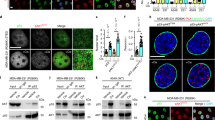
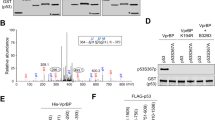
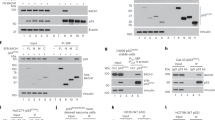
Abstract
Only a few p53 regulators have been shown to participate in the selective control of p53-mediated cell cycle arrest or apoptosis. How p53-mediated apoptosis is negatively regulated remains largely unclear. Here we report that Apak (ATM and p53-associated KZNF protein), a Krüppel-associated box (KRAB)-type zinc-finger protein, binds directly to p53 in unstressed cells, specifically downregulates pro-apoptotic genes, and suppresses p53-mediated apoptosis by recruiting KRAB-box-associated protein (KAP)-1 and histone deacetylase 1 (HDAC1) to attenuate the acetylation of p53. Apak inhibits p53 activity by interacting with ATM, a previously identified p53 activator. In response to stress, Apak is phosphorylated by ATM and dissociates from p53, resulting in activation of p53 and induction of apoptosis. These findings revealed Apak to be a negative regulator of p53-mediated apoptosis and showed the dual role of ATM in p53 regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Laptenko, O. & Prives, C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 13, 951–961 (2006).
Braithwaite, A. W., Del Sal, G. & Lu, X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ. 13, 984–993 (2006).
Das, S., et al. Hzf determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell 130, 624–637 (2007).
Tanaka, T., Ohkubo, S., Tatsuno, I. & Prives, C. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell 130, 638–650 (2007).
Trigiante, G. & Lu, X. ASPP and cancer. Nature Rev. Cancer 6, 217–226 (2006).
Sullivan, A. & Lu, X. ASPP: a new family of oncogenes and tumour suppressor genes. Br. J. Cancer 96, 196–200 (2007).
Huntley, S. et al. A comprehensive catalog of human KRAB-associated zinc finger genes: insights into the evolutionary history of a large family of transcriptional repressors. Genome Res. 16, 669–677 (2006).
Friedman, J. R. et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 10, 2067–2078 (1996).
Urrutia, R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 4, 231 (2003).
Peng, H. et al. Reconstitution of the KRAB-KAP-1 repressor complex: a model system for defining the molecular anatomy of RING-B box-coiled-coil domain-mediated protein-protein interactions. J. Mol. Biol. 295, 1139–1162 (2000).
Schultz, D. C., Friedman, J. R. & Rauscher, F. J. 3rd. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2α subunit of NuRD. Genes Dev. 15, 428–443 (2001).
Schultz, D. C., Ayyanathan, K., Negorev, D., Maul, G. G. & Rauscher, F. J. 3rd. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of enchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16, 919–932 (2002).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Ziv, Y. et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nature Cell Biol. 8, 870–876 (2006).
Wang, J. et al. Protein interaction data set highlighted with human Ras-MAPK/PI3K signaling pathways. J. Proteome Res. 7, 3879–3889 (2008).
Shen, Z., Pardington-Purtymun P. E., Comeaux, J. C., Moyzis, R. K. & Chen, D. J. Associations of UBE2I with RAD52, UBL1, p53, and RAD51 proteins in a yeast two-hybrid system. Genomics 37, 183–186 (1996).
Kahyo, T., Nishida, T. & Yasuda, H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol. Cell 8, 713–718 (2001).
Schmidt, D. & Muller, S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc. Natl Acad. Sci. USA 99, 2872–2877 (2002).
Kim, S. T., Lim, D. S., Canman, C. E. & Kastan, M. B. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 274, 37538–37543 (1999).
Tang, Y., Zhao, W., Chen, Y., Zhao, Y. & Gu, W. Acetylation is indispensable for p53 activation. Cell 133, 612–626 (2008).
Luo, J., Su, F., Chen, D., Shiloh, A. & Gu, W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408, 377–381 (2000).
Luo, J. et al. Negative control of p53 by Sir2a promotes cell survival under stress. Cell 107, 137–148 (2001).
Ito, A. et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20, 1331–1340 (2001).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Wang, C. et al. MDM2 interaction with nuclear corepressor KAP-1 contributes to p53 inactivation. EMBO J. 24, 3279–3290 (2005).
Zhang, L. et al. CKIP-1 recruits nuclear ATM partially to the plasma membrane through interaction with ATM. Cell. Signal. 18, 1386–1395 (2006).
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004).
Khosravi, R. et al. Rapid ATM-dependent phosphorylation of Mdm2 precedes p53 accumulation in response to DNA damage. Proc. Natl Acad. Sci. USA 96, 14973–14977 (1999).
Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677 (1998).
Li, J. et al. ZNF307, a novel zinc finger gene suppresses p53 and p21 pathway. Biochem. Biophys. Res. Commun. 363, 895–900 (2007).
Rui, Y. et al. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 23, 4583–4594 (2004).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Li, L. et al. PACT is a negative regulator of p53 and essential for cell growth and embryonic development. Proc. Natl Acad. Sci. USA 104, 7951–7956 (2007).
Liu, X. et al. JAB1 accelerates mitochondrial apoptosis by interaction with proapoptotic BclGs. Cell. Signal. 20, 230–240 (2008).
Lu, K. et al. Targeting of WW domains linker of HECT type ubiquitin ligase Smurf1 for activation by CKIP-1. Nature Cell Biol. 10, 994–1002 (2008).
Li, L. et al. NuSAP is degraded by APC/C-Cdh1 and its overexpression results in mitotic arrest dependent of its microtubules' affinity. Cell. Signal. 19, 2046–2055 (2007).
Andersen, J. S. et al. Nucleolar proteome dynamics. Nature 433, 77–83 (2005).
Zhang, L. et al. The PH domain containing protein CKIP-1 binds to IFP35 and Nmi and is involved in cytokine signaling. Cell. Signal. 19, 932–944 (2007).
Wang, J. et al. ATM-dependent CHK2 activation induced by anticancer agent, irofulven. J. Biol. Chem. 279, 39584–39592 (2004).
Kurki, S. et al. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell 5, 465–475 (2004).
Shiotani, B. et al. Involvement of the ATR- and ATM-dependent checkpoint responses in cell cycle arrest evoked by pierisin-1. Mol. Cancer Res. 4, 125–133 (2006).
Xie, P. et al. Histone methyltransferase protein SETD2 interacts with p53 and selectively regulates its downstream genes. Cell. Signal. 20, 1671–1678 (2008).
Huang, J. et al. Repression of p53 activity by Smyd2-mediated methylation. Nature 444, 629–632 (2006).
Hermeking, H. et al. 14-3-3σ is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1, 3–11 (1997).
Kastan, M. B. et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71, 587–597 (1992).
Shi, X. et al. Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol. Cell 27, 636–646 (2007).
Kaeser, M. D. & Iggo, R. D. Chromatin immunoprecipitation analysis fails to support the latency model for regulation of p53 DNA binding activity in vivo. Proc. Natl Acad. Sci. USA 99, 95–100 (2002).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Cliby, W. A. et al. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17, 159–169 (1998).
Acknowledgements
We are grateful to Bert Vogelstein, Michael B. Kastan, Qimin Zhan, Wei Gu, Jiandong Chen, Yue Xiong, Shengcai Lin, Gang Pei and Weiguo Zhu for providing materials; Yi Tie, Heping Pan, Qiao Sun, Juntao Yang, Xiushan Yin, Dahu Li, Bo Dong, Shaofei Zhong, Bin Li and Yanzhi Yuan for technical assistance; and Wei Gu, Yue Xiong, Shengcai Lin, Zigang Dong, Yi Rao and Chengrong Lu for critical reading of the manuscript. This study was supported by Chinese National Basic Research Programs 2007CB914601 (L.Z.) and 2006CB910802 (F.H., L.Z.), Chinese National Natural Science Foundation Projects 30621063 (F.H.) and 30600310, 30871373 (C.T.), and the Beijing Science and Technology NOVA Program 2007A063 (L.Z.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1790 kb)
Rights and permissions
About this article
Cite this article
Tian, C., Xing, G., Xie, P. et al. KRAB-type zinc-finger protein Apak specifically regulates p53-dependent apoptosis. Nat Cell Biol 11, 580–591 (2009). https://doi.org/10.1038/ncb1864
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1864
This article is cited by
-
Genome-wide analysis toward the epigenetic aetiology of myelodysplastic syndrome disease progression and pharmacoepigenomic basis of hypomethylating agents drug treatment response
Human Genomics (2023)
-
ZNF498 promotes hepatocellular carcinogenesis by suppressing p53-mediated apoptosis and ferroptosis via the attenuation of p53 Ser46 phosphorylation
Journal of Experimental & Clinical Cancer Research (2022)
-
ZNF322A-mediated protein phosphorylation induces autophagosome formation through modulation of IRS1-AKT glucose uptake and HSP-elicited UPR in lung cancer
Journal of Biomedical Science (2020)
-
KRAB-type zinc-finger proteins PITA and PISA specifically regulate p53-dependent glycolysis and mitochondrial respiration
Cell Research (2018)
-
The complexity of TRIM28 contribution to cancer
Journal of Biomedical Science (2017)