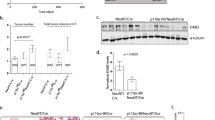
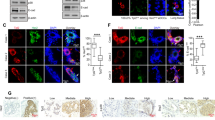
Abstract
Overexpression of the human epidermal growth factor receptor 2 (HER2) is the cause of HER2-positive breast cancer (BC). Although HER2-inactivating therapies have benefited BC patients, development of resistance and disease recurrence have been the major clinical problems, pointing to a need for alternative therapeutic strategies. For that to happen, proteins that play critical roles in the biology of HER2-induced tumorigenesis have to be identified and characterized. Here, we show that the Src homology phosphotyrosyl phosphatase 2 (Shp2) encoded by the Ptpn11 gene is a requisite for ErbB2-induced tumorigenesis. We report that conditional knockout of Shp2 alleles in the ErbB2 BC model mice abrogates mammary tumorigenesis by blocking the expression of the ErbB2 transgene. We also show that inhibition of SHP2 encoded by the PTPN11 gene in the HER2-amplified BC cells induces a normal-like cellular phenotype and suppresses tumorigenesis and metastasis by blocking HER2 overexpression. These findings demonstrate that ErbB2-induced tumors in mice or xenograft tumors induced by transplantation of HER2-amplified BC cells are vulnerable to SHP2 inhibition since it abrogates the expression of the very oncogene that causes of the disease. This report paves the way for developing SHP2-targeting therapies for BC treatment in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pegram MD, Konecny G, Slamon DJ. The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer. Cancer Treat Res. 2000;103:57–75.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Brandt BH, Roetger A, Dittmar T, Nikolai G, Seeling M, Merschjann A, et al. c-erbB-2/EGFR as dominant heterodimerization partners determine a motogenic phenotype in human breast cancer cells. FASEB J: Off Publ Fed Am Soc Exp Biol. 1999;13:1939–49.
Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–73.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–87.
Pawson T, Gish GD. SH2 and SH3 domains: from structure to function. Cell. 1992;71:359–62.
Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30.
Difiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. Erbb-2 is a potent oncogene when overexpressed in Nih/3t3 Cells. Science. 1987;237:178–82.
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82.
Dent S, Oyan B, Honig A, Mano M, Howell S. HER2-targeted therapy in breast cancer: a systematic review of neoadjuvant trials. Cancer Treat Rev. 2013;39:622–31.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23:7875–86.
Ahmed Z, George R, Lin CC, Suen KM, Levitt JA, Suhling K, et al. Direct binding of Grb2 SH3 domain to FGFR2 regulates SHP2 function. Cell Signal. 2010;22:23–33.
Frearson JA, Alexander DR. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J Exp Med. 1998;187:1417–26.
Zhou X, Agazie YM. Molecular mechanism for SHP2 in promoting HER2-induced signaling and transformation. J Biol Chem. 2009;284:12226–34.
Feng GS, Shen R, Heng HH, Tsui LC, Kazlauskas A, Pawson T. Receptor-binding, tyrosine phosphorylation and chromosome localization of the mouse SH2-containing phosphotyrosine phosphatase Syp. Oncogene. 1994;9:1545–50.
Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–73.
Matalkah F, Martin E, Zhao H, Agazie YM. SHP2 acts both upstream and downstream of multiple receptor tyrosine kinases to promote basal-like and triple-negative breast cancer. Breast Cancer Res: Bcr. 2016;18:2.
Zhao H, Agazie YM. Inhibition of SHP2 in basal-like and triple-negative breast cells induces basal-to-luminal transition, hormone dependency, and sensitivity to anti-hormone treatment. BMC Cancer. 2015;15:109.
Zhou X, Coad J, Ducatman B, Agazie YM. SHP2 is up-regulated in breast cancer cells and in infiltrating ductal carcinoma of the breast, implying its involvement in breast oncogenesis. Histopathology. 2008;53:389–402.
Zhou XD, Agazie YM. Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ. 2008;15:988–96.
Muenst S, Obermann EC, Gao F, Oertli D, Viehl CT, Weber WP, et al. Src homology phosphotyrosyl phosphatase-2 expression is an independent negative prognostic factor in human breast cancer. Histopathology. 2013;63:74–82.
Fornaro M, Burch PM, Yang W, Zhang L, Hamilton CE, Kim JH, et al. SHP-2 activates signaling of the nuclear factor of activated T cells to promote skeletal muscle growth. J Cell Biol. 2006;175:87–97.
Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–70.
Hartman ZR, Schaller MD, Agazie YM. The tyrosine phosphatase SHP2 regulates focal adhesion kinase to promote EGF-induced lamellipodia persistence and cell migration. Mol Cancer Res: MCR. 2013;11:651–64.
Burks J, Agazie YM. Modulation of alpha-catenin Tyr phosphorylation by SHP2 positively effects cell transformation induced by the constitutively active FGFR3. Oncogene. 2006;25:7166–79.
Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92.
Kim YS, Jung MJ, Ryu DW, Lee CH. Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer. 2014;17:121–8.
Lan L, Holland JD, Qi J, Grosskopf S, Vogel R, Gyorffy B, et al. Shp2 signaling suppresses senescence in PyMT-induced mammary gland cancer in mice. EMBO J. 2015;34:1493–508.
Ke Y, Lesperance J, Zhang EE, Bard-Chapeau EA, Oshima RG, Muller WJ, et al. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J Biol Chem. 2006;281:34374–80.
Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–21.
Deblois G, Chahrour G, Perry MC, Sylvain-Drolet G, Muller WJ, Giguere V. Transcriptional control of the ERBB2 amplicon by ERRalpha and PGC-1beta promotes mammary gland tumorigenesis. Cancer Res. 2010;70:10277–87.
Cleghon V, Feldmann P, Ghiglione C, Copeland TD, Perrimon N, Hughes DA, et al. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol Cell. 1998;2:719–27.
Hartman Z, Zhao H, Agazie YM. HER2 stabilizes EGFR and itself by altering autophosphorylation patterns in a manner that overcomes regulatory mechanisms and promotes proliferative and transformation signaling. Oncogene. 2013;32:4169–80.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70.
Acknowledgements
This work was supported by a grant (CA124940) from the National Cancer Institute (NCI), a component of the National Institute of Health (NIH) to YMA. The Flow Core and Imaging facilities are supported by grants from NIH (GM103488, GM104842, GM103434, P20RR016440, and P30RR032138/P30GM103488.). We would like to thank Dr. Benjamin Neel and Dr. Timothy Lane for providing the SHP2-floxed and the MMTV-Cre mice, respectively. Also, we thank Dr. Karen Martin, Dr. Amanda Ammer, and Mrs. Sarah McLaughlin for their support in microscopic and ultrasound imaging and Dr. Katherine Brundage for her support in FACS analyses.
Author contributions
HZ carried out the genetic studies and tissue processing, including H&E and IF staining, and microscopic imaging. EM was responsible for cell-based studies, including mammosphere formation and RT-PCR analyses. FM contributed in cell-based studies, including immunoblotting, cell transformation, and cancer stem cell studies. AI contributed in the RT-PCR analyses and manuscript editing. JMR contributed in the genetic studies and manuscript editing. NS and PL contributed in xenograft tumorigenesis studies and manuscript editing. YMA was responsible for designing, overseeing, directing the whole project, and preparation of the manuscript. In addition, YMA participated in data acquisition in the genetic and cell culture studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, H., Martin, E., Matalkah, F. et al. Conditional knockout of SHP2 in ErbB2 transgenic mice or inhibition in HER2-amplified breast cancer cell lines blocks oncogene expression and tumorigenesis. Oncogene 38, 2275–2290 (2019). https://doi.org/10.1038/s41388-018-0574-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0574-8