Key Points
-
Memory lymphocytes have an indispensable role in eradicating pathogens. They mediate their effects by responding more robustly with repeated infections.
-
Studies of immune memory have primarily focused on effector B cells and T cells. However, recent work has identified persistently expanded populations of antigen-specific regulatory T (TReg) cells.
-
It is hypothesized that memory TReg cells are generated to regulate memory effector responses and to mitigate collateral damage to tissues in the face of these robust immune reactions.
-
Memory TReg cells have been shown to have major roles in animal models of autoimmunity, antimicrobial host defence and maternal–fetal tolerance. In addition, there is evidence that these cells exist in humans.
-
Lack of definitive markers has hampered the phenotypic and the functional characterization of memory TReg cells. Comprehensive transcriptional profiling and detailed examination of epigenetic and metabolic signatures will be essential in defining the functional role that memory TReg cells have in both health and disease.
Abstract
Memory for antigen is a defining feature of adaptive immunity. Antigen-specific lymphocyte populations show an increase in number and function after antigen encounter and more rapidly re-expand upon subsequent antigen exposure. Studies of immune memory have primarily focused on effector B cells and T cells with microbial specificity, using prime–challenge models of infection. However, recent work has also identified persistently expanded populations of antigen-specific regulatory T cells that protect against aberrant immune responses. In this Review, we consider the parallels between memory effector T cells and memory regulatory T cells, along with the functional implications of regulatory memory in autoimmunity, antimicrobial host defence and maternal–fetal tolerance. In addition, we discuss emerging evidence for regulatory T cell memory in humans and key unanswered questions in this rapidly evolving field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chang, J. T., Wherry, E. J. & Goldrath, A. W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 15, 1104–1115 (2014).
Harty, J. T. & Badovinac, V. P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8, 107–119 (2008).
Wakim, L. M. & Bevan, M. J. From the thymus to longevity in the periphery. Curr. Opin. Immunol. 22, 274–278 (2010).
Homann, D., Teyton, L. & Oldstone, M. B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7, 913–919 (2001).
Seder, R. A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4, 835–842 (2003).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Gasper, D. J., Tejera, M. M. & Suresh, M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 34, 121–146 (2014).
Pepper, M. & Jenkins, M. K. Origins of CD4+ effector and central memory T cells. Nat. Immunol. 12, 467–471 (2011).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor FOXP3. Science 299, 1057–1061 (2003). This is a landmark paper that shows that FOXP3 is the master transcription factor driving development of T Reg cells.
Yamaguchi, T., Wing, J. B. & Sakaguchi, S. Two modes of immune suppression by FOXP3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 23, 424–430 (2011).
Rosenblum, M. D. et al. Response to self antigen imprints regulatory memory in tissues. Nature 480, 538–542 (2011). This work phenotypically and functionally defines memory T Reg cells in a mouse model of autoimmunity.
Rowe, J. H., Ertelt, J. M., Xin, L. & Way, S. S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012). This work phenotypically and functionally defines memory T Reg cells in a mouse model of fetal–maternal tolerance.
Gratz, I. K. et al. Cutting edge: self-antigen controls the balance between effector and regulatory T cells in peripheral tissues. J. Immunol. 192, 1351–1355 (2014). This paper shows that persistent self antigen expression in tissues leads to the preferential accumulation of T Reg cells instead of effector T cells. It also shows that memory T Reg cells can be generated from peripherally derived T Reg cells.
Brincks, E. L. et al. Antigen-specific memory regulatory CD4+FOXP3+ T cells control memory responses to influenza virus infection. J. Immunol. 190, 3438–3446 (2013).
Sanchez, A. M., Zhu, J., Huang, X. & Yang, Y. The development and function of memory regulatory T cells after acute viral infections. J. Immunol. Baltim. Md. 1950 189, 2805–2814 (2012). References 14 and 15 phenotypically and functionally define memory T Reg cells in a mouse model of infection.
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Cerottini, J. C. & MacDonald, H. R. The cellular basis of T-cell memory. Annu. Rev. Immunol. 7, 77–89 (1989).
Reinhardt, R. L., Bullard, D. C., Weaver, C. T. & Jenkins, M. K. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J. Exp. Med. 197, 751–762 (2003).
Kondrack, R. M. et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198, 1797–1806 (2003).
Lenz, D. C. et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl Acad. Sci. USA 101, 9357–9362 (2004).
Van, V. Q. et al. CD47low status on CD4 effectors is necessary for the contraction/resolution of the immune response in humans and mice. PLoS ONE 7, e41972 (2012).
Marshall, H. D. et al. Differential expression of LY6C and T-bet distinguish effector and memory TH1 CD4+ cell properties during viral infection. Immunity 35, 633–646 (2011).
Youngblood, B., Hale, J. S. & Ahmed, R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 139, 277–284 (2013).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Liu, T., Soong, L., Liu, G., König, R. & Chopra, A. K. CD44 expression positively correlates with FOXP3 expression and suppressive function of CD4+ TReg cells. Biol. Direct 4, 40 (2009).
Firan, M., Dhillon, S., Estess, P. & Siegelman, M. H. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood 107, 619–627 (2006).
Chen, X. & Oppenheim, J. J. Resolving the identity myth: key markers of functional CD4+FOXP3+ regulatory T cells. Int. Immunopharmacol. 11, 1489–1496 (2011).
Schmetterer, K. G., Neunkirchner, A. & Pickl, W. F. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 26, 2253–2276 (2012).
Gratz, I. K. et al. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013). This work shows that memory T Reg cells in mouse skin are dependent on IL-7 and not on IL-2.
Sanchez Rodriguez, R. et al. Memory regulatory T cells reside in human skin. J. Clin. Invest. 124, 1027–1036 (2014). The paper phenotypically characterizes memory T Reg cells in normal human skin and in the skin of patients with psoriasis.
Huang, H.-Y. & Luther, S. A. Expression and function of interleukin-7 in secondary and tertiary lymphoid organs. Semin. Immunol. 24, 175–189 (2012).
Morikawa, H. & Sakaguchi, S. Genetic and epigenetic basis of TReg cell development and function: from a FOXP3-centered view to an epigenome-defined view of natural TReg cells. Immunol. Rev. 259, 192–205 (2014).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the FOXP3 locus. Cell 158, 749–763 (2014).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a STAT3-dependent manner. Science 326, 986–991 (2009).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458, 351–356 (2009).
Weissler, K. A. & Caton, A. J. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of FOXP3+ regulatory T cells. Immunol. Rev. 259, 11–22 (2014).
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 14, 1007–1013 (2013).
Loblay, R. H., Pritchand-Briscoe, H. & Basten, A. Suppressor T-cell memory. Nature 272, 620–622 (1978). This is the first paper to define the phenomenon of regulatory memory.
Gratz, I. K. & Campbell, D. J. Organ-specific and memory TReg cells: specificity, development, function, and maintenance. Front. Immunol. 5, 333 (2014).
Hori, S., Haury, M., Coutinho, A. & Demengeot, J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl Acad. Sci. USA 99, 8213–8218 (2002).
MacLeod, M. K., Kappler, J. W. & Marrack, P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 130, 10–15 (2010).
Zhao, J. et al. IFN-γ- and IL-10-expressing virus epitope-specific FOXP3+ TReg cells in the central nervous system during encephalomyelitis. J. Exp. Med. 208, 1571–1577 (2011).
Shafiani, S. et al. Pathogen-specific TReg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity 38, 1261–1270 (2013).
Johanns, T. M., Ertelt, J. M., Rowe, J. H. & Way, S. S. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 6, e1001043 (2010).
Jiang, T. T. et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J. Immunol. 192, 4949–4956 (2014).
Erlebacher, A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 13, 23–33 (2013).
Campbell, D. M., MacGillivray, I. & Carr-Hill, R. Pre-eclampsia in second pregnancy. Br. J. Obstet. Gynaecol. 92, 131–140 (1985).
Trupin, L. S., Simon, L. P. & Eskenazi, B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiol. Camb. Mass 7, 240–244 (1996).
Bianchi, D. W., Zickwolf, G. K., Weil, G. J., Sylvester, S. & DeMaria, M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc. Natl Acad. Sci. USA 93, 705–708 (1996).
Nelson, J. L. The otherness of self: microchimerism in health and disease. Trends Immunol. 33, 421–427 (2012).
Kinder, J. M. et al. Pregnancy-induced maternal regulatory T cells, bona fide memory or maintenance by antigenic reminder from fetal cell microchimerism? Chimerism 5, 16–19 (2014).
Kinder, J. M. et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell 162, 505–515 (2015). This works shows that microchimeric maternal cells provide a source of cognate antigen required for sustaining the postnatal accumulation of memory T Reg cells with specificity for non-inherited maternal antigens in the offspring.
Mold, J. E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008).
Dutta, P. et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 114, 3578–3587 (2009).
Dutta, P. & Burlingham, W. J. Tolerance to noninherited maternal antigens in mice and humans. Curr. Opin. Organ. Transplant. 14, 439–447 (2009).
Uzonna, J. E., Wei, G., Yurkowski, D. & Bretscher, P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167, 6967–6974 (2001).
Belkaid, Y., Piccirillo, C. A., Mendez, S., Shevach, E. M. & Sacks, D. L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420, 502–507 (2002).
Nelson, R. W., McLachlan, J. B., Kurtz, J. R. & Jenkins, M. K. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J. Immunol. 190, 2828–2834 (2013).
Hermiston, M. L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 (2003).
Trowbridge, I. S. & Thomas, M. L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 12, 85–116 (1994).
Booth, N. J. et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J. Immunol. 184, 4317–4326 (2010).
Henson, S. M., Riddell, N. E. & Akbar, A. N. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 24, 476–481 (2012).
Sallusto, F., Mackay, C. R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FOXP3 transcription factor. Immunity 30, 899–911 (2009). This paper phenotypically and functionally defines resting and effector T Reg cells in human blood.
Seddiki, N. et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107, 2830–2838 (2006).
van der Geest, K. S. M. et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 60, 190–196 (2014).
Dong, S. et al. Multiparameter single-cell profiling of human CD4+FOXP3+ regulatory T-cell populations in homeostatic conditions and during graft-versus-host disease. Blood 122, 1802–1812 (2013).
Moriya, N., Sanjoh, K., Yokoyama, S. & Hayashi, T. Mechanisms of HLA-DR antigen expression in phytohemagglutinin-activated T cells in man. Requirement of T cell recognition of self HLA-DR antigen expressed on the surface of monocytes. J. Immunol. 139, 3281–3286 (1987).
Katzman, S. D. et al. Opposing functions of IL-2 and IL-7 in the regulation of immune responses. Cytokine 56, 116–121 (2011).
Pepper, M., Pagán, A. J., Igyártó, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the BCL-6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011).
Dooms, H., Wolslegel, K., Lin, P. & Abbas, A. K. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7Rα-expressing cells. J. Exp. Med. 204, 547–557 (2007).
Furtado, G. C., Curotto de Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. Interleukin-2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 196, 851–857 (2002).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014). This work phenotypically and functionally defines resting and effector T Reg cell subsets in mice. The authors show that resting T Reg cells are highly dependent on IL-2 for survival, whereas effector T Reg cells are dependent on signalling through ICOS.
Obar, J. J. et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J. Immunol. 187, 4967–4978 (2011).
Best, J. A. et al. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat. Immunol. 14, 404–412 (2013).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Oestreich, K. J. et al. BCL-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15, 957–964 (2014).
Banerjee, A. et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992 (2010).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Kallies, A., Xin, A., Belz, G. T. & Nutt, S. L. BLIMP1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Rutishauser, R. L. et al. Transcriptional repressor BLIMP1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Linterman, M. A. et al. FOXP3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Cretney, E. et al. The transcription factors BLIMP1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Vahedi, G. et al. STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993 (2012).
Zediak, V. P., Johnnidis, J. B., Wherry, E. J. & Berger, S. L. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J. Immunol. 186, 2705–2709 (2011).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Pearce, E. L., Poffenberger, M. C., Chang, C.-H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
van der Windt, G. J. W. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
O'Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TReg cells. J. Exp. Med. 208, 1367–1376 (2011).
Battaglia, M., Stabilini, A. & Roncarolo, M.-G. Rapamycin selectively expands CD4+CD25+FOXP3+ regulatory T cells. Blood 105, 4743–4748 (2005).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish TReg cell function. Nature 499, 485–490 (2013).
Coe, D. J., Kishore, M. & Marelli-Berg, F. Metabolic regulation of regulatory T cell development and function. Front. Immunol. 5, 590 (2014).
Powell, J. D., Heikamp, E. B., Pollizzi, K. N. & Waickman, A. T. A modified model of T-cell differentiation based on mTOR activity and metabolism. Cold Spring Harb. Symp. Quant. Biol. 78, 125–130 (2013).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16, 219–230 (2002).
Abbas, A. K. et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14, 307–308 (2013).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Zhang, Y., Joe, G., Hexner, E., Zhu, J. & Emerson, S. G. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 11, 1299–1305 (2005).
Farber, D. L., Yudanin, N. A. & Restifo, N. P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14, 24–35 (2014).
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Acknowledgements
S.S.W. is supported by the NIH through awards R01AI100934, R01AI120202 and R21AI112186, the March of Dimes Foundation and the Investigator in the Pathogenesis of Infectious Disease program from the Burroughs Wellcome Fund. M.D.R. is supported by the NIH through awards DP2AR068130, K08AR062064, R21AR066821 and UM1AI110498, by the Burroughs Wellcome Fund Career Award for Medical Scientists, the Scleroderma Research Foundation, the National Psoriasis Foundation and the Dermatology Foundation Stiefel Scholar Award in Autoimmune &/or Connective Tissue Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Memory TReg cells
-
Previously activated regulatory T (TReg) cells that persist in the absence of antigen expression or in the presence of intermittent low-level antigen expression. It is currently unknown whether central memory T cell, effector memory T cell or tissue-resident memory T cell subsets of memory TReg cells exist.
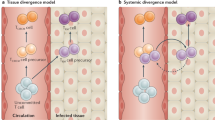
- Central memory T cells
-
(TCM cells). Generated in secondary lymphoid tissues and reside in secondary lymphoid tissues in the absence of antigen.
- Effector memory T cells
-
(TEM cells). Generated in secondary lymphoid tissues and recirculate between blood and non-lymphoid tissues in the absence of antigen.
- Tissue-resident memory T cells
-
(TRM cells). Generated in non-lymphoid tissues and stably reside in these tissues in the absence of antigen.
- Tissue-restricted self antigens
-
Self antigens that are expressed in specific tissues during defined periods of time. Hair follicle-associated antigens are an example of tissue-restricted self antigens in skin.
Rights and permissions
About this article
Cite this article
Rosenblum, M., Way, S. & Abbas, A. Regulatory T cell memory. Nat Rev Immunol 16, 90–101 (2016). https://doi.org/10.1038/nri.2015.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.1
This article is cited by
-
Identifying the sensor elements of regulatory T cells in atherosclerosis
Nature Cardiovascular Research (2024)
-
IL-23 stabilizes an effector Treg cell program in the tumor microenvironment
Nature Immunology (2024)
-
CD4+ T cell memory
Nature Immunology (2023)
-
IL-2 immunotherapy for targeting regulatory T cells in autoimmunity
Genes & Immunity (2023)
-
Soluble factors from TLR4- or TCR-activated cells contribute to stability of the resting phenotype and increase the expression of CXCR4 of human memory CD4 T cells
Immunologic Research (2023)