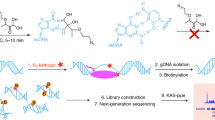
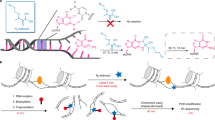
Abstract
4sUDRB-seq separately measures, on a genomic scale, the distinct contributions of transcription elongation speed and rate of RNA polymerase II (Pol II) transition into active elongation (TAE) to the overall mRNA production rate. It uses reversible inhibition of transcription elongation with 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB), combined with a pulse of 4-thiouridine (4sU), to tag newly transcribed RNA. After DRB removal, cells are collected at several time points, and tagged RNA is biotinylated, captured on streptavidin beads and sequenced. 4sUDRB-seq enables the comparison of elongation speeds between different developmental stages or different cell types, and it allows the impact of specific transcription factors on transcription elongation speed versus TAE to be studied. RNA preparation takes ∼4 d to complete, with deep sequencing requiring an additional ∼4–11 d plus 1–3 d for bioinformatics analysis. The experimental protocol requires basic molecular biology skills, whereas data analysis requires knowledge in bioinformatics, particularly MATLAB and the Linux environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, Z., Gerstein, M. & Snyder, M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Dori-Bachash, M., Shalem, O., Manor, Y.S., Pilpel, Y. & Tirosh, I. Widespread promoter-mediated coordination of transcription and mRNA degradation. Genome Biol. 13, R114 (2012).
Tani, H. & Akimitsu, N. Genome-wide technology for determining RNA stability in mammalian cells: historical perspective and recent advantages based on modified nucleotide labeling. RNA Biol. 9, 1233–1238 (2012).
Rabani, M. et al. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 29, 436–442 (2011).
Dolken, L. et al. High-resolution gene expression profiling for simultaneous kinetic parameter analysis of RNA synthesis and decay. RNA 14, 1959–1972 (2008).
Core, L.J., Waterfall, J.J. & Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Mondal, T., Rasmussen, M., Pandey, G.K., Isaksson, A. & Kanduri, C. Characterization of the RNA content of chromatin. Genome Res. 20, 899–907 (2010).
Wang, I.X. et al. RNA-DNA differences are generated in human cells within seconds after RNA exits polymerase II. Cell Rep. 6, 906–915 (2014).
Churchman, L.S. & Weissman, J.S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011).
Sims, R.J. III, Belotserkovskaya, R. & Reinberg, D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18, 2437–2468 (2004).
Fuchs, G. et al. 4sUDRB-seq: measuring genomewide transcriptional elongation rates and initiation frequencies within cells. Genome Biol. 15, R69 (2014).
Singh, J. & Padgett, R.A. Rates of in situ transcription and splicing in large human genes. Nat. Struct. Mol. Biol. 16, 1128–1133 (2009).
Melvin, W.T., Milne, H.B., Slater, A.A., Allen, H.J. & Keir, H.M. Incorporation of 6-thioguanosine and 4-thiouridine into RNA. Application to isolation of newly synthesised RNA by affinity chromatography. Eur. J. Biochem. 92, 373–379 (1978).
Fuchs, G. et al. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol. Cell 46, 662–673 (2012).
Chen, T. & Dent, S.Y. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet. 15, 93–106 (2014).
Bester, A.C. et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 145, 435–446 (2011).
Ward, P.S. & Thompson, C.B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Lin, C. et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol. Cell 37, 429–437 (2010).
Marazzi, I. et al. Suppression of the antiviral response by an influenza histone mimic. Nature 483, 428–433 (2012).
Ehrensberger, A.H., Kelly, G.P. & Svejstrup, J.Q. Mechanistic interpretation of promoter-proximal peaks and RNAPII density maps. Cell 154, 713–715 (2013).
Veloso, A. et al. Rate of elongation by RNA polymerase II is associated with specific gene features and epigenetic modifications. Genome Res. 24, 896–905 (2014).
Saponaro, M. et al. RECQL5 controls transcript elongation and suppresses genome instability associated with transcription stress. Cell 157, 1037–1049 (2014).
Jonkers, I., Kwak, H. & Lis, J.T. Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife 3, e02407 (2014).
Palangat, M. & Larson, D.R. Complexity of RNA polymerase II elongation dynamics. Biochim. Biophys. Acta 1819, 667–672 (2012).
Windhager, L. et al. Ultrashort and progressive 4sU tagging reveals key characteristics of RNA processing at nucleotide resolution. Genome Res. 22, 2031–2042 (2012).
Fuchs, G., Hollander, D., Voichek, Y., Ast, G. & Oren, M. Cotranscriptional histone H2B monoubiquitylation is tightly coupled with RNA polymerase II elongation rate. Genome Res. 24, 1572–1583 (2014).
Cleary, M.D., Meiering, C.D., Jan, E., Guymon, R. & Boothroyd, J.C. Biosynthetic labeling of RNA with uracil phosphoribosyltransferase allows cell-specific microarray analysis of mRNA synthesis and decay. Nat. Biotechnol. 23, 232–237 (2005).
Danko, C.G. et al. Signaling pathways differentially affect RNA polymerase II initiation, pausing, and elongation rate in cells. Mol. Cell 50, 212–222 (2013).
Acknowledgements
We thank D.R. Bublik, E. Kotler and L. Golomb for helpful discussions. This work was supported in part by grant 293438 (RUBICAN) from the European Research Council and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. M.O. is an incumbent of the Andre Lwoff chair in molecular biology.
Author information
Authors and Affiliations
Contributions
G.F., M.R. and M.O. developed the protocol; G.F., Y.V. and M.O. wrote the manuscript; Y.V., S.B., S.G. and I.A. contributed to protocol development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1: Primer sequences.
List of primers and their sequences used for qRT-PCR validation (Steps 16 and 62) examples shown in Figures 3 and 4. (XLSX 11 kb)
Supplementary Software: Scripts for bioinformatics analysis.
Compressed file containing the scripts: reads2chr.cc and find_boundary_4sUDRBseq.m, needed for the bioinformatics analysis (Steps 66-72). (ZIP 3 kb)
Rights and permissions
About this article
Cite this article
Fuchs, G., Voichek, Y., Rabani, M. et al. Simultaneous measurement of genome-wide transcription elongation speeds and rates of RNA polymerase II transition into active elongation with 4sUDRB-seq. Nat Protoc 10, 605–618 (2015). https://doi.org/10.1038/nprot.2015.035
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.035
This article is cited by
-
Temporal-iCLIP captures co-transcriptional RNA-protein interactions
Nature Communications (2023)
-
Ageing-associated changes in transcriptional elongation influence longevity
Nature (2023)
-
Using TTchem-seq for profiling nascent transcription and measuring transcript elongation
Nature Protocols (2020)
-
Pre‐mRNA modifications and their role in nuclear processing
Quantitative Biology (2018)
-
Model-based genome-wide determination of RNA chain elongation rates in Escherichia coli
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.