Abstract
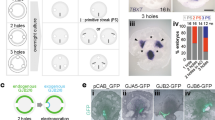
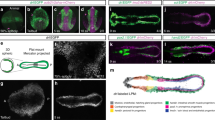
During gastrulation, a single epithelial cell layer, the ectoderm, generates two others: the mesoderm and the endoderm. In amniotes (birds and mammals), mesendoderm formation occurs through an axial midline structure, the primitive streak1, the formation of which is preceded by massive ‘polonaise’ movements2,3 of ectoderm cells. The mechanisms controlling these processes are unknown. Here, using multi-photon time-lapse microscopy of chick (Gallus gallus) embryos, we reveal a medio-lateral cell intercalation confined to the ectodermal subdomain where the streak will later form. This intercalation event differs from the convergent extension movements of the mesoderm described in fish and amphibians (anamniotes)4,5,6,7,8: it occurs before gastrulation and within a tight columnar epithelium. Fibroblast growth factor from the extraembryonic endoderm (hypoblast, a cell layer unique to amniotes) directs the expression of Wnt planar-cell-polarity pathway components to the intercalation domain. Disruption of this Wnt pathway causes the mesendoderm to form peripherally, as in anamniotes1,9. We propose that the amniote primitive streak evolved from the ancestral blastopore by acquisition of an additional medio-lateral intercalation event, preceding gastrulation and acting independently of mesendoderm formation to position the primitive streak at the midline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stern, C. D. Gastrulation: from cells to embryo (Cold Spring Harbor Laboratory Press, New York, 2004)
Gräper, L. Die Primitiventwicklung des Hünchens nach stereokinematographischen Untersuchungen, kontrolliert durch vitale Farbmarkierung und verglichen mit der Entwicklung anderer Wierbeltiere. Arch. EntwMech. Org. 116, 382–429 (1929)
Wetzel, R. Untersuchungen am Hühnchen. Die Entwicklung des Keims während der ersten beiden Bruttage. Arch. EntwMech. Org. 119, 188–321 (1929)
Heisenberg, C. P. et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 (2000)
Tada, M., Concha, M. L. & Heisenberg, C. P. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin. Cell Dev. Biol. 13, 251–260 (2002)
Wallingford, J. B., Fraser, S. E. & Harland, R. M. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2, 695–706 (2002)
Keller, R. & Davidson, L. in Gastrulation: from cells to embryo (ed. Stern, C. D.) 291–304 (Cold Spring Harbor Laboratory Press, New York, 2004)
Solnica-Krezel, L. Conserved patterns of cell movements during vertebrate gastrulation. Curr. Biol. 15, R213–R228 (2005)
Arendt, D. & Nubler-Jung, K. Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mech. Dev. 81, 3–22 (1999)
Wei, Y. & Mikawa, T. Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127, 87–96 (2000)
Cui, C., Yang, X., Chuai, M., Glazier, J. A. & Weijer, C. J. Analysis of tissue flow patterns during primitive streak formation in the chick embryo. Dev. Biol. 284, 37–47 (2005)
Chuai, M. et al. Cell movement during chick primitive streak formation. Dev. Biol. 296, 137–149 (2006)
Lawson, A. & Schoenwolf, G. C. Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis 29, 188–195 (2001)
Eyal-Giladi, H. & Kochav, S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 49, 321–337 (1976)
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951)
Bodenstein, L. & Stern, C. D. Formation of the chick primitive streak as studied in computer simulations. J. Theor. Biol. 233, 253–269 (2005)
Glickman, N. S., Kimmel, C. B., Jones, M. A. & Adams, R. J. Shaping the zebrafish notochord. Development 130, 873–887 (2003)
Klein, T. J. & Mlodzik, M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 21, 155–176 (2005)
Medina, A., Reintsch, W. & Steinbeisser, H. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev. 92, 227–237 (2000)
Skromne, I. & Stern, C. D. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128, 2915–2927 (2001)
Rothbächer, U. et al. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19, 1010–1022 (2000)
Domingos, P. M. et al. The Wnt/β-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev. Biol. 239, 148–160 (2001)
Foley, A. C., Skromne, I. & Stern, C. D. Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127, 3839–3854 (2000)
Bertocchini, F. & Stern, C. D. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev. Cell 3, 735–744 (2002)
Waddington, C. H. Experiments on the development of chick and duck embryos cultivated in vitro . Phil. Trans. R. Soc. Lond. B 221, 179–230 (1932)
Schoenwolf, G. C. & Smith, J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development 109, 243–270 (1990)
Wang, J. et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133, 1767–1778 (2006)
Ybot-Gonzalez, P. et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789–799 (2007)
Hertwig, O. Lehrbuch der Entwicklungsgeschichte des Menschen und der Wirbeltiere (Verlag Gustav Fischer, Jena, 1910)
Pasteels, J. Un aperçu comparitif de la gastrulation chez les Chordés. Biol. Rev. Camb. Philos. Soc. 15, 59–106 (1940)
Acknowledgements
We thank C. Formstone, C. Marcelle, M. Tada, N. Itasaki and V. Papaioannou for reagents; C. Thrasivoulou for advice on imaging; A. Nieto for sharing unpublished information and S. Fraser, M. Tada and A. Streit for comments on the manuscript. This study was funded by grants from the BBSRC, the Medical Research Council and the European Union FP6 Network of Excellence ‘Cells into Organs’. O.V. was supported by an HFSP fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures S5-S6 with Legends, Supplementary Methods and Legends to Supplementary Videos S1-S4. (PDF 1744 kb)
Supplementary Video S1
This file contains Supplementary Video S1 which shows chick embryo development and cell movements at stages XIII to 5. (MOV 8472 kb)
Supplementary Video S2
This file contains Supplementary Video S2 which shows cell divisions during primitive streak formation. (MOV 339 kb)
Supplementary Video S3
This file contains Supplementary Video S3 which shows cell movements during formation of the primitive streak. (MOV 1328 kb)
Supplementary Video S4
This file contains Supplementary Video S4 which shows relative movements of cells during formation of the primitive streak. (MOV 883 kb)
Rights and permissions
About this article
Cite this article
Voiculescu, O., Bertocchini, F., Wolpert, L. et al. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449, 1049–1052 (2007). https://doi.org/10.1038/nature06211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06211
This article is cited by
-
Self-enhanced mobility enables vortex pattern formation in living matter
Nature (2024)
-
Basal stem cell progeny establish their apical surface in a junctional niche during turnover of an adult barrier epithelium
Nature Cell Biology (2023)
-
Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure
Cellular and Molecular Life Sciences (2022)
-
Exploring the roles of FGF/MAPK and cVG1/GDF signalling on mesendoderm induction and convergent extension during chick primitive streak formation
Development Genes and Evolution (2022)
-
Endocytosis in the context-dependent regulation of individual and collective cell properties
Nature Reviews Molecular Cell Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.