Abstract
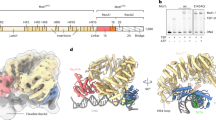
The three-dimensional structure of a TATA box-binding protein (TBP) from Arabidopsis thaliana complexed with a fourteen base pair oligonucleotide bearing the Adenovirus major late promoter TATA element has been refined at 1.9 Å resolution, giving a final crystallographic R-factor of 19.4%. Binding of the monomeric, saddle-shaped α/β protein induces an unprecedented conformational change in the DNA. A detailed structural and functional analysis of this unusual protein-DNA complex is presented, with particular emphasis on the mechanisms of DNA deformation, TATA element recognition, and preinitiation complex assembly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sawadogo, M. & Sentenac, A. RNA polymerase B (II) and general transcription factors. Rev. Biochem. 59, 711–754 (1990).
Zawel, L. & Reinberg, D. Advances in RNA polymerase II transcription. Curr. Opin. Cell Biol. 4, 488–495 (1992).
Kaufmann, J. & Smale, S.T Direct recognition of initiator elements by a component of the transcription factor IID complex. Genes Dev. 8, 821–829 (1994).
Purnell, B.A., Emanuel, P.A. & Gilmour, D.S. TFIID sequence recognition of the initiator and sequences farther downstream in Drosophila class II genes. Genes Dev. 8, 830–842 (1994).
Verrijzer, C., Yokomori, K., Chen, J.-L. & Tjian, R. Drosophila TAFII150: Similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science 264, 933–941 (1994).
Roeder, R.G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends biochem. Sci. 16, 402–408 (1991).
Nikolov, D.B. & Burley, S.K. 2.1 Å Resolution refined structure of a TATA box-binding protein. Nature struct. Biol. 1, 621–637 (1994).
Buratowski, S. & Zhow, H. Transcription factor IID mutants defective for interaction with transcription factor IIA. Science 255, 1030–1032 (1992).
Lee, D.K., DeJong, J., Hashimoto, S., Horikoshi, M. & Roeder, R.G. TFIIA induces conformational changes in TFIID via interactions with the basic repeat. Molec. Cell. Biol. 12, 5189–5196 (1992).
Ha, I. et al. Multiple functional domains of human transcription factor IIB: distinct interactions with two general transcription factors and RNA polymerase II. Genes Dev. 7, 1021–1032 (1993).
Usheva, A. et al. Specific interactions between the nonphosphorylated form of RNA polymerase II and the TATA-binding protein. Cell 69, 871–881 (1992).
Koleske, A.J., Buratowski, S., Nonet, M. & Young, R.A. A Novel transcription factor reveals a functional link between the RNA polymerase II CTD and TFIID. Cell 69, 883–894 (1992).
Hernandez, N. TBP, a universal transcription factor? Genes Dev. 7, 1291–1308 (1993).
Hahn, S., Buratowski, S., Sharp, P.A. & Guarente, L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc. natn. Acad. Sci. U.S.A. 86, 5718–5722 (1989).
Horikoshi, M. et al. Transcription factor TFIID induces DNA bending upon binding to the TATA element. Proc. natn. Acad. Sci. U.S.A. 89, 1060–1064 (1992).
Starr, D.B. & Hawley, D.K. TFIID binds in the minor groove of the TATA box. Cell 67, 1231–1240 (1991).
Lee, D.K., Horikoshi, M. & Roeder, R.G. Interaction of TFIID in the minor groove of the TATA element. Cell 67, 1241–1250 (1991).
Nikolov, D.B. et al. Crystal structure of TFIID TATA-box binding protein. Nature 360, 40–46 (1992).
Chasman, D.I., Flaherty, K.M., Sharp, P.A. & Kornberg, R.D. Crystal structure of yeast TATA-binding protein and model for interaction with DNA. Proc. natn. Acad. Sci. U.S.A. 90, 8174–8178 (1993).
Geiger, J.H., Kim, Y., Hahn, S. & Sigler, P.B. Crystal structure of yeast TBP at 2.1 Å resolution. Biochemistry 33, in the press (1994).
Kim, J.L., Nikolov, D.B. & Burley, S.K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 365, 520–527 (1993).
Kim, Y., Geiger, J.H., Hahn, S. & Sigler, P.B. Crystal structure of a yeast TBP/TATA-box complex. Nature 356, 512–520 (1993).
Nagawa, F. & Fink, G.R. The relationship between the “TATA” sequence and transcription initiation sites at the HIS4 gene of Saccharomyces cerevisiae. Proc. natn. Acad. Sci. U.S.A. 82, 8557–8561 (1985).
Luzzati, P.V. Traitement statistique des erreurs dans la determination des structures cristallines. Acta. crystallogr. 5, 802–810 (1952).
Lorch, Y. & Kornberg, R.D. Near-zero linking difference upon transcription factor IId binding to promoter DNA. Molec. Cell. Biol. 13, 1872–1875 (1993).
Nelson, H.C.M., Finch, J.T., Luisi, B.F. & Klug, A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature 330, 221–226 (1987).
DiGabriele, A.D., Sanderson, M.R. & Steitz, T.A. Crystal lattice packing is important in determining the bend of a DNA dodecamer containing an adenine tract. Proc. natn. Acad. Sci. U.S.A. 86, 1816–1820 (1989).
DiGabriele, A.D. & Steitz, T.A. A DNA dodecamer containing an adenine tract crystallizes in a unique lattice and exhibits a new bend. J. molec. Biol. 231, 1024–1039 (1993).
Aggarwal, A.K., Rodgers, D.W., Drottar, M., Ptashne, M. & Harrison, S.C. Recognition of a DNA operator by the represser of Phage 434: A view at high resolution. Science 242, 899–907 (1988).
Winkler, F.K. et al. The crystal structure of EcoRV endonuclease and of its complexes with cognate and non-cognate DNA fragments. EMBO J. 12, 1781–1795 (1993).
Suck, D., Lahm, A. & Oefner, C. Structure refined to 2 Å of a nicked DNA octanucleotide complex with DNase I. Nature 332, 464–468 (1988).
Hodel, A., Kim, S.-H. & Brunger, A.T. Model bias in macromolecular crystal structures. Acta crystallogr. A48, 851–858 (1992).
Yamamoto, T. et al. A bipartite DNA binding domain composed of direct repeats in the TATA box binding factor TFIID. Proc. natn. Acad. Sci. U.S.A. 89, 2844–2848 (1992).
Lilley, D.M.J. HMG has DNA wrapped up. Nature 357, 282–283 (1992).
Crothers, D.M. Architectural elements in nucleoprotein complexes. Curr. Biol. 3, 675–676 (1993).
Yang, C.-C. & Nash, H.A. The interaction of E. coli IHF protein with its specific binding sites. Cell 57, 869–880 (1989).
Pil, P.M., Chow, C.S. & Lippard, S.J. High-mobility-group 1 protein mediates DNA bending as determined by ring closures. Proc. natn. Acad. Sci. U.S.A. 90, 9465–9469 (1993).
Giese, K., Cox, J. & Grosschedl, R. The HMG domain of lympnoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell 69, 185–195 (1992).
Weir, H.M. et al. Structure of the HMG box motif in the B-domain of HMG-1. EMBO J. 12, 1311–1319 (1993).
Read, C.M., Cary, P.D., Crane-Robinson, C., Driscoll, P.C. & Norman, D.G. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 21, 3427–3436 (1993).
King, C.-Y. & Weiss, M.A. The SRY high-mobility-group box recognizes DNA by partial intercalation in the minor groove: A topological mechanism of sequence specificity. Proc. natn. Acad. Sci. U.S.A. 90, 11990–11994 (1993).
Tanaka, I., Appelt, K., Dijk, J., White, S.W. & Wilson, K.S. 3-Å resolution structure of a protein with histone-like properties in prokaryotes. Nature 310, 376–381 (1984).
Steitz, T.A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q. Rev. Biophys. 23, 205–280 (1990).
Harrison, S.C. A structural taxonomy of DNA-binding domains. Nature 353, 715–719 (1991).
Wolberger, C. Transcription Factor Structure and DNA Binding. Curr. Opin. struct. Biol. 3, 3–10 (1993).
Burley, S.K. DNA-binding motifs from eukaryotic transcription factors. Curr. Opin. struct. Biol. 4, 3–11 (1994).
Spolar, R. & Record, M.T., Jr Science 263, 777–784 (1994).
Ladbury, J., Wright, J., Sturtevant, J. & Sigler, P. A thermodynamic study of the trp repressor-operator interaction. J. molec. Biol. 238, 669–681 (1994).
Livingston, J., Spolar, R. & Record, M.T., Jr. Biochemistry 30, 4237–4244 (1991).
Hoopes, B.C., LeBlanc, J.F. & Hawley, D.K. Kinetic analysis of yeast TFIID-TATA box complex formation suggests a multi-step pathway. J. biol. Chem. 267, 11539–11546 (1992).
Shakked, Z. et al. Sequence-dependent conformation of an A-DNA double helix. J. molec. Biol. 166, 183–201 (1983).
Ornstein, R.L., Rein, R., Breen, D.L. & MacElroy, R.D. An optimized potential function for the calculation of nucleic acid interaction energies. I. Base stacking. Biopolymers 17, 2341–2361 (1978).
Yuan, H., Quintana, J. & Dickerson, R. Alternative structures for alternating poly(dA-DT) tracts: the structure of the B-DNA decamer C-G-A-T-A-T-A-T-C-G. Biotechemistry 31, 8009–8021 (1992).
Wobbe, C.R. & Struhl, K. Yeast and human TATA-binding proteins have nearly identical DNA sequence requirements for transcription in vitro. Molec. Cell. Biol. 10, 3859–3867 (1990).
Poon, D. et al. Genetic and biochemical analyses of yeast TATA-binding protein mutants. J. biol. Chem. 268, 5005–5013 (1993).
Strubin, M. & Struhl, K. Yeast and human TFIID with altered DNA-binding specificity for TATA elements. Cell 68, 721–730 (1992).
Roy, A., Malik, S., Meisterernst, M. & Roeder, R. An alternative pathway for transcription initiation involving TFII-I. Nature 365, 355–359 (1993).
Usheva, A. & Shenk, T. TATA-binding protein-independent initiation: YY1, TFIIB, and RNA polymerase II direct basal transcription on supercoiled template DNA. Cell 76, 1115–1121 (1994).
Drew, H.R. & Travers, A.A. DNA bending and its relation to nucleosome positioning. J. molec. Biol. 186, 773–790 (1985).
Satchwell, S.C., Drew, H.R. & Travers, A.A. Sequence periodicities in chicken nucleosome core DNA. J. molec. Biol. 191, 659–675 (1986).
Workman, J.L. & Roeder, R.G. Binding of transcription factor TFIID to the major late promoter during In vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell 51, 613–622 (1987).
Meisterernst, M., Horikoshi, M. & Roeder, R.G. Recombinant yeast TFIID, a general transcription factor, mediates activation by the gene-specific factor USF in a chromatin assembly assay. Proc. natn. Acad. Sci. U.S.A. 87, 9153–9157 (1990).
Prioleau, M.-N., Huet, J., Sentenac, A. & Mechali, M. Competition between chromatin and transcription complex assembly regulates gene expression during early development. Cell 77, 439–449 (1994).
Kopka, M., Yoon, C., Goodsell, D., Pjura, P. & Dickerson, R. The binding of an antitumor drug to DNA. Netropsin and CGCGAATTBrCGCG. J. molec. Biol. 183, 553–563 (1985).
Coll, M., Aymami, J., Marel, G.v.d., Boom, J.v. & Wang, A.-J. Molecular structure of the netropsin-d(CGCGATATCGCG) complex: DNA conformation in an alternating AT segment. Biochemistry 28, 310–320 (1989).
Chen, X., Ramakrishnan, B., Sambhorao, T. & Sundaralingam, M. Binding of two distamycin A molecules in the minor groove of an alternating B-DNA duplex. Nature struct. Biol. 1, 169–175 (1994).
Chiang, S.-Y., Welch, J., Rauscher, F. & Beerman, T. Effects of minor groove binding drugs on the interaction of TATA box binding protein and TFIIA with DNA. Biochemistry 33, 7033–7040 (1994).
Schultz, S.C., Shields, G.C. & Steitz, T.A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 °. Science 253, 1001–1007 (1991).
Brunger, A.T. XPLOR Manual (Yale University, New Haven, 1992).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta crystallogr. A47, 110–119 (1991).
Brunger, A.T. The free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–474 (1992).
Ramachandran, G.N. & Sasisekharan, V. Conformation of polypeptides and proteins. Advan. prot. Chem. 23, 283–437 (1968).
Kraulis, P.J. Molscript: a program to produce both detailed and schematic plots of protein structures. J. appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Gilson, M., Sharp, K. & Honig, B. Calculating the electrostatic potential of molecules in solution: method and error assessment. J. comput. Chem. 9, 327–335 (1988).
Lavery, R. & Sklenar, H. Defining the structure of irregular nucleic acids: conventions and principles. J. biomolec. Struct. Dynamics 6, 655–667 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, J., Burley, S. 1.9 Å resolution refined structure of TBP recognizing the minor groove of TATAAAAG. Nat Struct Mol Biol 1, 638–653 (1994). https://doi.org/10.1038/nsb0994-638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0994-638
This article is cited by
-
Structural basis of the complete poxvirus transcription initiation process
Nature Structural & Molecular Biology (2021)
-
An in vitro characterisation of the Trichomonas vaginalis TATA box-binding proteins (TBPs)
Parasitology Research (2019)
-
Structural features of DNA that determine RNA polymerase II core promoter
BMC Genomics (2016)
-
Structural basis of transcription initiation by RNA polymerase II
Nature Reviews Molecular Cell Biology (2015)
-
Role of indirect readout mechanism in TATA box binding protein–DNA interaction
Journal of Computer-Aided Molecular Design (2015)