Abstract
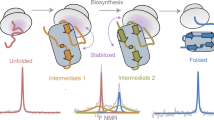
The folding of the β-sheet protein, interleukin-1β, was examined at pH 5.0 and 25 °C using pulse-labelling hydrogen exchange and electrospray ionization mass spectrometric analysis, as well as stopped-flow circular dichroism and fluorescence spectroscopies. The first detectable event is the formation of a partially folded intermediate in a kinetic step with a relaxation time of 126 ± 26 ms. There is a lag in native protein production of at least 400 ms. Optical studies indicate that the intermediate is converted to the native species in a reaction with a relaxation time of 43 ± 5 s. The kinetic rates determined from stopped-flow fluorescence, circular dichroism and pulse-labelling experiments are similar and consistent with a simple sequential model for the folding pathway of interleukin-1β at pH 5.0 and 25 °C. Taken together, our data provide kinetic evidence that formation of the native state of interleukin-1β proceeds through an obligatory intermediate. We explain our results in terms of the classical and new views of protein folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sosnick, T.R., Hiller, M.R. & Englander, S.W. The barriers in protein folding. Nature Struct. Biol. 1, 149–156 (1994).
Creighton, I.E. The energetic ups and downs of protein folding. Nature Struct. Biol. 1, 135–138 (1994).
Creighton, T.E. Up the kinetic pathway. Nature 356, 194–195 (1992).
Creighton, T.E. How important is the molten globule for correct protein folding? Trends Biochem. Sci. 22, 6–10 (1997).
Baldwin, R.L. On-pathway versus off-pathway folding intermediates. Folding and Design 1, R1–R8 (1996).
Baldwin, R.L. The nature of protein folding pathways: the classical versus the new view. J.Biomol.NMR 5, 103–109 (1995).
Kiefhaber, T. Kinetic traps in lysozyme folding. Proc. Natl. Acad. Sci. USA 92, 9029–9033 (1995).
Dill, K.A. & Chan, H.S. From Levinthal to pathways to funnels. Nature Struct. Biol. 4, 10–19 (1997).
Wolynes, P.G., Luthey-Schulten, Z. & Onuchic, J.N. Fast-folding experiments and the topography of protein folding energy landscapes. Chem. and Biol. 3, 425–432 (1996).
Zitzewitz, J.A. & Matthews, R.C. Protein engineering strategies in examining protein folding intermediates. Curr. Op. Struct. Biol. 3, 594–600 (1993).
Bai, Y., Sosnick, T.R., Mayne, L. & Englander, S.W. Protein folding intermediates: Native-state hydrogen exchange. Science 269, 192–197 (1995).
Chamberlain, A.K., Handel, T.M. & Marqusee, S. Detection of rare partially folded molecules in equilibrium with the native conformation of RNaseH. Nature Struct. Biol. 3, 782–787 (1996).
Baldwin, R.L. Finding intermediates in protein folding. Bioessays 16, 207–210 (1994).
Kuwajima, K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins: Struct. Funct. Genet. 6, 87–103 (1989).
Matthews, C.R. Pathways of protein folding. Annu. Rev. Biochem. 62, 653–683 (1993).
Miranker, A., Robinson, C.V., Radford, S.E., Aplin, R.T. & Dobson, C.M. Detection of transient protein folding populations by mass spectrometry. Science 262, 896–900 (1993).
Khorasanizadeh, S., Peters, I.D. & Roder, H. Evidence for a three-state model of protein folding from kinetic analysis of ubiquitin variants with altered core residues. Nature Struct. Biol. 3, 193–205 (1996).
Pain, R.H. Mechanisms of Protein Folding (eds Hames, B. D., & Glover, D.M.,) 15–17 (IRL Press, New York; 1994).
Meyers, C.A. et al. Purification and characterization of human recombinant interleukin-1 beta. J. Biol. Chem. 262, 11176–11181 (1987).
Zhang, J.-X. & Goldenberg, D.P. Amino acid replacement that eliminates kinetic traps in the folding pathway of pancreatic trypisin inhibitor. Biochemistry 32, 14075–14081 (1993).
Dobson, C.M. Solid evidence for molten globules. Curr. Biol. 4, 636–640 (1994).
Miranker, A., Radford, S.E., Karplus, M. & Dobson, C.M. Demonstration by NMR of folding domains in lysozyme. Nature 349, 633–636 (1991).
Radford, S.E., Dobson, C.M. & Evans, P.A. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 358, 302–307 (1992).
Elöve, G.A. & Roder, H. Structure and stability of cytochrome c folding intermediates. in Protein Refolding (ed Georgiou, G. a.D.-B.-.C, E.) 51–63 (American Chemical Society, Washington, D.C.; 1991).
Chrunyk, B.A., Evans, J., Lillquist, J., Young, P. & Wetzel, R. Inclusion body formation and protein stability in sequence variants of interleukin-1 β. J. Biol. Chem. 268, 18053–18061 (1993).
Chrunyk, B.A. & Wetzel, R. Breakdown in the relationship between thermal and thermodynamic stability in an interleukin-1β point mutant modified in a surface loop. Prat. Engng. 6, 733–738 (1993).
Chrunyk, B.A., Evans, J. & Wetzel, R. Probing the Role of Protein Folding in Inclusion Body Formation. in Protein folding in vivo and in vitro (ed. Cleland, J.L.) 46–58 (American Chemical Society, Washington, D.C.; 1993).
Varley, P. et al. Kinetics of folding of the all-β sheet protein interleukin-1 β. Science 260, 1110–1113 (1993).
Craig, S., Schmeissner, U., Wingfield, P. & Pain, R.H. Conformation, stability, and folding of interleukin 1β. Biochemistry 26, 3570–3576 (1987).
Jennings, P.A. & Wright, P.E. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 262, 892–896 (1993).
Kuwajima, K., Garvey, E.P., Finn, B.E., Matthers, C.R. & Sugai, S. Transient intermediate in the folding of dihydrofolate reductase as detected by far-ultraviolet circular dichroism spectroscopy. Biochemistry 30, 7693–7703 (1991).
Kuwajima, K., Mitani, M. & Sugai, S. Characterization of the critical state in protein folding. Effects of guanidine hydrochloride and specific Ca2+ binding on the folding kinetics of α-lactalbumin. J. Mol. Biol. 206, 547–561 (1989).
Raschke, T.M. & Marqusee, S.M. The kinetic folding intermediate of ribonuclease H resembles the acid molten globule and partially unfolded molecules detected under native conditions. Nature Struct. Biol. 4, 298–304 (1997).
Knutson, J.R., Beecham, J.M. & Brand, L. Simultaneous analysis of multiple fluorescence decay curves: a global approach. Chem. Phys. Lett. 102, 501–507 (1983).
Kim, P.S. & Baldwin, R.L. Structural intermediates trapped during the folding of ribonuclease A by amide proton exchange. Biochemistry 19, 6124–6129 (1980).
Jones, B.E. & Matthews, C.R. Early intermediates in the folding of dihydrofolate reductase from Escherichia coli detected by hydrogen exchange and NMR. Prot. Sci. 4, 167–177 (1994).
Englander, S.W. & Mayne, L. Protein folding studied using hydrogen-exchange labeling and two–dimensional NMR. Annu. Rev. Biophys. Biomol. Struct. 21, 243–265 (1992).
Katta, V. & Chait, B.T. Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Comm. Mass Spec. 5, 214–217 (1991).
Kiefhaber, T., Quaas, R., Hahn, U. & Schmid, F.X. Folding of robonuclease T1. 1. Existence of multiple unfolded states created by proline isomerization. Biochemistry 29, 3053–3061 (1990).
Kiefhaber, T., Grunert, H.-P., Hahn, U. & Schmid, F.X. Replacement of a cis proline simplifies the mechanism of ribonuclease Tl folding. Biochemistry 29, 6475–6480 (1990).
Kiefhaber, T. & Schmid, F.X. Kinetic coupling between protein folding and prolyl isomerization. I. Folding of robonuclease A and ribonuclease Tl. J. Mol. Biol. 224, 231–240 (1992).
Ikeguchi, M., Kuwajima, K., Mitani, M. & Sugai, S. Evidence for identity between the equilibrium unfolding intermediate and a transient folding intermediate: a comparative study of the folding reaction of α-lactoalbumin and lysozyme. Biochemistry 25, 6965–6972 (1986).
Roder, H., Elöve, G.A. & Englander, S.W. Structural characterization of folding intermediates in cytochrome C by H-exchange labelling and proton NMR. Nature 335, 700–704 (1988).
Udgaonkar, J.B. & Baldwin, R.L. Early folding intermediate of ribonuclease A. Proc. Natl. Acad. Sci. USA 87, 8197–8201 (1990).
Utiyama, H. & Baldwin, R.L. Kinetic Mechanisms of Protein Folding. Meth. Enz. 131, 51–70 (1986).
Kuwajima, K., Hiraoka, Y., Ikeguchi, M. & Sugai, S. Comparison of the transient folding intermediates in lysozyme and α-lactalbumin. Biochemistry 24, 874–881 (1985).
Jones, B.E., Jennings, P.A., Pierre, R.A. & Matthews, C.R. Development of nonpolar surfaces in the folding of Escherichia coli dihydrofolate reductase detected by 1-anilinonaphthalene-8-sulfonate binding. Biochemistry 33, 15250–15258 (1994).
Jones, B.E., Beechem, J.M. & Matthews, C.R. Local and global dynamics during the folding of Escherichia coli dihydrofolate reductase by time-resolved fluorsecence spectroscopy. Biochemistry 34, 1867–1877 (1995).
Mann, C.J., Shao, X. & Matthews, C.R. Characterization of the slow folding reactions of trp aporepressor from Escherichia coli by mutational analysis of prolines and catalysis by a peptidyl-prolyl isomerase. Biochemistry 34, 14573–14580 (1995).
Smith, D.L. & Zhang, Z. Probing monovalent structural features of proteins by mass spectrometry. Mass. Specfrom. Rev. 13, 411–429 (1994).
Maddams, W.F. The scope and limitations of curve fitting. Appl. Spectroscopy 34, 245–267 (1980).
Barshop, B.A., Wrenn, R.F. & Frieden, C. Analysis of numerical methods for computer simulation of kinetic processes: Development of KINSIM-A flexible, portable system. Anal. Biochem. 130, 134–145 (1983).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidary, D., Gross, L., Roy, M. et al. Evidence for an obligatory intermediate in the folding of lnterleukin-1β. Nat Struct Mol Biol 4, 725–731 (1997). https://doi.org/10.1038/nsb0997-725
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0997-725
This article is cited by
-
Effective Application of Bicelles for Conformational Analysis of G Protein-Coupled Receptors by Hydrogen/Deuterium Exchange Mass Spectrometry
Journal of the American Society for Mass Spectrometry (2015)
-
Folding pathway of interleukin-1β
Nature Structural Biology (1998)