Abstract
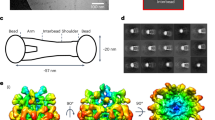
The C propeptides of fibrillar procollagens have crucial roles in tissue growth and repair by controlling both the intracellular assembly of procollagen molecules and the extracellular assembly of collagen fibrils. Mutations in C propeptides are associated with several, often lethal, genetic disorders affecting bone, cartilage, blood vessels and skin. Here we report the crystal structure of a C-propeptide domain from human procollagen III. It reveals an exquisite structural mechanism of chain recognition during intracellular trimerization of the procollagen molecule. It also gives insights into why some types of collagen consist of three identical polypeptide chains, whereas others do not. Finally, the data show striking correlations between the sites of numerous disease-related mutations in different C-propeptide domains and the degree of phenotype severity. The results have broad implications for understanding genetic disorders of connective tissues and designing new therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bateman, J.F., Boot-Handford, R.P. & Lamandé, S.R. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat. Rev. Genet. 10, 173–183 (2009).
Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978 (2011).
McLaughlin, S.H. & Bulleid, N.J. Molecular recognition in procollagen chain assembly. Matrix Biol. 16, 369–377 (1998).
Bottomley, M.J., Batten, M.R., Lumb, R.A. & Bulleid, N.J. Quality control in the endoplasmic reticulum. PDI mediates the ER retention of unassembled procollagen C-propeptides. Curr. Biol. 11, 1114–1118 (2001).
Boudko, S.P., Engel, J. & Bachinger, H.P. The crucial role of trimerization domains in collagen folding. Int. J. Biochem. Cell Biol. 44, 21–32 (2012).
Exposito, J.Y., Valcourt, U., Cluzel, C. & Lethias, C. The fibrillar collagen family. Int. J. Mol. Sci. 11, 407–426 (2010).
Lees, J.F., Tasab, M. & Bulleid, N.J. Identification of the molecular recognition sequence which determines the type-specific assembly of procollagen. EMBO J. 16, 908–916 (1997).
Kadler, K.E., Holmes, D.F., Trotter, J.A. & Chapman, J.A. Collagen fibril formation. Biochem. J. 316, 1–11 (1996).
Canty, E.G. & Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 118, 1341–1353 (2005).
Muir, A. & Greenspan, D.S. Metalloproteinases in Drosophila to humans that are central players in developmental processes. J. Biol. Chem. 286, 41905–41911 (2011).
Vadon-Le Goff, S. et al. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide only. J. Biol. Chem. 286, 38932–38938 (2011).
Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 (2007).
Wu, C.H., Walton, C.M. & Wu, G.Y. Propeptide-mediated regulation of procollagen synthesis in IMR- 90 human lung fibroblast cell cultures. J. Biol. Chem. 266, 2983–2987 (1991).
Mizuno, M., Fujisawa, R. & Kuboki, E. The effect of carboxyl-terminal propeptide of type I collagen (C-propeptide) on collagen synthesis of preosteoblasts and osteoblasts. Calcif. Tissue Int. 67, 391–399 (2000).
Davies, D. et al. Molecular characterisation of integrin-procollagen C-propeptide interactions. Eur. J. Biochem. 246, 274–282 (1997).
Lindahl, K. et al. COL1 C-propeptide cleavage site mutations cause high bone mass osteogenesis imperfecta. Hum. Mutat. 32, 598–609 (2011).
Van der Rest, M., Rosenberg, L.C., Olsen, B.R. & Poole, A.R. Chondrocalcin is identical with the C-propeptide of type II procollagen. Biochem. J. 237, 923–925 (1986).
Lee, E.R., Smith, C.E. & Poole, A.R. Ultrastructural localization of the C-propeptide released from type II procollagen in fetal bovine growth plate cartilage. J. Histochem. Cytochem. 44, 433–443 (1996).
Palmieri, D. et al. Procollagen I COOH-terminal fragment induces VEGF-A and CXCR4 expression in breast carcinoma cells. Exp. Cell Res. 314, 2289–2298 (2008).
Vincourt, J.B. et al. C-propeptides of procollagens I α1 and II that differentially accumulate in enchondromas versus chondrosarcomas regulate tumor cell survival and migration. Cancer Res. 70, 4739–4748 (2010).
McAlinden, A. α-helical coiled-coil oligomerization domains are almost ubiquitous in the collagen superfamily. J. Biol. Chem. 278, 42200–42207 (2003).
Ricard-Blum, S. et al. Interaction properties of the procollagen C-proteinase enhancer protein shed light on the mechanism of stimulation of BMP-1. J. Biol. Chem. 277, 33864–33869 (2002).
Holm, L. & Rosenstrom, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Byers, P.H. Folding defects in fibrillar collagens. Philos. Trans. R. Soc. Lond. B Biol. Sci 356, 151–157 (2001).
Pace, J.M., Kuslich, C.D., Willing, M.C. & Byers, P.H. Disruption of one intra-chain disulphide bond in the carboxyl-terminal propeptide of the proα1(I) chain of type I procollagen permits slow assembly and secretion of overmodified, but stable procollagen trimers and results in mild osteogenesis imperfecta. J. Med. Genet. 38, 443–449 (2001).
Lim, A.L., Doyle, S.A., Balian, G. & Smith, B.D. Role of the pro-α(I) COOH-terminal region in assembly of type I collagen: truncation of the last 10 amino acid residues of pro-α2(I) chain prevents assembly of type I collagen heterotrimer. J. Cell. Biochem. 71, 216–232 (1998).
Oliver, J.E., Thompson, E.M., Pope, F.M. & Nicholls, A.C. Mutation in the carboxy-terminal propeptide of the pro α1(1) chain of type I collagen in a child with severe osteogenesis imperfecta (OI type III): possible implications for protein folding. Hum. Mutat. 7, 318–326 (1996).
Zankl, A. et al. Dominant negative mutations in the C-propeptide of COL2A1 cause platyspondylic lethal skeletal dysplasia, torrance type, and define a novel subfamily within the type 2 collagenopathies. Am. J. Med. Genet. A. 133A, 61–67 (2005).
Lamandé, S.R. et al. Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the pro α1(I) chain carboxyl- terminal propeptide which impair subunit assembly. J. Biol. Chem. 270, 8642–8649 (1995).
Nishimura, G. et al. Identification of COL2A1 mutations in platyspondylic skeletal dysplasia, Torrance type. J. Med. Genet. 41, 75–79 (2004).
Chessler, S.D., Wallis, G.A. & Byers, P.H. Mutations in the carboxyl-terminal propeptide of the pro-α-1(I) chain of type-I collagen result in defective chain association and produce lethal osteogenesis imperfecta. J. Biol. Chem. 268, 18218–18225 (1993).
De Paepe, A., Nuytinck, L., Hausser, I., Anton-Lamprecht, I. & Naeyaert, J.M. Mutations in the COL5A1 gene are causal in the Ehlers-Danlos syndromes I and II. Am. J. Hum. Genet. 60, 547–554 (1997).
Myllyharju, J. & Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20, 33–43 (2004).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Gouet, P., Courcelle, E., Stuart, D.I. & Metoz, F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999).
Bourhis, J.M. et al. Production and crystallization of the C-propeptide trimer from human procollagen III. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. (in the press).
Aricescu, A.R., Lu, W. & Jones, E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 (2006).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Terwilliger, T.C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D Biol. Crystallogr. 65, 582–601 (2009).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Vagin, A.A. et al. REFMAC5 dictionary: organisation of prior chemical knowledge and guidelines for its use. Acta Crystallogr. D Biol. Crystallogr. 60, 2185–2195 (2004).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
We thank F. Delolme, D. Eichenberger, K. El Omari, P. Gouet, R. Haser, R. Liddington, G. Parsiegla, X. Robert, G. Stranzl, S. Vadon-Le Goff, M. van Rest and T. Walter for their help and suggestions at different stages of the project. We also thank A. Chaboud and I. Grosjean of the Protein Production and Analysis facility (Unité Mixte de Service Biosciences Gerland-Lyon Sud 3444) as well as staff of Diamond Light Source for technical support. This work was funded by the Fondation de France (11878 to D.J.S.H.), the Agence National de la Recherche (ANR 07 PHYSIO 022 01 to D.J.S.H.; ANR 2010 BLAN 1526 01 to C.M. and N.A.), the European Commission (227764 to E.Y.J.), the Medical Research Council UK (G09 000 84 to E.Y.J.), Cancer Research UK (A5261 to E.Y.J.) and the Centre National de la Recherche Scientifique, Université Lyon 1 and Lyonbiopôle.
Author information
Authors and Affiliations
Contributions
J.-M.B., N.M., Y.Z., K.H. and D.J.S.H. designed and performed the research; J.M.B., J.-Y.E., E.Y.J., C.M., N.A. and D.J.S.H. analyzed the data; and D.J.S.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
This work forms part of a US patent application by J.M.B., N.M., C.M., N.A. and D.J.S.H.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 1130 kb)
Supplementary Movie 1
Positions of known missense mutations in the C-propeptides of fibrillar procollagens I, II, III and V mapped on to the structure of the proa1(III) C-propeptide (MPG 6429 kb)
Rights and permissions
About this article
Cite this article
Bourhis, JM., Mariano, N., Zhao, Y. et al. Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat Struct Mol Biol 19, 1031–1036 (2012). https://doi.org/10.1038/nsmb.2389
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2389
This article is cited by
-
Expanding the clinical spectrum of COL2A1 related disorders by a mass like phenotype
Scientific Reports (2022)
-
Extracellular matrix-inspired hydrogel of hyaluronan and gelatin crosslinked via a Link module with a transglutaminase reactive sequence
Communications Materials (2022)
-
Trivalent soluble TNF Receptor, a potent TNF-α antagonist for the treatment collagen-induced arthritis
Scientific Reports (2018)
-
A cysteine-based molecular code informs collagen C-propeptide assembly
Nature Communications (2018)
-
Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification
Scientific Reports (2017)