Abstract
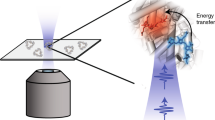
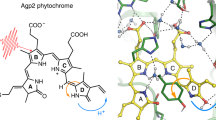
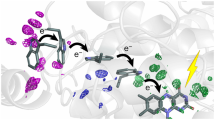
The role of conformational changes in explaining the huge catalytic power of enzymes is currently one of the most challenging questions in biology1,2,3,4,5,6,7. Although it is now widely regarded that enzymes modulate reaction rates by means of short- and long-range protein motions3,4,5,6,7, it is almost impossible to distinguish between conformational changes and catalysis. We have solved this problem using the chlorophyll biosynthetic enzyme NADPH:protochlorophyllide (Pchlide) oxidoreductase, which catalyses a unique light-driven reaction involving hydride and proton transfers8. Here we report that prior excitation of the enzyme-substrate complex with a laser pulse induces a more favourable conformation of the active site, enabling the coupled hydride and proton transfer reactions to occur. This effect, which is triggered during the Pchlide excited-state lifetime and persists on a long timescale, switches the enzyme into an active state characterized by a high rate and quantum yield of formation of a catalytic intermediate. The corresponding spectral changes in the mid-infrared following the absorption of one photon reveal significant conformational changes in the enzyme, illustrating the importance of flexibility and dynamics in the structure of enzymes for their function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Benkovic, S. J. & Hammes-Schiffer, S. A perspective on enzyme catalysis. Science 301, 1196–1202 (2003)
Villà, J. & Warshel, A. Energetics and dynamics of enzymatic reactions. J. Phys. Chem. B 105, 7887–7907 (2001)
Masgrau, L. et al. Atomic description of an enzyme reaction dominated by proton tunneling. Science 312, 237–241 (2006)
Kohen, A., Cannio, R., Bartolucci, S. & Klinman, J. P. Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature 399, 496–499 (1999)
Eisenmesser, E. Z. et al. Intrinsic dynamics of an enzyme underlies catalysis. Nature 438, 117–121 (2005)
Agarwal, P. K. Role of protein dynamics in reaction rate enhancement by enzymes. J. Am. Chem. Soc. 127, 15248–15256 (2005)
Wang, L., Goodey, N. M., Benkovic, S. J. & Kohen, A. Coordinated effects of distal mutations on environmentally coupled tunneling in dihydrofolate reductase. Proc. Natl Acad. Sci. USA 103, 15753–15758 (2006)
Heyes, D. J. & Hunter, C. N. Making light work of enzyme catalysis: protochlorophyllide oxidoreductase. Trends Biochem. Sci. 30, 642–649 (2005)
Eisenmesser, E. Z., Bosco, D. A., Akke, M. & Kern, D. Enzyme dynamics during catalysis. Science 295, 1520–1523 (2002)
Flomenbom, O. et al. Stretched exponential decay and correlations in the catalytic activity of fluctuating single lipase molecules. Proc. Natl Acad. Sci. USA 102, 2368–2372 (2005)
Boehr, D. D., McElheny, D., Dyson, H. J. & Wright, P. E. The dynamic energy landscape of dihydrofolate reductase catalysis. Science 313, 1638–1642 (2006)
Lebedev, N. & Timko, M. P. Protochlorophyllide photoreduction. Photosyn. Res. 58, 5–23 (1998)
Griffiths, W. T. Reconstruction of chlorophyllide formation by isolated etioplast membranes. Biochem. J. 174, 681–692 (1978)
Wilks, H. M. & Timko, M. P. A light-dependent complementation system for analysis of NADPH:protochlorophyllide oxidoreductase. Identification and mutagenesis of two conserved residues that are essential for enzyme activity. Proc. Natl Acad. Sci. USA 92, 724–728 (1995)
Valera, V., Fung, M., Wessler, A. N. & Richards, W. R. Synthesis of 4R- and 4S-tritium labeled NADPH for the determination of the coenzyme stereospecificity of NADPH-protochlorophyllide oxidoreductase. Biochem. Biophys. Res. Commun. 148, 515–520 (1987)
Begley, T. P. & Young, H. Protochlorophyllide reductase. 1. Determination of the regiochemistry and the stereochemistry of the reduction of protochlorophyllide to chlorophyllide. J. Am. Chem. Soc. 111, 3095–3096 (1989)
Heyes, D. J., Hunter, C. N., van Stokkum, I. H. M., van Grondelle, R. & Groot, M. L. Ultrafast enzymatic reaction dynamics in protochlorophyllide oxidoreductase. Nature Struct. Biol. 10, 491–492 (2003)
Heyes, D. J., Ruban, A. V., Wilks, H. M. & Hunter, C. N. Enzymology below 200K: the kinetics and thermodynamics of the photochemistry catalysed by protochlorophyllide oxidoreductase. Proc. Natl Acad. Sci. USA 99, 11145–11150 (2002)
Heyes, D. J. & Hunter, C. N. Identification and characterization of the product release steps within the catalytic cycle of protochlorophyllide oxidoreductase. Biochemistry 43, 8265–8271 (2004)
Heyes, D. J. et al. The first catalytic step of the light-driven enzyme protochlorophyllide oxidoreductase proceeds via a charge transfer complex. J. Biol. Chem. 281, 26847–26853 (2006)
Heyes, D. J., Ruban, A. V. & Hunter, C. N. protochlorophyllide oxidoreductase: ‘Dark’ reactions of a light-driven enzyme. Biochemistry 42, 523–528 (2003)
Zhao, G.-J. & Han, K.-L. Site-specific solvation of the photoexcited protochlorophyllide a in methanol: formation of the hydrogen-bonded intermediate state induced by hydrogen-bond strengthening. Biophys. J. 94, 38–46 (2008)
Lu, H. P., Xun, L. & Xie, X. S. Single-molecule enzymatic dynamics. Science 282, 1877–1882 (1998)
Townley, H. E., Sessions, R. B., Clarke, A. R., Dafforn, T. R. & Griffiths, W. T. Protochlorophyllide oxidoreductase: A homology model examined by site-directed mutagenesis. Proteins 44, 329–335 (2001)
Groot, M. L., Breton, J., van Wilderen, L. J. G. W., Dekker, J. P. & van Grondelle, R. Femtosecond visible/visible and visible/mid-IR pump-probe study of the photosystem II core antenna complex CP47. J. Phys. Chem. B 108, 8001–8006 (2004)
van Stokkum, I. H. M., Larsen, D. S. & van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004)
Barth, A. & Zscherp, C. What vibrations tell us about proteins. Q. Rev. Biophys. 35, 369–430 (2002)
Iwaki, M., Cotton, N. P., Quirk, P. G., Rich, P. R. & Jackson, J. B. Molecular recognition between protein and nicotinamide dinucleotide in intact, proton-translocating transhydrogenase studied by ATR-FTIR Spectroscopy. J. Am. Chem. Soc. 128, 2621–2629 (2006)
Nabedryk, E., Leonhard, M., Mäntele, W. & Breton, J. Fourier transform infrared difference spectroscopy shows no evidence for an enolization of chlorophyll a upon cation formation either in vitro or during P700 photooxidation. Biochemistry 29, 3242–3247 (1990)
Hartwich, G., Geskes, C., Scheer, H., Heinze, J. & Maentele, W. Fourier transform infrared spectroscopy of electrogenerated anions and cations of metal-substituted bacteriochlorophyll a. J. Am. Chem. Soc. 117, 7784–7790 (1995)
Mäntele, W. G., Wollenweber, A. M., Nabedryk, E. & Breton, J. Infrared spectroelectrochemistry of bacteriochlorophylls and bacteriopheophytins: Implications for the binding of the pigments in the reaction center from photosynthetic bacteria. Proc. Natl Acad. Sci. USA 85, 8468–8472 (1988)
Acknowledgements
This research was supported by The Netherlands Organization for Scientific Research through the Dutch Foundation for Earth and Life Sciences (Investment Grant no. 834.01.002). M.L.G. is grateful to NWO-ALW for providing financial support with a long-term fellowship (Grant no. 831.00.004) and O.S. received support from NWO-CW (Grant no. 700.53.307). D.J.H. and C.N.H. gratefully acknowledge support from the Biotechnology and Biological Sciences Research Council, UK. We thank H. Fidder and F. van Mourik for reading the manuscript.
Author Contributions O.A.S., D.J.H., M.T.A. and M.L.G. produced the samples and performed all of the experiments. O.A.S., I.H.M.v.S. and M.L.G. analysed the data. D.J.H., C.N.H., R.v.G. and M.L.G. coordinated the study, designed the experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S6 with Legends. (PDF 852 kb)
Rights and permissions
About this article
Cite this article
Sytina, O., Heyes, D., Hunter, C. et al. Conformational changes in an ultrafast light-driven enzyme determine catalytic activity. Nature 456, 1001–1004 (2008). https://doi.org/10.1038/nature07354
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07354
This article is cited by
-
Photocatalysis as the ‘master switch’ of photomorphogenesis in early plant development
Nature Plants (2021)
-
Elaborating and modulating the excited state intramolecular proton transfer behavior for 2-benzothiazole-2-yl-5-hex-1-ynyl-phenol
Theoretical Chemistry Accounts (2020)
-
Consensus model of a cyanobacterial light-dependent protochlorophyllide oxidoreductase in its pigment-free apo-form and photoactive ternary complex
Communications Biology (2019)
-
NADPH:protochlorophyllide oxidoreductase B (PORB) action in Arabidopsis thaliana revisited through transgenic expression of engineered barley PORB mutant proteins
Plant Molecular Biology (2017)
-
Theoretical Study of the ESIPT Process for a New Natural Product Quercetin
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.