although a correlation of antibody levels with clinical manifestations could suggest a direct role in pathogenesis, changes may be incidental to the clinical outcome, with a decrease, for example, resulting from the action of immunosuppressive agents that attenuate disease by their effects on other processes or cell types (for example, T cells)12. Nevertheless, quantitative assessment of autoantibody production can provide a valuable biomarker for clinical decision-making, as illustrated by the antibody response to PLA2R in membranous nephropathy13,14.
In the absence of a concerted search for autoantibodies, the role of autoimmunity in a condition may not be appreciated. For example, studies now suggest that certain forms of chronic pain can be associated with antibodies that induce pain behaviour in mice. Similar considerations may apply to other neuropsychiatric diseases, including psychosis, although complex psychological phenomena are difficult to model in animals15,16,17.
Identifying autoantigens for assays
The diverse clinical presentations of autoimmune disease requires exploration of autoreactivity, particularly for poorly understood conditions. In these investigations, the first step is a search for autoantigens with which to provide a framework to consider the role of B and/or T cell autoreactivity in disease pathogenesis.
Molecular identification of autoantigens
The identification of autoantibodies and autoantigens is closely intertwined since autoantibody assays depend on knowledge of the target antigen. Currently, screening for autoantigens is accomplished using large protein arrays, peptide arrays or phage display libraries for target identification by antibodies in patient sera18,19. These techniques allow the screening of thousands of proteins or peptide fragments for autoantigenicity; they are also agnostic and can complement traditional immunochemical techniques that use extracts of the tissues involved as a source of antigens.
In general, large-scale screening efforts indicate a greater diversity of autoantibody specificities than has conventional serology, including the presence of autoantibodies found in only some affected individuals. In addition, these screens can identify autoantibodies in otherwise healthy individuals and in diseases that are not usually considered autoimmune (such as COVID-19)20. The origin of so-called ‘common’ autoantibodies in sera of healthy individuals is not known but their presence can complicate the search for autoantibodies in patients with presumed autoimmune disease21.
Although large-scale screening and other array techniques can reveal potential target antigens for many diseases, these approaches may fail to detect autoreactivity, especially when the antigenicity of a molecule depends on its conformation. The appropriate conformation of an antigen for antibody binding may require an intact protein structure (in contrast to a peptide or fragment) or the interaction of a putative autoantigen with another macromolecular component. Along with the use of protein or peptide arrays, molecular techniques can facilitate the identification of autoantigens at sites of tissue injury. For example, microdissection can be used to retrieve proteins from immune deposits in tissue samples, with subsequent ultrasensitive mass spectrometry analysis to identify the antigen. The discovery of neural adhesion molecule 1 (NCAM1) as a target antigen in membranous lupus nephritis exemplifies this approach22.
Animal models can also be used to identify potential target antigens. For example, studies in the Heymann nephritis model of membranous nephropathy provided initial evidence for a role for autoantibody binding to podocytes in disease pathogenesis23. Subsequent studies in patients demonstrated that autoantibodies to neutral endopeptidase can lead to the development of neonatal membranous nephritis in infants born to mothers lacking expression of this protein; in this case, disease relates to alloimmunization, supporting the idea that podocyte proteins are key targets in membranous disease, a mechanism first elucidated in the animal model23.
Searches for autoreactive antibodies have also demonstrated that autoantibodies of the same specificity can be expressed in different diseases. For example, antibodies to the protein glutamic acid decarboxylase (GAD) can occur in stiff person syndrome (a neurological condition characterized by muscle rigidity and spasms) as well as in type 1 diabetes mellitus (also known as immune dependent diabetes mellitus or IDDM)24. In such situations, it is likely that differences in the precise specificity of the autoantibodies for antigen or their amounts might induce different clinical manifestations. In addition, serological studies have documented a phenomenon known as latent autoimmunity in which autoantibodies may be present in patients with other diseases, including autoimmune conditions, in the absence of usual clinical manifestations25.
Limitations of serological assays
Despite the power of current technologies, screening assays may fail to identify autoantigens under conditions in which autoantibodies would be expected. In this context, as discussed above, the biochemical structure of a possible target molecule may affect its antigenicity in immunoassays, leading to a false-negative result. As well as false-negative results, false-positive results can occur with serological assays, emphasizing the need to interpret laboratory results in the context of the clinical setting and to consider alternative diagnoses in the event of a positive result26.
Despite the fact that many autoantibodies have been identified and characterized, assays are available for only a small fraction of them. In SLE, for example, over a hundred different autoantibody specificities have been reported, but assays for only a few are available for routine testing27. To be used clinically, autoantibody assays should display certain properties as described by the SSSMAART criteria (Box 1). In the future, rather than assays of single autoantibodies, antigen arrays may enable simultaneous detection of a large number of different autoantibodies, many of which are currently considered ‘orphan’ (Supplementary Box 1).
Antigenicity of nuclear molecules
Among autoimmune diseases, a group of related systemic diseases is characterized by the expression of antibodies to macromolecular components of the cell nucleus (antinuclear antibodies)28. These antibodies target proteins, nucleic acids (DNA and RNA) and proteins complexed with DNA or RNA and characterize conditions known as rheumatic diseases. In addition to serological disturbances, these conditions have prominent involvement of the skin, joints and vasculature; kidney and pulmonary manifestations are also common. Antinuclear antibodies are also present in other non-rheumatic conditions, such as autoimmune hepatitis. As a group, the antigens targeted by these antinuclear antibodies share characteristic molecular features that may confer immunogenicity (Supplementary Box 2)29.
The role of post-translation modifications
Post-translational modifications can profoundly affect the antigenicity of proteins and their activity as neoantigens30. For example, citrullination can generate autoantigenic determinants on proteins that are targeted in rheumatoid arthritis. Citrullination is catalysed by the enzyme peptidyl arginine deiminase and probably affects the immunogenicity of the modified proteins (for example, vimentin, α-enolase or fibrinogen). Antibodies directed to citrullinated proteins are termed anti-cyclic citrinullated peptide antibodies (anti-CCPs) or anti-citrullinated protein/peptide antibodies (ACPAs). In IDDM, hybrid insulin peptides can form between a fragment of insulin and another peptide from a beta cell protein. This hybrid can be recognized as a neoepitope and stimulate T cell reactivity31.
Although studies of autoimmune disease usually assume that autoantibodies initiate inflammation and damage, it is nevertheless possible that these antibodies arise as a consequence of damage induced by some other mechanism; for example, local inflammation may perturb cells and expose or unmask autoantigenic sites to allow autoantibody induction. Without more detailed testing in in vitro models or transfer systems, it can be difficult to determine whether autoantibody production is primary or secondary. Furthermore, although autoantibodies mark the course of a clinical condition, disease manifestations may actually result from the action of T cells, as may be the case for IDDM.
Mechanisms of tissue inflammation and injury
Autoantibodies can interact with autoantigens in many different ways, contributing to the wide variety of clinical manifestations and functional disturbances that characterize autoimmune disease.
The pathogenicity of autoantibodies
Autoantibodies can mediate disease through their antigen binding site (Fab) or the crystallizable fragment (Fc, a portion of the immunoglobulin molecule that can activate complement as well as bind to Fc receptors on cells to heighten inflammation). Through these actions, autoantibodies can interact with cell surface receptors to alter cell function. In Graves’ disease, for example, antibodies to the thyrotropin receptor function as agonists; by contrast, antibodies to the acetylcholine receptor in myasthenia gravis have an antagonistic function, although these antibodies may modulate receptor function by mechanisms other than blocking ligand interaction. An autoantibody can also alter physiological processes simply by eliminating a cell population by complement-mediated lysis or antibody-dependent cell cytotoxicity; eliminating the targeted structure may also occur by antibody-mediated endocytosis and degradation.
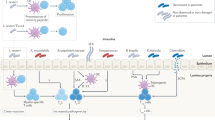
Another important mechanism by which autoantibodies mediate disease is through the formation of immune complexes32,33 (Box 2). Immune complexes are an amalgam of antibodies and antigens that vary in size and structure depending on the physical–chemical properties of the constituents, including the antibody affinity. Immune complexes can deposit within tissue, particularly in the kidney, and activate complement to drive inflammation through the recruitment of neutrophils and other myeloid and lymphoid cells to the affected tissue. Although immune complexes can form in the blood, they may preferentially localize in certain tissue sites (for example, the glomerular basement membrane) because of their size or physical-chemical properties (for example, the charge of the antibody or the antigen).
In some conditions, rather than assembling in the circulation, immune complexes may form locally in the kidney, with antigen deposited or ‘planted’ from the circulation or released by kidney cells. In lupus nephritis, a local decrease in the activity of DNase enzymes can increase the availability of DNA to form complexes with anti-DNA antibodies, resulting in the activation of complement and inflammation34. In membranous nephropathy, the release of podocyte molecules provides a source of antigens for immune complexes that deposit on the basement membrane (Fig. 1). Immune complexes can also stimulate the production of cytokines through interaction of nucleic acid components of nuclear antigens with internal nucleic acid sensors (Supplementary Box 2).
A common mechanism for immune-mediated kidney disease involves the formation and deposition of immune complexes followed by the activation of complement and ensuing inflammation. The mechanism for the formation of immune complexes varies among diseases and may involve: the formation of immune complexes in the blood followed by their glomerular deposition; binding of antibody to an autoantigen that is bound to the basement membrane (or has been ‘planted’); the formation of complexes within the glomerulus by locally generated antigen; or direct binding of antibody to a site on the basement membrane. Adapted from ref. 32, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
For the glomerulonephritides, immunohistochemistry can help to delineate disease pathogenesis. In SLE, immunofluorescent microscopy of kidney biopsy samples can show the so-called full-house pattern with the presence of immunoglobulins IgG, IgA and IgM and complement in the glomerulus; electron microscopy can further localize immune complexes to either the sub-epithelial or sub-endothelial side of the basement membrane. In contrast to the ‘lumpy bumpy’ pattern of immunoreactants in lupus nephritis, the immunohistochemistry of kidney biopsy samples from patients with Goodpasture syndrome (also known as anti-GBM disease; a pulmonary-renal syndrome) shows a linear pattern of staining, which results from the binding of autoantibodies to the alpha 3 domain of type IV collagen present in the glomerular basement membrane and lung. In anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, immunofluorescence staining fails to detect appreciable amounts of antibody or complement, leading to the designation of ANCA vasculitis as so-called pauci-immune glomerulonephritis3. Nevertheless, complement may have a role in ANCA-associated vasculitis, as demonstrated by the efficacy of avocapan — an inhibitor of the C5a receptor35. We note that in some cases, such as in minimal change disease, the level of staining for immunoreactants may be very limited, diverting attention away from an autoimmune mechanism despite its role in disease pathogenesis.
The clinical manifestations of ANCA vasculitis, in particular, may be an indirect consequence of the action of autoantibodies36. Under these conditions, antibodies to myeloperoxidase or proteinase 3 can activate neutrophils to extrude neutrophil extracellular traps (NETs), which are large aggregates of DNA with bound granule proteins. NETs have antibacterial actions and also can damage endothelial and epithelial cells to promote lung and vascular injury37. Autoantibodies can also act by inhibiting cell–cell interactions. For example, in the blistering skin conditions pemphigus vulgaris and pemphigus foliaceus, autoantibodies to desmoglein proteins bind at the desmosome to block cell attachment in the epithelial layer of the skin38.
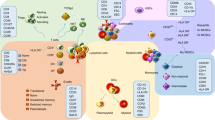
In addition to targeting cellular antigens, autoantibodies can also bind to soluble molecules in the blood. Antibodies to cytokines can modulate immune function by inhibiting key regulatory interactions involved in host defence, as may occur with antibodies to interferon (IFN)-γ in SARS-CoV-2 infection and SLE39,40. In lupus nephritis, autoantibodies to the complement protein C1q can bind to immune complexes in the kidney and amplify complement activation and inflammation41. In another example, autoantibodies to components of the alternative complement pathway can lead to C3 glomerulonephritis (including dense deposit disease), which is characterized by isolated or predominant staining of C3 on renal biopsy42 (Fig. 2).
Autoantibodies can disturb the function of cells or promote their damage or death through diverse mechanisms. These mechanisms include actions as receptor agonists (for example, as occurs in Graves’ disease), receptor antagonists (for example, as occurs in myasthenia gravis), ligand antagonists (for example, as occurs in immunodeficiency), cytotoxicity (for example, as occurs in haemolytic anaemia), neutrophil activation (with indirect damage to endothelium and epithelium) and acantholysis (for example, as occurs in pemphigus). ACh, acetylcholine; AChR, acetylcholine receptor; Fc, crystallizable fragment. Adapted from ref. 176, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
The role of T cells in autoimmune disease
Like autoantibodies, T cells can mediate autoimmune disease. T cells can be categorized in terms of their phenotypic markers, transcriptional control and functional properties (for example, cytokine production)43. In the context of autoimmune disease, antigen-specific CD4+ T helper cells (TH) cells can stimulate autoantibody production by B cells whereas cytotoxic CD8+ T cells can damage or kill cells. T cells may also produce factors (for example, circulating permeability factors) that can drive disease in diseases such as focal segmental glomerulosclerosis44.
Assays of T cell autoreactivity are challenging because of the way in which T cells recognize antigen and the huge diversity of molecules encoded in the major histocompatibility complex (MHC) region (discussed below). The technical issues involved in characterizing T cells in autoimmunity should in no way minimize the probably critical role of these cells in certain diseases. Moreover, a change in the physiological state of a tissue or organ can influence the extent of autoimmune injury in the presence of B and/or T cell autoreactivity to constituent molecules45. For example, the destruction of pancreatic beta cells in the context of IDDM reduces the capacity for insulin production. Subsequent attempts by the remaining beta cells to meet the demand for insulin may promote cell stress, thereby increasing their sensitivity to further injury by antibodies or autoreactive T cells.
Disturbances in tolerance
B and/or T cell reactivity signifies a disturbance of immune tolerance and is essential to the development of autoimmunity. The extent of these disturbances as well as the underlying mechanism may vary by disease, however, and can contribute to the heterogeneity of autoimmunity.
Mechanisms of tolerance
The generation of autoreactivity represents the loss of self-tolerance — a fundamental breakdown in immune regulation. In this context, the ‘self’ represents the molecular constituents of the organism, while ‘nonself’ represents the constituents of foreign organisms (bacteria, viruses or fungi). Tolerance is a state of immunological non-responsiveness resulting from mechanisms that occur both centrally and peripherally and involve both B cells and T cells46,47,48.
The establishment of central tolerance begins during development in the bone marrow for B cells and in the thymus for T cells49,50. In both locales, interaction with antigen initiates functional changes that determine cell fate. These processes differ for B cell and T cells because of the respective mechanisms of antigen binding. B cells bind antigen directly via their B cell receptor, which is a surface immunoglobulin. By contrast, T cells recognize peptide fragments of antigens that have been enzymatically cleaved and then presented in the context of an MHC molecule, either class I or class II, depending on whether the antigen is endogenous or exogenous.
The creation of the B cell and T cell repertoires involves gene rearrangements and other molecular changes to generate the large array of receptor molecules that is essential for host defence. In the thymus, T cells undergo both positive and negative selection to create a T cell repertoire that is broad enough to respond to foreign antigens but nonetheless restricted enough to prevent responses to self-antigens. For T cells to recognize antigen, a T cell receptor requires some level of binding to an MHC molecule, regardless of the structure of the bound peptide. Developing T cells with sufficiently strong interactions of this kind undergo positive selection, whereas T cells with insufficient binding to MHC molecules are culled.
Negative selection to establish tolerance necessitates the interaction of developing T cells with the myriad molecules that constitute the ‘self’. The display of peptide fragments of these molecules on thymic epithelial cells occurs through the action of a transcriptional regulator called autoimmune regulator (AIRE)51. AIRE promotes the expression of tissue-restricted antigens, which are otherwise selectively expressed by differentiated cells; the transcription factor Fezf2 also regulates the expression of tissue-restricted antigens by a mechanism distinct from that of AIRE52. In the event of a high-affinity T cell receptor interaction, the T cell undergoes negative selection and is not released into the periphery. Thus, depending on the strength of their interactions with MHC molecules and their bound peptides, T cells can undergo positive or negative selection. Only a minority of T cells enters the periphery after these selection processes.
Like T cell development, B cell development involves the generation of a large number of receptor types to combat foreign antigen challenge while avoiding autoreactivity53,54. In response to foreign antigen challenge, the variable region genes of the heavy and light chains undergo somatic mutation. These mutational events occur in the complementarity-determining regions and give rise to variants with increased affinity for foreign antigens to promote host defence. B cells that can bind to autoantigens can also arise during this process, leading to a potential source of pathogenic specificities. These mutational and selection events can take place in germinal centres, which are organized structures that allow the interaction of B cells and T cells. The specialized T cells that facilitate these responses are known as T follicular helper cells55. B cell responses can also develop outside germinal centres through so-called extrafollicular pathways. Furthermore, in the context of autoimmune disease, autoreactive B cells can develop in tertiary lymphoid structures at sites of tissue inflammation56.
As shown in studies of both humans and mice, B cell development involves steps denoted as checkpoints that can alter the content of autoreactive cells in the repertoire53. The elimination of autoreactive B cells can occur by deletion, anergy and a process known as receptor editing. In receptor editing of a B cell expressing a self-antigen receptor, the cell undergoes additional gene rearrangements to generate a receptor that has lost autoreactivity.
Although tolerance can occur centrally in the thymus and bone marrow, it can also occur in the periphery in lymphoid tissues57. In the periphery, regulatory cells — deriving from both the B cell and T cell lineages — have a key role in restraining autoreactivity by downregulating responses through various mechanisms, including via the production of immunosuppressive cytokines58,59,60. A characteristic feature of regulatory T cells (Treg) cells is the expression of a transcription factor called FOXP3, which can serve as a marker of this cell population58.
Depending on the autoimmune disease, the origin and nature of these disturbances in tolerance varies, leading to differences in the number of targets of autoreactivity as well as their tissue distribution. In some diseases, autoreactivity is discrete and tissue-specific whereas, in other conditions (for example, SLE), the array of autoantibodies is diverse, pointing to a more extensive disturbance in tolerance pathways.
Genetics of autoimmune disease
The current model of autoimmune disease posits that disease develops in a genetically susceptible individual in response to an environmental trigger. Evidence for genetic determination is longstanding, with the occurrence of autoimmune disease in family members pointing to a contribution of hereditary factors. Observational studies of multiplex families have further suggested the inheritance of a general tendency for autoimmunity rather than a specific disease. For example, one member of a family may have rheumatoid arthritis while another might have autoimmune thyroid disease61.
The role of HLA in autoimmunity
Screening for genes associated with autoimmune disease has become increasingly precise as studies of genetic variation in the human population have advanced62,63,64; these studies have consistently delineated the important contribution of MHC genes to many diseases65. Although genes in the MHC region encode a variety of molecules that have immune activity (such as complement components and cytokines), the genetic association of MHC alleles with autoimmune diseases probably reflects the role of MHC class I and class II molecules in presenting self-antigen determinants for T cell recognition. As genes in this region are in linkage disequilibrium, the precise determination of the contribution of different genes can be challenging.
In humans, molecules of the HLA complex (the human MHC) are enormously polymorphic, with different class I and class II molecules able to present different peptides for T cell recognition. Such polymorphism is vital for host defence and increases the ability of the host to generate immune reactivity against infecting organisms. Even if the capacity of an individual to respond to a particular antigen from an infecting organism is limited, the diversity of responses in a population confers overall protection. The polymorphism of HLA molecules, however, might also lead to autoreactivity in some individuals, according to their expression of specific alleles.
The contribution of genes to disease pathogenesis
Although dissecting the genetic architecture of autoimmunity is a huge undertaking, an increase in the number of both patients enrolled in studies and genetic markers tested has enabled the identification of genes that confer only a limited risk of disease66. In this regard, studies have shown that the same genes may increase the risk of different autoimmune diseases, consistent with an underlying propensity to autoreactivity. Genetic studies can also provide a unique perspective on disease mechanisms. For example, although the disease neuromyelitis optica is clinically similar to multiple sclerosis, it is genetically more similar to SLE67. Neuromyelitis optica is characterized by autoantibodies to the protein aquaporin 4 that lead to manifestations such as blindness or limb paralysis.
Several important conclusions have emerged from genetic studies. The first is that many genes contribute to a predisposition to autoimmunity. For diseases like rheumatoid arthritis, SLE and IDDM, over 100 different genetic loci may confer risk. Variations in the MHC region usually confer the greatest risk, although linkage disequilibrium among genes in this region may increase the magnitude of this effect. For other genes, the contribution of each locus is smaller, with a relative risk of the order of 1.2 not unusual. The number of genes identified further suggests that disease susceptibility is multigenic, with an ensemble of genes affecting processes such as antigen presentation, chemotaxis or B cell and T cell signalling. In a multi-hit process, the effects of gene variants may be more than additive, especially if they act along the same pathway68.
A second important conclusion is that gene variants in general do not affect the coding sequences of proteins69,70. Rather, the variants associated with autoimmune diseases seem to regulate the level of gene expression that could influence, for example, the downstream effects of cytokine stimulation not only on the magnitude of the inflammatory response but also with regard to their ability to induce direct damage to target cells such as the beta cells of the pancreas71. Although a candidate locus may be in the vicinity of a structural gene that is involved in immune responsiveness, it is often not possible to determine whether a causative link exists; patterns of gene transcription as well as epigenetic changes in individual cell types may provide further insights72. In this regard, epigenetic factors can modify the expression of genes that predispose to autoimmunity, strengthening the relevance of these genes to disease pathogenesis73,74.
These studies have also identified the roles of genes that are associated with different autoimmune diseases. For example, studies have demonstrated a contribution of PTPN22 to several different diseases, such as rheumatoid arthritis, SLE and or IDDM, in which it represents one of the strongest susceptibility genes other than the genes of the MHC region. PTPN22 encodes the tyrosine phosphatase nonreceptor type 2, an enzyme that can remove phosphate groups from regulatory proteins that determine cell signalling75,76. In the case of the Arg620Trp variant, which predisposes to autoimmunity, enhanced phosphatase activity may increase T cell receptor or B cell receptor signalling, affecting tolerance and promoting autoreactivity. The situation is not simple, however, because the biology of PTPN22 is complex. Its encoded protein is expressed in different lymphoid and myeloid cell types and participates in a number of distinct interactions that affect immune responsiveness. Furthermore, PTPN22 polymorphisms can also decrease the risk of some inflammatory diseases, such as Crohn’s disease, while not affecting others77.
Finally, although genetic polymorphisms might affect the function of immune cells or antigen recognition, variants of genes that encode antigens might also affect disease susceptibility78. In membranous nephropathy, for example, genes in both the HLA region and the PLA2R1 locus represent the major genetic determinants for this disease; however, this disease is unusual in that only four loci account for a major part of the genetic risk78,79.
The role of ancestry in disease pathogenesis
Genetic studies have also identified ancestry as an important determinant of disease although, in some regions of the world, socioeconomic status can complicate assessment of this impact. In SLE, patients of African ancestry have more severe kidney disease than patients of other ancestries, as well as more abundant autoantibody production, as exemplified by higher levels of antibodies to RNA binding proteins80. Ancestral groups may also differ in the array of cellular immune disturbances demonstrable in peripheral blood. For example, although gene variants may influence the expression of myeloid cells in European Americans with SLE, in patients of African ancestry, gene variants may be associated with functional changes in B cells as well as other biological processes such cellular stress81,82,83. Gene variants may also affect the outcome of disease and response to certain therapeutic agents84,85.
Since genes for autoimmunity probably evolved for defence against infection, differences in the endemic infections in any given locale may lead to the selection of gene variants that affect disease risk or outcome. Variants of APOL1 (which encodes apolipoprotein L1) are a key example of such selection. Although APOL1 variants are not associated with SLE itself, they are associated with worse outcomes in patients with lupus nephritis of African ancestry86. The postulated role of APOL1 in defence against trypanosomiasis may have maintained the presence of risk alleles in some populations despite their association with kidney disease risk.
Current studies can account for only some of the heritability of autoimmune disease, because rare variants that increase disease risk may not be detected by current analytic approaches. In the future, complete sequencing of the genomes of affected patients will probably identify additional variants that increase disease risk and thereby reduce the missing heritability.
Single-gene models of autoimmunity
Most autoimmune diseases are multigenic; nevertheless single-gene mutations can powerfully influence disease susceptibility87. For example, deficiency of the complement component C1q seems to be almost invariably associated with SLE. Although a complement deficiency would seem to be protective in a disease characterized by the deposition of immune complexes, complement has other actions (for example, in the clearance of dead and dying cells). Deficient clearance of such material could stimulate autoantibody responses, leading to autoimmunity. Sequence and copy number variations in genes that encode components of the alternative complement pathway (for example, complement-factor H related proteins) may also drive disease in C3 glomerulonephritis, lowering complement levels much as do the autoantibodies to these proteins32,88.
Other single-gene systems that contribute to SLE include those that encode the enzymes that degrade intracellular nucleic acids. The impaired degradation of these nucleic acids in the context of infection or cell stress can lead to a rise in the intracellular levels of DNA and RNA, allowing their interaction with cytoplasmic nucleic acid receptors. Stimulation of these receptors can induce cytokines such as type 1 interferon and their downstream effects, including autoreactivity. Diseases of this kind have inflammatory and autoimmune features and have been termed interferonopathies89.
Abnormalities in single genes can also drive T cell autoimmunity. Mutations in AIRE can lead to an autosomal recessive disease known as autoimmune polyglandular syndrome type I or autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED)90,91. This condition classically presents with Whitaker’s triad (candidiasis, hypoparathyroidism and adrenocortical insufficiency), among other features. The absence of AIRE leads to the decreased expression of tissue-restricted antigens, resulting in impaired negative selection; the situation is more complicated because AIRE can also affect Treg cells. An interesting feature of this disease concerns the occurrence of candidiasis, which can result from autoantibodies to interferon, with autoimmunity leading to immunodeficiency.
Other monogenic diseases involve Treg cells and the action of FOXP3 — which, as mentioned earlier, is a transcription factor that regulates the development and expression of Treg cells. Mutations in FOXP3 are associated with a clinical syndrome called IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome)92. Along with this triad, patients may have other manifestations such as hepatitis or thyroiditis. In addition, abnormalities in other genes that contribute to Treg cell function (for example, the genes that encode CD25 or the IL-2 receptor) can lead to related autoimmune syndromes known as tregopathies93.
Copy-number variations can also underlie the mechanism by which products of single-gene loci or gene families can increase disease susceptibility. For example, several different genes encode the two forms of the complement protein C4; these forms are called C4a and C4b and differ in their biochemical interactions with antigen during complement activation. Among the rheumatic diseases, copy-number variations in the genes encoding C4a determine the levels of C4a in the blood as well as susceptibility to SLE and the production of autoantibodies to autoantigens known as SSA/Ro and SSB/La94. As in the case of C1q deficiency, the impact of C4a copy-number variations may reflect the role of complement in the clearance of immunostimulatory material, whether self or foreign.
The role of various single genes in the development of autoimmunity can be verified in animal models by knocking out single genes. As these studies have demonstrated, single genes can act at various steps in tolerance, both centrally and peripherally. Furthermore, these studies have indicated the role of positive and negative selection not only for the development of conventional TH cells but also for Treg populations. Studies in animal models have indicated that enhancement of B cell signalling frequently leads to anti-DNA production and lupus-like disease. By contrast, disturbances in Treg function lead to an array of clinical manifestations and serological findings in humans and mice. For example, patients with IPEX can produce autoantibodies to gut-associated target molecules such as harmonin or villin; the production of antinuclear antibodies is not a prominent feature of these diseases despite the widespread disturbances in T cell regulation95,96.
The role of sex
Many autoimmune diseases occur with a higher frequency in women than men, with sex representing a major genetic risk factor97,98,99. Since some diseases (for example, SLE) characteristically affect women in their child-bearing years, the influence of sex may relate to the action of hormones such as oestrogens and progestins. Both classes of sex hormones have important immunological activities; oestrogens, in particular, are able to boost immune activity. On the other hand, certain diseases (for example, rheumatoid arthritis) may improve during pregnancy, highlighting the complexity of hormonal interactions. Sex hormone activity is not always deleterious, however, since women demonstrate increased levels of resistance to infection as well as heightened responses to vaccines compared with those of men100,101. The greater susceptibility of women to autoimmune disease may therefore represent a trade-off for more robust responses to infection. Of note, unlike cancer (for example, breast cancer and prostate cancer), sex hormones have not been a major focus of attention in the development of treatments of autoimmune disease.
The influence of sex may also involve genes on the X chromosome. Although men have a single X chromosome and women have two, the level of expression of X-linked genes in women is normalized by the inactivation of one of these chromosomes at the level of each somatic cell. X chromosome inactivation may be incomplete, however, allowing both copies of the X-linked genes to be expressed. For genes encoding proteins with immune activity, incomplete X chromosome inactivation could lead to increased protein production and the downstream effects of such proteins.
Evidence for the influence of the number of X chromosomes on disease derives from studies on the occurrence of SLE in patients with Klinefelter’s syndrome102. This condition is characterized by an additional X chromosome so that affected individuals are genotypically XXY. Despite the presence of the Y chromosome in these patients, the prevalence of SLE is similar to that in women. Of the genes that can be affected by X chromosome inactivation, Toll-like receptor (TLR) 7 may be particularly important, given that this internal nucleic acid sensor is stimulated by single-stranded RNA, which can be present in immune complexes in SLE. Of note, a gain-of-function missense mutation in TLR7 (TLR7Y264H) has been identified in a child with SLE, with in vitro and animal studies pointing to a role in B cell activation and survival103. Other genes encoding proteins with immune activity — including FOXP3, CD40L and IRAK1 — are also found on the X chromosome, highlighting the important contribution of the X chromosome to immunity.
The difference in disease occurrence between men and women may relate to differences in underlying disease mechanisms as well as in response to therapy; men and women may receive different therapy because of concerns about pregnancy, for example104. Interestingly, men and women may also differ in the transmission of genetic risk since children of fathers with IDDM are more likely to develop disease than children of mothers with this disease105.
Triggers of autoimmunity
Gene variants can create a predisposition to immune dysregulation in patients, but environmental factors seem to be necessary to induce B and/or T cell autoreactivity and clinical disease manifestations. The number and kind of these factors are unknown and, for the individual patient, they may be multiple and act over time.
Unlike the genes that predispose to autoimmunity, the environmental factors that can act as triggers are more difficult to ascertain. One reason is the large number of potentially causative factors given the ubiquity of infective organisms and chemical agents in the environment. The time element is also important given that a relevant exposure may occur years or even decades prior to disease onset. Birth by Caesarean section, for instance, can affect the eventual development of IDDM years or decades later106. We note that the frequency of autoreactivity may be increasing in the general population, as indicated by assays for antinuclear antibodies107. Furthermore, changes in the environment may be increasing the prevalence of diseases such as IDDM as observed in Western countries; these changes may also affect the contribution of genetic factors to disease since stronger environmental influences may reduce added risk conferred by gene variants108,109.
The role of microorganisms
Of environmental factors, interaction with microorganisms remains the most likely direct trigger of autoimmune disease through at least two different mechanisms110,111. The first is through molecular mimicry based on structural similarity between self and foreign molecules. Despite being directed against a foreign antigen, antibodies induced during infection may also bind self-antigens. For example, rheumatic fever is an autoimmune process that can result from cross-reactive autoantibodies, in this case to group A streptococcus. Assessing the role of molecular mimicry in disease pathogenesis must consider the biochemistry of these cross-reactions, because sequence homology among proteins is not uncommon. It is therefore important to demonstrate the occurrence of infection or colonization in the autoimmune individual when antibodies that bind homologous sequences are found.
The second mechanism is through nonspecific stimulation of the immune system. In general, infection exposes the host to pathogen-associated molecular patterns (PAMPs), which can bind to pattern recognition receptors to stimulate innate immune responses and potentiate adaptive immune responses112. The action of PAMPs such as endotoxin or lipopolysaccharide can affect multiple cell types, enhancing responses to self as well as to foreign molecules by enhancing the stimulation of B or T cell responses. These responses can be elicited by different infections that can be symptomatic or asymptomatic.
The impact of some infections on disease processes can have both specific and nonspecific components. For example, certain Epstein–Barr virus (EBV) proteins display structural similarity to RNA binding proteins (RBPs)113; thus, an aberrant antibody response to an EBV protein can lead to cross-reactivity to an RBP. Once an anti-RBP antibody is established, it can promote disease by forming immune complexes that drive cytokine production and potentiate antigen-specific responses. EBV can also lead to B cell activation and antibody responses.
The microbiota comprises all commensal organisms (bacteria, viruses and fungi) that coexist with the host and represents an important source of foreign antigens110,111. The composition of microbiota varies by site (for example, in the gut, mouth and skin) and contains a huge variety of different organisms. Indeed, there are more bacterial cells than mammalian cells in the ordinary person. Disturbances in the composition of the microbiome can lead to dysbiosis and disturbances in homeostatic functions, especially of the immune system114.
As in the case of infection, dysbiosis can induce both nonspecific and specific immune responses that underpin autoreactivity. In rheumatoid arthritis, expansion of Prevotella copri in the gut is associated with disease expression, with bacterial P. copri peptides showing homology to self antigens that can bind to the shared epitope (see below). In SLE, expansions of Ruminococcus gnavus are associated with disease activity as well as the presence of antibodies against lipoglycan antigens of this organism. The composition of the microbiome may also influence treatment response owing to effects on drug metabolism115,116,117,118.
Among other effects of the microbiome, bacteria from the gut can translocate into the circulation and localize in tissue to provide a nidus of microorganisms that can trigger autoantibody production; disturbances in the barrier function of the gut wall can increase translocation119. Although the body has traditionally been considered to be sterile except for the gut, sensitive molecular techniques have demonstrated the presence of organisms in a variety of different tissues. Interestingly, gut organisms that act as pathobionts can evolve in the host and develop mutations that influence their capacity for translocation120.
In addition to its role as a source of antigen, gut microbiota can affect the immune system through its effects on metabolism121,122,123. Gut bacteria have a key role in the metabolism of short-chain fatty acids, which can interact with G protein coupled receptors on immune cells to modulate their function124. The microbiome can also alter the metabolism of amino acids such as tryptophan. Since tryptophan can be converted to kynurenine, which has immunomodulatory effects, gut dysbiosis can lead to immune system changes through the actions of this mediator125.
Although both bacterial and viral infection can drive autoimmunity, the overall level of exposure may have countervailing effects. The hygiene hypothesis posits that the frequency of autoimmune and allergic diseases in the population is accelerating, especially in the high-income regions, because of a relatively ‘clean’ environment that limits early life exposures to microbial organisms. Such environmental changes may shift the balance of the immune system processes, creating a predisposition to autoreactivity. In this schema, diet is another factor that can shape the immune system since the composition of diet (for example, the amount of fibre) can affect the microbiome, gut wall integrity and mediator production126,127.
Smoking
The impact of smoking on the development of rheumatoid arthritis is one of the clearest examples of the interplay between genes and the environment. Among genetic factors, the so-called HLA shared epitope dramatically increases the risk of rheumatoid arthritis. Smoking exacerbates the risk of rheumatoid arthritis, and the combination of smoking and presence of the shared epitope dramatically increases disease risk by approximately 10-fold128. The causative factor in smoke is not clear, although an increase in protein citrullination in the lung may elicit ACPA production, initiating a process that ultimately targets the joints.
Drugs
Drugs represent another exposure that can trigger autoreactivity, with SLE and autoimmune thyroid disease showing evidence of disease induction by a variety of therapeutic agents. Among widely used agents, statins have been associated with immune-mediated necrotizing myopathy (IMNM), which is characterized by antibodies to 3-hydroxy-3-methylglutaryl-CoA reductase, the target of statins129. The association of IMNM with certain HLA antigens provides further evidence for the interplay between genetic and environmental factors in disease aetiology. As another example, antibiotics may affect pathogenic processes via their effects on microbiota. Certain drugs (for example, NSAIDs) may be associated with distinct forms of kidney disease (for example, acute interstitial nephritis and membranous nephropathy), although the frequency of NSAID use in the population can complicate assessment of the effect on immune-mediated disease. Prostaglandins have complex immunological actions and may be anti-inflammatory in certain settings; as a result, their inhibition through NSAID use may provoke nephritis130,131.
Malignancy and its treatment
The systemic effects of malignancy include the induction of autoreactivity. As the host mounts a defence against tumour growth, the targeting of a neo-antigen can induce a cross-reactive response to non-mutated self-antigen and clinical features such as severe neurological manifestations. The development of encephalitis from antibodies to the N-methyl-d-aspartate glutamate (NMDA) receptor in women with an ovarian tumour is a classic example of this process132,133. These manifestations, which are associated with characteristic antibodies, can sometimes precede the tumour and provide a clue to the underlying malignancy. In this regard, certain chemotherapeutic agents may cause ‘immunogenic cell death’, which can provoke an antitumour response — an example of the way that a cell state (for example, involving cell damage or stress) can determine autoimmune reactivity134.
The treatment of cancer with immune checkpoint inhibitors is an increasingly common setting for the development of autoimmunity132,135. In this context, the goal of therapy is to increase immunity to the tumour, which has been restrained by immune checkpoints that also prevent autoreactivity. Removal or attenuation of the restraints through the administration of therapeutic antibodies to CTLA-4, PD1 or PD1 ligand (alone or in combination) can induce a wide range of autoimmune syndromes; gastrointestinal and endocrine manifestations are particularly common. Although autoantibodies can occur in this setting, the development of antinuclear antibodies of the kind found in SLE is infrequent, providing another example of the differing consequences of aberrant B cell and T cell autoreactivity.
Stages of autoimmunity disease
Autoimmune disease is dynamic, with clinical manifestations leading to the culmination of a process that develops over many years. Defining the stages of disease is important for devising new strategies for early diagnosis, treatment and prevention; staging may also be important in determining the need for ongoing immunosuppressive therapy, especially if remission has occurred.
The concept of pre-clinical autoimmunity
One of most important outcomes of research on autoimmune disease is the concept of ‘pre-clinical autoimmunity’. Pre-clinical autoimmunity defines a period of time in which autoreactivity or autoimmunity (usually assessed by the expression of autoantibodies) is present in the absence of classic signs and symptoms of disease136. The boundary between pre-clinical autoimmunity and autoimmune disease is vague and depends on the degree of patient symptomatology, availability of laboratory data and appropriate recognition by the provider. For some conditions, the term ‘incomplete’ disease is used, suggesting that, although clinical findings are present, they do not fully meet diagnostic or classification criteria for ‘complete’ disease137.
Regardless of the terminology, the period of pre-clinical autoimmunity signifies that autoimmune disease entails a process that develops over time; a duration of years may not be unusual138,139,140. This dynamic differs greatly from that of a condition like acute rheumatic fever in which signs and symptoms rapidly follow infection. Many autoimmune diseases can therefore be best understood as diseases in which chronicity arises not just from the sequelae of an autoimmune attack (for example, insulin dependence in IDDM) but also from the gradual induction of the effector elements themselves, whether B cells or T cells (Fig. 3).
Autoimmune disease develops over a time course and its trajectory may be affected by the presence of genetic, epigenetic and environmental factors. The presence of certain genetic risk factors, may, for example, cause disturbances in B cell and T cell signalling, even early in life. After a while, the impact of these disturbances becomes manifest, potentially as a consequence of environmental exposures (such as infection); in women, sex hormones may heighten these disturbances. Over time, evidence of autoimmunity (for example, elevated levels of autoantibodies or cytokines) increases and patients enter a stage that can be called asymptomatic or benign autoimmunity. The subsequent accumulation of other causative factors induces a transition to a stage of pre-clinical autoimmunity in which functional changes (for example, dysglycaemia) or symptoms (for example, arthralgia) occur but remain below a threshold that would cause the patient to seek medical attention or prompt the provider to order more testing. Once such a threshold has been reached, clinical autoimmune disease can be diagnosed and therapy started. The various stages probably represent a continuum, with more intensive screening moving each of the stages back in time. Post-clinical autoimmunity refers to the changes that may occur after clinical recognition and initiation of therapy. For diseases such as immune dependent diabetes mellitus, therapy might focus on agents such as insulin that aim to correct the functional disturbance without monitoring for autoimmunity or treatment with immunosuppressive agents. For other diseases, such as anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis and systemic lupus erythematosus, management may involve immunosuppressive therapy of varying intensity, in some instances guided by biomarkers such as anti-DNA, ANCA or complement. It is useful to distinguish between clinical autoimmunity and post-clinical autoimmunity because current therapy can induce remission in at least some diseases (for example, rheumatoid arthritis).
Pre-clinical autoimmunity can be recognized in various ways. The first involves retrospective analysis of blood from patients collected as part of longitudinal population-based studies such as that performed by the USA military. For patients who develop disease, the stored samples can be tested for autoantibodies to determine when they arise138. Pre-clinical autoimmunity can also be characterized prospectively as part of public health efforts to improve disease outcomes by early detection and treatment141. Although an entire population could be monitored, a more efficient approach involves testing of individuals who are at high risk of disease on the basis of family history or the presence of disease in a first-degree relative; identical twins are the most informative relatives but they are not common142.
Pre-clinical autoimmunity can also be discovered accidentally or incidentally. In the evaluation of patients with musculoskeletal complaints, providers may order tests such as the rheumatoid factor (an IgM anti-IgG antibody), anti-CCP, or an antinuclear antibody (Supplementary Box 3). Since these tests may be ordered in patients with a low pre-test probability of disease (for example, patients with degenerative arthritis or widespread pain), positive results are most probably false-positive. Some positive results, however, may represent the pre-clinical autoimmune state.
Both retrospective and prospective studies confirm that the presence of autoantibodies can pre-date disease by many years and that, during the pre-clinical autoimmune setting, autoantibody production can evolve and lead to the expression of antibodies to either more epitopes (epitope spreading) on a target antigen or additional antigens, which may accompany the progression to clinical disease. For instance, several different autoantibodies (including anti-GAD, anti-insulin, anti-islet antigen-2 and anti-z-transporter 8) are associated with IDDM. The likelihood of developing clinical diabetes increases with the number of different autoantibodies present139. Similarly, in rheumatic diseases, the chance of developing SLE rises as the number of different antinuclear antibodies increases over time. In addition to expanding serological findings, the pre-clinical autoimmune state can be associated with evidence of immune dysfunction (for example, increased cytokine production) that can intensify as clinical findings develop138,143.
A transition from pre-clinical autoimmunity to clinical disease may not be inevitable. Prospective studies may facilitate the identification of factors that drive this transition and may be targeted for early therapy144. Interestingly, studies have demonstrated that autoantibody responses may revert, with the risk of disease diminishing145. Better prediction of the transition from pre-clinical to clinical disease may require adjunctive information from genetic and genomic studies to identify more precisely the individuals at greatest risk.
Early or preventive therapy
The opportunity to initiate early or pre-emptive therapy depends on serological tests that can predict disease as well as the availability of treatments with a favourable efficacy-to-safety ratio. This approach also depends on the extent of the organ damage that has occurred by the time pre-clinical autoimmunity can be recognized by laboratory findings or symptomatology. Nevertheless, early treatment can have the enormous benefit of limiting subsequent tissue injury. The term ‘window of opportunity’ denotes the time period in which effective treatment may attenuate or even halt autoimmunity to prevent progression to permanent or persistent disease activity.
IDDM and rheumatoid arthritis are two diseases that enable the testing of strategies to treat pre-clinical autoimmunity. In IDDM, family members can be screened for evidence of autoantibody production; the function of pancreatic beta cells can be assessed by testing for glucose tolerance and insulin production, and a number of studies are under way to identify therapeutic agents that are effective during early stages of autoimmunity. One of these agents, teplizumab (an Fc receptor nonbinding humanized anti-CD3 monoclonal antibody) has received approval from the US FDA to delay the onset of stage 3 IDDM (clinical diabetes) in high-risk individuals with stage 2 IDDM (that is, in individuals with two or more autoantibodies and elevated blood glucose levels) who are 8 years of age or older. Use of this agent is associated with an increase in the frequency of a population of CD8+ T cells that bear the features of ‘partial exhaustion’. For the pancreas as a targeted organ, various approaches, including insulin therapy, are being tested to decrease stress and thereby blunt the effects of autoimmune attack146,147,148,149,150,151.
In contrast to IDDM, for which several marker autoantibodies herald the onset of autoimmunity, anti-CCP antibodies are the only specific marker of rheumatoid arthritis; rheumatoid factors are much less specific. In the absence of a functional test for rheumatoid arthritis comparable to a glucose tolerance test in IDDM, pain (that is, arthralgia) can serve as a marker of incipient disease although, arguably, pain may indicate ongoing synovitis. Treatments such as hydroxychloroquine, methotrexate or rituximab have all been explored to prevent inflammatory arthritis in patients with arthralgia at risk of rheumatoid arthritis, although early treatment may only delay disease onset rather than foster a more significant shift in the immunological profiles of patients152,153.
In considering early or preventive therapy, benefits attributable to immunomodulatory therapies or immunosuppressive agents may actually reflect spontaneous remission, which can occur in certain autoimmune diseases depending on factors such as patient age and serological status14,154,155. Once disease manifestations occur, however, therapy may be necessary to prevent organ damage, limiting the ability to observe spontaneous remission and the immunological changes that terminate autoreactivity and restore homeostasis. In this regard, for remissions induced by therapy, autoantibody production may continue despite clinical quiescence.
The concept of post-clinical autoimmunity
Not surprisingly, research on autoimmune disease has focused on factors that initiate autoreactivity and induce inflammation. Fewer studies have addressed subsequent events in disease, particularly the long-term impact of treatment on underlying B cell and T cell disturbances. Studies of this kind require informative biomarkers as well as an understanding of the mode of action of immunosuppressive therapies. Many currently available agents have broad actions, with anti-proliferative agents capable of modifying the number or function of both B cells and T cells. Even agents that seemingly target a specific cell population can have complex and unexpected actions. For instance, although rituximab can eliminate B cells, it can also affect T cell function because B cells are effective antigen-presenting cells and are required for the maintenance of T follicular helper cells156.
For some autoimmune diseases, once irreversible damage has occurred, the need for ongoing immunosuppressive treatment lessens or disappears. In these conditions, long-term treatment focuses on the organ dysfunction (for example, thyroid replacement for hypothyroidism from thyroiditis, or insulin therapy for IDDM). In instances in which autoimmune damage is permanent, further serological testing would be mostly of academic interest. For other diseases such as rheumatoid arthritis or multiple sclerosis, autoreactivity and inflammation can persist for many years at varying intensity, waxing and waning during flares. The basis of flares is unknown, although flares may result from a nonspecific increase in immune reactivity (for example, from infection or stress) or a change in the exposure of the immune system to self-antigen.
The role of B cells in determining disease course
For autoimmune diseases mediated by B cells, characteristic changes in B cell populations can occur and signal the diverse roles of these cells in antigen presentation, cytokine production and autoantibody production. Among these changes, age-associated B cells can increase in number and distribution. Age-associated B cells are present in the context of ageing, infection and autoimmunity; they can be defined according to their expression of the T-bet transcription factor as well as cell surface markers (CD11c+ and CD21–/low) and represent a heterogeneous population of activated cells that can be induced by TLR7 or TLR9 engagement157,158. These cells have unique functional and migratory properties and may also be found in tissue (for example, in synovium or synovial fluid). The expansion of age-associated B cells during active SLE and rheumatoid arthritis suggests that the expression of these cells contributes to disease initiation and persistence159,160.
In addition to changes in certain B cell populations, such as the decrease in anergic B cells in IDDM161, an important determinant of disease course is the nature of the autoantibody-producing cells and the respective roles of newly emergent B cells, memory populations and long-lived plasma cells (LLPCs)162. Unlike the transient role of plasmablasts or short-lived plasma cells, antibody production by LLPCs can persist for many years, with antibody production to some antigens being essentially lifelong. The duration of antibody production by LLPCs can vary and may reflect the strength of the activating signals during induction that ‘imprint’ the lifespan of the response163,164,165. LLPCs can survive in specialized niches in the bone marrow as well as secondary lymphoid organs; LLPCs may also reside in sites of inflammation such as the kidney in SLE or the joints in rheumatoid arthritis166.
Since LLPCs do not express CD20, they are not affected by rituximab, a monoclonal anti-CD20 agent. Other approaches aimed at eliminating plasma cells include proteasome inhibitors such as bortezomid and monoclonal antibodies to CD38 or SLAMF7 — agents developed to treat the plasma cell malignancy multiple myeloma. The elimination of LLPCs can affect protective antibody responses as well as autoantibodies167,168.
The role of LLPCs in many autoimmune responses is unclear because sequential autoantibody testing is often not performed after disease onset owing to the uncertainty of the role of antibodies as markers of disease activity or prognosis. For example, it has been difficult to establish whether ANCA testing helps to predict disease activity in ANCA vasculitis; the relationship between ANCAs and disease activity may vary depending on clinical manifestations (renal versus non-renal), the specificity of the autoantibodies (myeloperoxidase versus proteinase 3) and the performance characteristics of the assays169.
The role of autoantibodies as a biomarker has been more extensively explored in SLE. In this disease, levels of anti-DNA antibodies fluctuate widely and can rise and fall with disease activity and therapy. In some patients, these antibodies can essentially vanish only to reappear months or years later170. This variability may reflect antibody secretion by plasmablasts derived from a memory cell population or the emergence of new B cells. By contrast, antibodies to RBPs show a more stable level of expression, consistent with their production by LLPCs171. In general, levels of anti-RBPs are not assessed in patient follow-up, although antibodies to both DNA and RBPs have been associated with the formation of immune complexes that induce interferon.
The term ‘post-clinical autoimmunity’ or ‘post-clinical-onset autoimmunity’ can be used to denote events in autoimmune disease after a clinical (or laboratory) threshold has been met, the diagnosis established and therapy implemented. This terminology does not mean that autoimmunity has ended or that B and T cell reactivity is eliminated; rather, it focuses attention on the subsequent disease course, especially the effects of therapy. Thus, anti-inflammatory or immunosuppressive agents may attenuate or modify the immune system changes that led to disease onset. The natural history of disease may also determine the post-clinical autoimmune state since some diseases (for example, autoimmune haemolytic anaemia) can be monophasic and may remit after a single course of therapy; other diseases may enter into a state of low disease activity or even remission with therapy172.
Immune system changes in post-clinical autoimmunity
Studies of SLE point to the heuristic value of the concept of post-clinical onset autoimmunity. During the development of belimumab — a monoclonal antibody against the B cell factor BAFF (also known as BLyS) — many patients (about 30%) who entered the phase II clinical trials did not have detectable antinuclear antibodies despite active disease173. Importantly, in the phase II trials, patients who were seropositive had much better treatment responses than those who were seronegative173. The phase III trials enrolled only seropositive patients (antinuclear antibodies, anti-DNA and/or anti-Sm) and were successful, leading to regulatory approval of belimumab174. Subsequent trials of other agents have followed this approach and entered only patients with ‘autoantibody positive, clinically active’ SLE175. Thus, although at disease onset, essentially all patients with active SLE are autoantibody positive, in the post-clinical-onset state, some patients may have active disease despite seronegativity. Some aspect of the immune system has changed profoundly in patients with this state, either as a consequence of therapy, natural disease history or both.
In trials for new agents for SLE and probably other autoimmune diseases (such as rheumatoid arthritis), at the time of enrollment, patients might already have had the disease for 5 to 10 years depending on the manifestation. Much can happen during this time, including reductions in autoantibody production in response to therapy. Without periodic assessment, it can be difficult to understand the relationship of autoantibodies and disease manifestations given that both may evolve during the course of disease. It is possible that T cell autoreactivity also ebbs and flows.
Conclusions
Autoimmune diseases are a diverse collection of conditions that arise from a breakdown in the mechanisms of tolerance and self–nonself discrimination. Tolerance is enormously complicated and involves many different mechanisms and interactions, perhaps accounting for the heterogeneity of the various diseases classified as autoimmune. Future studies will delineate more precisely both the genetic and genomic architecture of the different diseases in the hopes of developing more effective, more targeted and less toxic therapies for both treatment and prevention.
References
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Slight-Webb, S., Bourn, R. L., Holers, V. M. & James, J. A. Shared and unique immune alterations in pre-clinical autoimmunity. Curr. Opin. Immunol. 61, 60–68 (2019).
Sethi, S., De Vriese, A. S. & Fervenza, F. C. Acute glomerulonephritis. Lancet 399, 1646–1663 (2022).
Beck, L. H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Sethi, S. New ‘antigens’ in membranous nephropathy. J. Am. Soc. Nephrol. 32, 268–278 (2021).
Watts, A. J. B. et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J. Am. Soc. Nephrol. 33, 238–252 (2022).
Rose, N. R. & Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol. Today 14, 426–430 (1993).
Mecoli, C. A. & Casciola-Rosen, L. An update on autoantibodies in scleroderma. Curr. Opin. Rheumatol. 30, 548–553 (2018).
McHugh, N. J. & Tansley, S. L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 14, 290–302 (2018).
Lazaridis, K. & Tzartos, S. J. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front. Immunol. 11, 212 (2020).
Moore, E. et al. Promise and complexity of lupus mouse models. Nat. Immunol. 22, 683–686 (2021).
de Moel, E. C. et al. In rheumatoid arthritis, changes in autoantibody levels reflect intensity of immunosuppression, not subsequent treatment response. Arth. Res. Ther. 21, 28 (2019).
van de Logt, A. E. et al. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 93, 1016–1017 (2018).
Wu, W. et al. The prognostic value of phospholipase A2 receptor autoantibodies on spontaneous remission for patients with idiopathic membranous nephropathy: a meta-analysis. Medicine 97, e11018 (2018).
Goebel, A. et al. The autoimmune aetiology of unexplained chronic pain. Autoimmun. Rev. 21, 103015 (2022).
Goebel, A. et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. https://doi.org/10.1172/jci144201 (2021).
Pollak, T. A. et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiat. 7, 93–108 (2020).
Larman, H. B. et al. Autoantigen discovery with a synthetic human peptidome. Nat. Biotechnol. 29, 535–541 (2011).
Wang, E. Y. et al. High-throughput identification of autoantibodies that target the human exoproteome. Cell Rep. Meth. https://doi.org/10.1016/j.crmeth.2022.100172 (2022).
Knight, J. S. et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. https://doi.org/10.1172/jci154886 (2021).
Shome, M. et al. Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep. 39, 110873 (2022).
Caza, T. N. et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 100, 171–181 (2021).
Ronco, P. & Debiec, H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J. Am. Soc. Nephrol. 16, 1205–1213 (2005).
Graus, F., Saiz, A. & Dalmau, J. GAD antibodies in neurological disorders — insights and challenges. Nat. Rev. Neurol. 16, 353–365 (2020).
Buzzetti, R. et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes 69, 2037–2047 (2020).
Rüster, M., Kiehntopf, M., Gröne, H. J. & Wolf, G. A Friday afternoon case of apparent anti-glomerular basement nephritis. Nephrol. Dial. Transplant. 21, 2328–2330 (2006).
Fritzler, M. J., Choi, M. Y., Satoh, M. & Mahler, M. Autoantibody discovery, assay development and adoption: death valley, the sea of survival and beyond. Front. Immunol. 12, 679613 (2021).
Pisetsky, D. S. & Lipsky, P. E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 16, 565–579 (2020).
Rosen, A. & Casciola-Rosen, L. Autoantigens as partners in initiation and propagation of autoimmune rheumatic diseases. Annu. Rev. Immunol. 34, 395–420 (2016).
Scherer, H. U., Huizinga, T. W. J., Krönke, G., Schett, G. & Toes, R. E. M. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat. Rev. Rheumatol. 14, 157–169 (2018).
Mitchell, A. M. et al. T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2019129118 (2021).
Poppelaars, F. & Thurman, J. M. Complement-mediated kidney diseases. Mol. Immunol. 128, 175–187 (2020).
Kant, S., Kronbichler, A., Sharma, P. & Geetha, D. Advances in understanding of pathogenesis and treatment of immune-mediated kidney disease: a review. Am. J. Kidney Dis. 79, 582–600 (2022).
Zykova, S. N., Tveita, A. A. & Rekvig, O. P. Renal Dnase1 enzyme activity and protein expression is selectively shut down in murine and human membranoproliferative lupus nephritis. PLoS One https://doi.org/10.1371/journal.pone.0012096 (2010).
Jayne, D. R. W., Merkel, P. A., Schall, T. J. & Bekker, P. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Alba, M. A., Jennette, J. C. & Falk, R. J. Pathogenesis of ANCA-associated pulmonary vasculitis. Semin. Respir. Crit. Care Med. 39, 413–424 (2018).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7, e32366 (2012).
Kasperkiewicz, M. et al. Pemphigus. Nat. Rev. Dis. Prim. 3, 17026 (2017).
Wang, E. Y. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021).
Puel, A., Bastard, P., Bustamante, J. & Casanova, J. L. Human autoantibodies underlying infectious diseases. J. Exp. Med. https://doi.org/10.1084/jem.20211387 (2022).
Beurskens, F. J., van Schaarenburg, R. A. & Trouw, L. A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 68, 6–13 (2015).
Bomback, A. S. et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 93, 977–985 (2018).
Yoshitomi, H. & Ueno, H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell. Mol. Immunol. 18, 523–527 (2021).
den Braanker, D. J. W. et al. Novel in vitro assays to detect circulating permeability factor(s) in idiopathic focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 36, 247–256 (2021).
Sims, E. K., Mirmira, R. G. & Evans-Molina, C. The role of beta-cell dysfunction in early type 1 diabetes. Curr. Opin. Endocrinol. Diab. Obes. 27, 215–224 (2020).
Hogquist, K. A., Baldwin, T. A. & Jameson, S. C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Bluestone, J. A. & Anderson, M. Tolerance in the age of immunotherapy. N. Engl. J. Med. 383, 1156–1166 (2020).
Tsubata, T. B-cell tolerance and autoimmunity. F1000 Res. 6, 391 (2017).
Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 17, 281–294 (2017).
Takaba, H. & Takayanagi, H. The mechanisms of T cell selection in the thymus. Trends Immunol. 38, 805–816 (2017).
Anderson, M. S. & Su, M. A. AIRE expands: new roles in immune tolerance and beyond. Nat. Rev. Immunol. 16, 247–258 (2016).
Takaba, H. et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 163, 975–987 (2015).
Meffre, E. & O’Connor, K. C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019).
Rawlings, D. J., Metzler, G., Wray-Dutra, M. & Jackson, S. W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 17, 421–436 (2017).
Mintz, M. A. & Cyster, J. G. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol. Rev. 296, 48–61 (2020).
Jamaly, S., Rakaee, M., Abdi, R., Tsokos, G. C. & Fenton, K. A. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 20, 102980 (2021).
ElTanbouly, M. A. & Noelle, R. J. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat. Rev. Immunol. 21, 257–267 (2021).
Wing, J. B., Tanaka, A. & Sakaguchi, S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50, 302–316 (2019).
Shevach, E. M. Foxp3+ T regulatory cells: still many unanswered questions — A perspective after 20 years of study. Front. Immunol. 9, 1048 (2018).
Dasgupta, S., Dasgupta, S. & Bandyopadhyay, M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell. Immunol. 352, 104076 (2020).
Criswell, L. A. et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am. J. Hum. Genet. 76, 561–571 (2005).
Parkes, M., Cortes, A., van Heel, D. A. & Brown, M. A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 14, 661–673 (2013).
Iwamoto, T. & Niewold, T. B. Genetics of human lupus nephritis. Clin. Immunol. 185, 32–39 (2017).
Robertson, C. C. et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat. Genet. 53, 962–971 (2021).
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Goulielmos, G. N. et al. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene 668, 59–72 (2018).
Estrada, K. et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat. Commun. 9, 1929 (2018).
Lenz, T. L. et al. Widespread non-additive and interaction effects within HLA loci modulate the risk of autoimmune diseases. Nat. Genet. 47, 1085–1090 (2015).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Cano-Gamez, E. & Trynka, G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 11, 424 (2020).
Benaglio, P. et al. Type 1 diabetes risk genes mediate pancreatic beta cell survival in response to proinflammatory cytokines. Cell Genom. 2, 100214 (2022).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464 (2015).
Coit, P. et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naïve CD4+ T cells from lupus patients. J. Autoimmun. 43, 78–84 (2013).
Coit, P. et al. Epigenetic reprogramming in naive CD4+ T cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares. Arth. Rheumatol. 68, 2200–2209 (2016).
Stanford, S. M. & Bottini, N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 10, 602–611 (2014).
Mustelin, T., Bottini, N. & Stanford, S. M. The contribution of PTPN22 to rheumatic disease. Arth. Rheumatol. 71, 486–495 (2019).
Tizaoui, K. et al. The role of PTPN22 in the pathogenesis of autoimmune diseases: a comprehensive review. Semin. Arth. Rheum. 51, 513–522 (2021).
Xie, J. et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 11, 1600 (2020).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364, 616–626 (2011).
Catalina, M. D. et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight https://doi.org/10.1172/jci.insight.140380 (2020).
Langefeld, C. D. et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat. Commun. 8, 16021 (2017).
Menard, L. C. et al. B cells from African American lupus patients exhibit an activated phenotype. JCI Insight 1, e87310 (2016).
Owen, K. A. et al. Analysis of trans-ancestral SLE risk loci identifies unique biologic networks and drug targets in African and European ancestries. Am. J. Hum. Genet. 107, 864–881 (2020).
Tesar, V. & Hruskova, Z. Lupus nephritis: a different disease in European patients. Kidney Dis. 1, 110–118 (2015).
Isenberg, D. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology 49, 128–140 (2010).
Yusuf, A. A., Govender, M. A., Brandenburg, J. T. & Winkler, C. A. Kidney disease and APOL1. Hum. Mol. Genet. 30, R129–r137 (2021).
Omarjee, O. et al. Monogenic lupus: dissecting heterogeneity. Autoimmun. Rev. 18, 102361 (2019).
Zipfel, P. F., Wiech, T., Stea, E. D. & Skerka, C. CFHR gene variations provide insights in the pathogenesis of the kidney diseases atypical hemolytic uremic syndrome and C3 glomerulopathy. J. Am. Soc. Nephrol. 31, 241–256 (2020).
Crow, Y. J. & Stetson, D. B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 22, 471–483 (2022).
Husebye, E. S., Anderson, M. S. & Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 1132–1141 (2018).
Ferre, E. M. et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy. JCI Insight https://doi.org/10.1172/jci.insight.88782 (2016).
Barzaghi, F. & Passerini, L. IPEX syndrome: improved knowledge of immune pathogenesis empowers diagnosis. Front. Pediatr. 9, 612760 (2021).
Cepika, A. M. et al. Tregopathies: monogenic diseases resulting in regulatory T-cell deficiency. J. Allergy Clin. Immunol. 142, 1679–1695 (2018).
Lundtoft, C. et al. Complement C4 copy number variation is linked to SSA/Ro and SSB/La autoantibodies in systemic inflammatory autoimmune diseases. Arth. Rheumatol. 74, 1440–1450 (2022).
Hoshino, A. et al. Identification of autoantibodies using human proteome microarrays in patients with IPEX syndrome. Clin. Immunol. 203, 9–13 (2019).
Lampasona, V. et al. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One 8, e78664 (2013).
Souyris, M., Mejía, J. E., Chaumeil, J. & Guéry, J. C. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 41, 153–164 (2019).
Nusbaum, J. S. et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin. Proc. 95, 384–394 (2020).
Webb, K. et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front. Immunol. 9, 3167 (2018).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. Usa. 111, 869–874 (2014).
Fischinger, S., Boudreau, C. M., Butler, A. L., Streeck, H. & Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 41, 239–249 (2019).
Scofield, R. H. et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arth. Rheum. 58, 2511–2517 (2008).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Bergstra, S. A. et al. Sex-associated treatment differences and their outcomes in rheumatoid arthritis: results from the METEOR register. J. Rheumatol. 45, 1361–1366 (2018).
Warram, J. H., Krolewski, A. S., Gottlieb, M. S. & Kahn, C. R. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N. Engl. J. Med. 311, 149–152 (1984).
Bonifacio, E., Warncke, K., Winkler, C., Wallner, M. & Ziegler, A. G. Cesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes 60, 3300–3306 (2011).
Dinse, G. E. et al. Increasing prevalence of antinuclear antibodies in the United States. Arth. Rheumatol. 72, 1026–1035 (2020).
Hermann, R. et al. Temporal changes in the frequencies of HLA genotypes in patients with type 1 diabetes — indication of an increased environmental pressure? Diabetologia 46, 420–425 (2003).
Gillespie, K. M. et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 364, 1699–1700 (2004).
Miller, A. L., Bessho, S., Grando, K. & Tükel, Ç. Microbiome or infections: amyloid-containing biofilms as a trigger for complex human diseases. Front. Immunol. 12, 638867 (2021).
Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18, 521–538 (2020).
Ogunrinde, E. et al. A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arth. Rheumatol. 71, 1858–1868 (2019).
Jog, N. R. & James, J. A. Epstein Barr virus and autoimmune responses in systemic lupus erythematosus. Front. Immunol. 11, 623944 (2020).
Manasson, J., Blank, R. B. & Scher, J. U. The microbiome in rheumatology: where are we and where should we go? Ann. Rheum. Dis. 79, 727–733 (2020).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Artacho, A. et al. The pretreatment gut microbiome is associated with lack of response to methotrexate in new-onset rheumatoid arthritis. Arth. Rheumatol. 73, 931–942 (2021).
Azzouz, D. et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 78, 947–956 (2019).
Pianta, A. et al. Identification of novel, immunogenic HLA-DR-presented Prevotella copri peptides in patients with rheumatoid arthritis. Arth. Rheumatol. 73, 2200–2205 (2021).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Yang, Y. et al. Within-host evolution of a gut pathobiont facilitates liver translocation. Nature 607, 563–570 (2022).
Mariño, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Zegarra-Ruiz, D. F. et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 25, 113–127.e116 (2019).
McPherson, A. C., Pandey, S. P., Bender, M. J. & Meisel, M. Systemic immunoregulatory consequences of gut commensal translocation. Trends Immunol. 42, 137–150 (2021).
Milligan, G., Shimpukade, B., Ulven, T. & Hudson, B. D. Complex pharmacology of free fatty acid receptors. Chem. Rev. 117, 67–110 (2017).
Brown, J., Robusto, B. & Morel, L. Intestinal dysbiosis and tryptophan metabolism in autoimmunity. Front. Immunol. 11, 1741 (2020).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
Meier, H. C. S. et al. Hygiene hypothesis indicators and prevalence of antinuclear antibodies in US adolescents. Front. Immunol. 13, 789379 (2022).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arth. Rheum. 54, 38–46 (2006).
Tiniakou, E. & Christopher-Stine, L. Immune-mediated necrotizing myopathy associated with statins: history and recent developments. Curr. Opin. Rheumatol. 29, 604–611 (2017).
Mérida, E. & Praga, M. NSAIDs and nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 14, 1280–1282 (2019).
Burkett, J. B., Doran, A. C. & Gannon, M. Harnessing prostaglandin E(2) signaling to ameliorate autoimmunity. Trends Immunol. 44, 162–171 (2023).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 16, 535–548 (2019).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes. Curr. Opin. Neurol. 25, 795–801 (2012).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500 (2022).
Weinmann, S. C. & Pisetsky, D. S. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 58, vii59–vii67 (2019).
Olsen, N. J., Okuda, D. T., Holers, V. M. & Karp, D. R. Editorial: Understanding the concept of pre-clinical autoimmunity. Front. Immunol. 13, 983310 (2022).
Choi, M. Y. & Costenbader, K. H. Understanding the concept of pre-clinical autoimmunity: prediction and prevention of systemic lupus erythematosus: identifying risk factors and developing strategies against disease development. Front. Immunol. 13, 890522 (2022).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Sosenko, J. M. et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 36, 2615–2620 (2013).
Jacobsen, L. M. et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 63, 588–596 (2020).
Haville, S. & Deane, K. D. Pre-RA: can early diagnosis lead to prevention? Best. Pract. Res. Clin. Rheumatol. 36, 101737 (2022).
Munroe, M. E. et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arth. Rheumatol. 69, 630–642 (2017).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Slight-Webb, S. et al. Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J. Allergy Clin. Immunol. 146, 1419–1433 (2020).
So, M., O’Rourke, C., Bahnson, H. T., Greenbaum, C. J. & Speake, C. Autoantibody reversion: changing risk categories in multiple-autoantibody-positive individuals. Diab. Care 43, 913–917 (2020).
Powers, A. C. Type 1 diabetes mellitus: much progress, many opportunities. J. Clin. Investig. https://doi.org/10.1172/jci142242 (2021).
Dayan, C. M., Korah, M., Tatovic, D., Bundy, B. N. & Herold, K. C. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet 394, 1286–1296 (2019).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Perdigoto, A. L. et al. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62, 655–664 (2019).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Hirsch, J. S. FDA approves teplizumab: a milestone in type 1 diabetes. Lancet Diab. Endocrinol. 11, 18 (2023).
Deane, K. D. & Holers, V. M. Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift. Arth. Rheumatol. 73, 181–193 (2021).
Krijbolder, D. I. et al. Intervention with methotrexate in patients with arthralgia at risk of rheumatoid arthritis to reduce the development of persistent arthritis and its disease burden (TREAT EARLIER): a randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet 400, 283–294 (2022).
Arroyo, H. A. & Torres, A. R. Spontaneous remission in juvenile myasthenia gravis: a cohort of 13 cases and review of the literature. Neuromuscul. Disord. 32, 213–219 (2022).
Watanabe, S. et al. Spontaneous remission of thrombospondin type-1 domain-containing-associated membranous nephropathy. Intern. Med. 60, 3125–3128 (2021).
Dörner, T. & Lipsky, P. E. B cells: depletion or functional modulation in rheumatic diseases. Curr. Opin. Rheumatol. 26, 228–236 (2014).
Phalke, S. & Marrack, P. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr. Opin. Immunol. 55, 75–80 (2018).
Mouat, I. C., Goldberg, E. & Horwitz, M. S. Age-associated B cells in autoimmune diseases. Cell. Mol. Life Sci. 79, 402 (2022).
Vidal-Pedrola, G. et al. Characterization of age-associated B cells in early drug-naïve rheumatoid arthritis patients. Immunology https://doi.org/10.1111/imm.13598 (2022).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Smith, M. J., Cambier, J. C. & Gottlieb, P. A. Endotypes in T1D: B lymphocytes and early onset. Curr. Opin. Endocrinol. Diab. Obes. 27, 225–230 (2020).
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Amanna, I. J. & Slifka, M. K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 236, 125–138 (2010).
Hale, M., Rawlings, D. J. & Jackson, S. W. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr. Opin. Immunol. 55, 81–88 (2018).
Chang, H. D. et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 61, 86–91 (2019).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Ostendorf, L. et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N. Engl. J. Med. 383, 1149–1155 (2020).
Morris, A. D., Rowbottom, A. W., Martin, F. L., Woywodt, A. & Dhaygude, A. P. Biomarkers in ANCA-associated vasculitis: potential pitfalls and future prospects. Kidney360 2, 586–597 (2021).
Schur, P. H. & Sandson, J. Immunologic factors and clinical activity in systemic lupus erythematosus. N. Engl. J. Med. 278, 533–538 (1968).
McCarty, G. A., Rice, J. R., Bembe, M. L. & Pisetsky, D. S. Independent expression of autoantibodies in systemic lupus erythematosus. J. Rheumatol. 9, 691–695 (1982).
Schett, G. et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann. Rheum. Dis. 75, 1428–1437 (2016).
Wallace, D. J. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arth. Rheum. 61, 1168–1178 (2009).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arth. Rheum. 63, 3918–3930 (2011).
Pisetsky, D. S., Rovin, B. H. & Lipsky, P. E. New perspectives in rheumatology: biomarkers as entry criteria for clinical trials of new therapies for systemic lupus erythematosus: the example of antinuclear antibodies and anti-DNA. Arth. Rheumatol. 69, 487–493 (2017).
Ludwig, R. J. et al. Mechanisms of autoantibody-induced pathology. Front. Immunol. 8, 603 (2017).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Philip Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat Rev Nephrol 19, 509–524 (2023). https://doi.org/10.1038/s41581-023-00720-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00720-1
This article is cited by
-
The immunoregulatory roles of non-haematopoietic cells in the kidney
Nature Reviews Nephrology (2024)
-
Antigen-specific Fab profiling achieves molecular-resolution analysis of human autoantibody repertoires in rheumatoid arthritis
Nature Communications (2024)
-
Activation of human endogenous retroviruses and its physiological consequences
Nature Reviews Molecular Cell Biology (2024)
-
Immunosuppressive Cyclotides: A Promising Approach for Treating Autoimmune Diseases
The Protein Journal (2024)
-
The diagnostic role of the systemic inflammation index in patients with immunological diseases: a systematic review and meta-analysis
Clinical and Experimental Medicine (2024)