Abstract
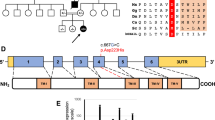
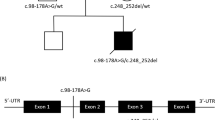
Mammalian cytochrome c oxidase (COX) catalyses the transfer of reducing equivalents from cytochrome c to molecular oxygen and pumps protons across the inner mitochondrial membrane1. Mitochondrial DNA (mtDNA) encodes three COX subunits (I–III) and nuclear DNA (nDNA) encodes ten. In addition, ancillary proteins are required for the correct assembly and function of COX (refs 2, 3, 4, 5, 6). Although pathogenic mutations in mtDNA-encoded COX subunits have been described7, no mutations in the nDNA-encoded subunits have been uncovered in any mendelian-inherited COX deficiency disorder8,9,10,11,12,13. In yeast, two related COX assembly genes, SCO1 and SCO2 (for synthesis of cytochrome c oxidase), enable subunits I and II to be incorporated into the holoprotein. Here we have identified mutations in the human homologue, SCO2, in three unrelated infants with a newly recognized fatal cardioencephalomyopathy and COX deficiency. Immunohistochemical studies implied that the enzymatic deficiency, which was most severe in cardiac and skeletal muscle, was due to the loss of mtDNA-encoded COX subunits. The clinical phenotype caused by mutations in human SCO2 differs from that caused by mutations in SURF1, the only other known COX assembly gene associated with a human disease, Leigh syndrome14,15.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Michel, H., Behr, J., Harrenga, A. & Kannt, A. Cytochrome c oxidase: structure and spectroscopy. Annu. Rev. Biophys. Biomol. Struct. 27, 329–356 ( 1998).
Pel, H.J., Tzagoloff, A. & Grivell, L.A. The identification of 18 nuclear genes required for the expression of the yeast mitochondrial gene encoding cytochrome c oxidase subunit 1. Curr. Genet. 21, 139– 146 (1992).
Glerum, D.M. & Tzagoloff, A. Isolation of a human cDNA for heme A:farnesyltransferase by functional complementation of a yeast cox10 mutant. Proc. Natl Acad. Sci. USA 91, 8452–8456 (1994).
Petruzzella, V. et al. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics 15, 494– 504 (1998).
Amaravadi, R., Glerum, D.M. & Tzagoloff, A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum. Genet. 99, 329–333 (1997).
Bonnefoy, N. et al. Cloning of a human gene involved in cytochrome oxidase assembly by functional complementation of an oxa1– mutation in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 91, 11978–11982 (1994).
Schon, E.A., Bonilla, E. & DiMauro, S. Mitochondrial DNA mutations and pathogenesis. J. Bioenerg. Biomembr. 29, 131–149 (1997).
DiMauro, S. et al. Fatal infantile mitochondrial myopathy and renal dysfunction due to cytochrome-c-oxidase deficiency. Neurology 30 , 795–804 (1980).
DiMauro, S. et al. Benign infantile mitochondrial myopathy due to reversible cytochrome c oxidase deficiency. Ann. Neurol. 14, 226–234 (1983).
Lombes, A. et al. Biochemical and molecular analysis of cytochrome c oxidase deficiency in Leigh's syndrome. Neurology 41 , 491–498 (1991).
DiMauro, S., Hirano, M., Bonilla, E., Moraes, C.T. & Schon, E.A. Cytochrome oxidase deficiency: progress and problems. in Mitochondrial Disorders in Neurology (eds Schapira, A.H.V. & DiMauro, S.) 91–115 (Butterworth-Heinemann, Oxford, 1994).
Adams, P.L., Lightowlers, R.N. & Turnbull, D.M. Molecular analysis of cytochrome c oxidase deficiency in Leigh's syndrome. Ann. Neurol. 41, 268–270 (1997).
Jaksch, M. et al. A systematic mutation screen of 10 nuclear and 25 mitochondrial candidate genes in 21 patients with cytochrome c oxidase (COX) deficiency shows tRNA(Ser)(UCN) mutations in a subgroup with syndromal encephalopathy. J. Med. Genet. 35, 895– 900 (1998).
Zhu, Z. et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nature Genet. 20, 337–343 (1998).
Tiranti, V. et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 63, 1609–1621 (1998).
Buchwald, P., Krummeck, G. & Rödel, G. Immunological identification of yeast SCO1 protein as a component of the inner mitochondrial membrane. Mol. Gen. Genet. 229, 413–420 ( 1991).
Schulze, M. & Rödel, G. SCO1, a yeast nuclear gene essential for accumulation of mitochondrial cytochrome c oxidase subunit II. Mol. Gen. Genet. 211, 492– 498 (1988).
Schulze, M. & Rödel, G. Accumulation of the cytochrome c oxidase subunits I and II in yeast requires a mitochondrial membrane-associated protein, encoded by the nuclear SCO1 gene. Mol. Gen. Genet. 216, 37–43 ( 1989).
Krummeck, G. & Rödel, G. Yeast SCO1 protein is required for a post-translational step in the accumulation of mitochondrial cytochrome c oxidase subunits I and II. Curr. Genet. 18, 13–15 (1990).
Glerum, D.M., Shtanko, A. & Tzagoloff, A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae . J. Biol. Chem. 271, 20531– 20535 (1996).
Glerum, D.M., Shtanko, A. & Tzagoloff, A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 271, 14504–14509 ( 1996).
Rentzsch, A. et al. Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr. Genet. 35, 103–108 (1999).
McEwen, J.E., Ko, C., Kloeckner-Gruissem, B. & Poyton, R.O. Nuclear functions required for cytochrome c oxidase biogenesis in Saccharomyces cerevisiae. J. Biol. Chem. 261, 11872–11879 (1986).
DiMauro, S. et al. Cytochrome c oxidase deficiency in Leigh syndrome. Ann. Neurol. 22, 498–506 (1987).
Merante, F. et al. A biochemically distinct form of cytochrome oxidase (COX) deficiency in the Saguenay-Lac-Saint-Jean region of Quebec. Am. J. Hum. Genet. 53, 481–487 (1993).
Taanman, J.-W., Herzberg, N.H., De Vries, H., Bolhuis, P.A. & Van den Bogert, C. Steady-state transcript levels of cytochrome c oxidase genes during human myogenesis indicate subunit switching of subunit VIa and co-expression of subunit VIIa isoforms. Biochim. Biophys. Acta 1139, 155–162 (1992).
Preiss, T. & Lightowlers, R.N. Post-transcriptional regulation of tissue-specific isoforms. A bovine cytosolic RNA-binding protein, COLBP, associates with messenger RNA encoding the liver-form isopeptides of cytochrome c oxidase. J. Biol. Chem. 268, 10659 –10667 (1993).
Paret, C., Ostermann, K., Krause-Buchholz, U., Rentzsch, A. & Rödel, G. Human members of the SCO1 gene family: complementation analysis in yeast and intracellular localization. FEBS Lett. 447, 65–70 (1999).
Sciacco, M. & Bonilla, E. Cytochemistry and immunocytochemistry of mitochondria in tissue sections. Methods Enzymol. 264, 509–521 (1996).
Acknowledgements
We thank F. Guo, P. Kranz-Eberle, F. Pallotti, P. Magalhães, G. Manfredi, R. Pons and S. Tadesse for technical assistance; E. Holme, M. Huttermann, B. Kadenbach and M. Tulinius for patient samples; and A. Tzagoloff for communicating unpublished data. This work was supported by grants from the National Institutes of Health (NS28828, NS32527, NS11766, HL59657 and HD32062), the Muscular Dystrophy Association and a Neil Hamilton Fairley NHMRC Postdoctoral Fellowship (C.M.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papadopoulou, L., Sue, C., Davidson, M. et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet 23, 333–337 (1999). https://doi.org/10.1038/15513
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15513
This article is cited by
-
Dystonia and Parkinsonism in COA7-related disorders: expanding the phenotypic spectrum
Journal of Neurology (2023)
-
PTD-mediated delivery of α-globin chain into Κ-562 erythroleukemia cells and α-thalassemic (HBH) patients’ RBCs ex vivo in the frame of Protein Replacement Therapy
Journal of Biological Research-Thessaloniki (2021)
-
The function of Scox in glial cells is essential for locomotive ability in Drosophila
Scientific Reports (2021)
-
The regulatory roles of p53 in cardiovascular health and disease
Cellular and Molecular Life Sciences (2021)
-
The molecular mechanisms of copper metabolism and its roles in human diseases
Pflügers Archiv - European Journal of Physiology (2020)