Abstract
Background
Cardiovascular (CV) complications are the most significant cause of mortality in adults with Cushing disease (CD); little is known about CV risk factors in children with CD. Measurement of lipoprotein particles by nuclear magnetic resonance (NMR) spectroscopy is a novel technology to assess CV risk. The objective of the current study is to analyze the NMR lipid profile in pediatric CD patients before and 1 year after remission.
Methods
NMR lipid profile was obtained via the Vantera NMR analyzer, using frozen serum samples from 33 CD patients (mean age 13.8 ± 4.0 years) evaluated between 1997 and 2017 at the National Institutes of Health (NIH) Clinical Center (CC).
Results
GlycA (glycosylated acute-phase proteins), triglyceride-rich particles (TRLP medium and very small sizes), low-density lipoprotein (LDL) particles (LDLP total and large size), high-density lipoprotein (HDL) particles (HDLP total, medium and small sizes), total cholesterol, LDL-cholesterol, HDL-cholesterol, GlycA inflammatory biomarker, and apolipoprotein B and apolipoprotein A1 (ApoA1) concentrations showed statistically significant changes after remission of CD (p < 0.05).
Conclusion
In our study population, most of the lipid variables improved post-CD remission, with the exception of HDL and ApoA1, indicating that NMR lipoprotein profile may be a helpful tool in assessing the CV risk in pediatric patients with CD.
Similar content being viewed by others
Introduction
Cushing disease (CD) is a rare disease in pediatrics, with an estimated incidence of 1 to 1.5 per million children per year.1 It is caused by adrenocorticotropin hormone (ACTH) overproduction from the pituitary, most often due to an ACTH-secreting pituitary microadenoma. The prolonged hypercortisolism results in the typical Cushing syndrome (CS) signs and symptoms: truncal obesity, growth deceleration, skin changes, muscle weakness, and hypertension.2,3 Cardiovascular complications are the most significant cause of mortality in adult patients with CD, with an estimated cardiovascular mortality rate 4–5 times higher than the general population.4,5 A recent meta-analysis of 14 studies reports higher prevalence of markers of early atherosclerosis in 332 adult patients with CS compared to 462 controls.6 Cardiovascular risk factors, including metabolic syndrome, atherosclerotic plaques, elevated low-density lipoprotein (LDL), and total cholesterol levels, may persist in adults even after surgical remission has been achieved.7
Pediatric studies of cardiovascular involvement in CD are limited. Our group had previously showed that pediatric CD patients exhibit a significant improvement of hypertension after remission is achieved; however, they remain at risk for residual hypertension.8 We also showed that pediatric CD patients are at increased risk for metabolic syndrome, with more than 60% of our cohort exhibiting signs of insulin resistance based on Homeostatic model assessment-insulin resistance (HOMA-IR) values.9
Measurement of lipoprotein particles using nuclear magnetic resonance (NMR) spectroscopy is a novel technology to assess cardiovascular risk. NMR spectroscopy measures the signals generated by the terminal methyl groups on lipids; lipoprotein subclass concentrations are then derived from deconvolution of the lipid methyl signal. The Vantera Clinical Analyzer is the first NMR device cleared by the Food and Drug Administration (FDA) for use as a clinical instrument; it measures particle concentrations for all lipoprotein classes (very LDL (VLDL), LDL, high-density lipoprotein (HDL)) and subclasses (large, medium, small), as well as particle sizes for VLDL, LDL, and HDL.10
The lipoprotein particles transport cholesterol and triglycerides in the bloodstream and are direct mediators of atherosclerosis; LDL particles (LDLPs) initiate formation of cholesterol-rich foam cells and atherosclerotic plaque, while HDL particles (HDLPs) potentially mediate the reverse cholesterol transport by inhibiting the oxidation of LDLPs and removing cholesterol from the foam cells for delivery back to the liver.11,12,13 The small LDLPs are considered more atherogenic than the large LDLPs with previous studies showing that lower levels of LDL and HDL mean particle sizes are associated with insulin resistance.14,15 Two recent large-scale studies in obese, pre-diabetic, and type 2 diabetic adolescents and young adults showed that NMR lipid profile is a better indicator of vascular damage compared to the traditional lipid profile.16,17
The goal of the current study was to assess the lipoprotein profile in pediatric patients with CD before treatment and 1 year after biochemical remission was achieved. We hypothesized that remission of CD would improve the NMR lipid variables. Furthermore, we investigated potential correlations between lipoprotein variables and clinical markers of CD severity, using the CD severity score and its individual components, as described previously.1
Patients, materials, and methods
Patient population
Out of 211 pediatric CD patients (<21 years old) evaluated under the research protocol 97-CH-0076 of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (January 1997 until August 2017), we identified 33 patients who remained in remission at 1 year after transsphenoidal surgery (TSS) and for whom frozen serum or plasma samples were available. The study was approved by the Investigational Review Board of NICHD and all patients and/or their parents gave written assent/consent. The National Institutes of Health (NIH) Clinical Research Information System was used to obtain medical records and clinical data. Samples were obtained at their initial evaluation and at the 1-year post-transsphenoidal resection (TSS) follow-up visit. For the purposes of this study, we defined remission of CD only based on biochemical criteria as follows: midnight cortisol <4.4 μg/dl and/or urinary free cortisol (UFC) levels within the normal range at 12 months post-TSS.
NMR spectroscopy
NMR spectroscopy profile was obtained via the Vantera analyzer (LipoScience/LabCorp Global Research Services) at the Department of Laboratory Medicine at the NIH Clinical Center, using the LP4 algorithm. All the measurements were performed on the same analyzer. Between-day coefficient of variation is <11%, as determined in the performing lab. The following six categories of lipid variables were obtained (Table 1): (1) triglyceride-rich particles (TRLP total, very large, large, medium, small, and very small) are reported as nanomoles per liter with mean reference range values at 125.2 ± 61.6, 0.4 ± 1.0, 2.9 ± 6.5, 17.9 ± 16.2, 56.5 ± 37.5, and 47.5 ± 46.9 nmol/l, respectively, (2) LDLP concentrations (LDLP total, large, medium, and small) are reported as nanomoles per liter with mean reference range values at 1454.0 ± 393, 309.0 ± 223, 676.0 ± 405, and 469.0 ± 431 nmol/l, respectively, (3) HDLP concentrations (HDLP total, large, medium, and small) are reported as micromoles per liter with mean reference range values at 24.0 ± 3.0, 2.5 ± 1.9, 7.7 ± 2.7, 13.8 ± 3.4 µmol/l, respectively, (4) mean particle sizes (triglyceride-rich—TRLZ, LDL—LDLZ, and HDL—HDLZ) are reported as nanometers with mean reference range values at 44.0 ± 8.4, 21.0 ± 0.5, and 8.98 ± 0.44 nm, respectively, (5) lipids and apolipoproteins (total triglycerides, total cholesterol, TRL triglyceride, LDL cholesterol, HDL cholesterol, apolipoprotein B (ApoB), and apolipoprotein A1 (ApoA1)) are reported as milligrams per deciliter with mean reference range values at 119.3 ± 89.8, 193.8 ± 36.5, 99.1 ± 88.7, 110.5 ± 30.7, 61.1 ± 14.4, 87.1 ± 23.6, and 156.8 ± 27.8 mg/dl, respectively, and finally (6) inflammatory biomarker GlycA (glycosylated acute-phase proteins) is reported as micromoles per liter with mean reference range value at 402.4 ± 65.8 µmol/l.
CD severity score
The CD severity score was previously introduced by our group to quantify the clinically relevant manifestations of cortisol exposure in children. Seven individual factors are used in the scoring (degree of hypercortisolemia, impaired glucose tolerance, hypertension, height Z-score, body mass index (BMI) Z-score, time to diagnosis, and tumor size) and each of these factors has been independently associated with worse outcome. A full description of the CD severity score is outlined in the paper by Gkourogianni et al.1
Statistical analysis
All summary statistics are reported as mean ± SD. All reported p values are two-tailed, with p < 0.05 is considered to be statistically significant. Paired comparisons utilizing either the paired t test or non-parametric Wilcoxon’s signed-rank test were performed to compare lipoprotein variables in cured CD patients before and 1 year after TSS. In these paired comparisons, stepdown Bonferroni corrections for multiple comparisons were carried out for groups of related measures, and corrected p values are reported where applicable. Correlation analyses were done using Pearson’s correlation coefficient or Spearman’s ρ, as appropriate, between CD severity score parameters and the patients’ lipoprotein profile. Data were analyzed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
Out of 211 pediatric CD patients (<21 years old) evaluated at NIH since 1997, we identified 33 patients (11 males, 22 females, mean age 13.8 ± 4.0 years) who achieved biochemical remission at 1 year post-TSS and for whom pre- and post-surgery frozen serum or plasma samples were available. Pre- and post-surgery samples were drawn between 0 and 12 months before TSS, and 3 and 13 months (except two at 48 and 49 months) after TSS. Clinical and biochemical characteristics of CD patients are summarized in Table 2.
NMR spectroscopy
Six categories of NMR lipid variables were analyzed in 33 CD patients in remission and their values were compared before and after surgery. As pediatric normative data for the NMR lipid parameters are not available in the literature thus far, we compared the Δ changes of each lipid variable before and after cure (Table 3). There were robust, statistically significant effects on several lipid variables: GlycA (Δ = −69.0, 95% confidence interval (CI) −115.6 to −22.4), TRLPs (medium: Δ = 6.2, 95% CI 1.9–10.5; very small: Δ = −27.1, 95% CI −44.1 to −10.2), LDLP (total: Δ = −271.6, 95% CI −465.4 to −77.7; large: Δ = −142.9, 95% CI −235.1 to −50.6), HDLP (total: Δ = −3.6, 95% CI −5.6 to −1.6; medium: Δ = −2.0, 95% CI −3.2 to −0.7; small: Δ = −1.9, 95% CI −3.1 to −0.8), TC (Δ = −31.5, 95% CI −53.7 to −9.3), LDLC (Δ = −23.1, 95% CI −39.3 to −6.8), HDLC (Δ = −7.5, 95% CI −13.2 to −1.9), ApoB (Δ = −16.5, 95% CI −28.3 to −4.8) and ApoA1 (Δ = −17.5, 95% CI −31.2 to −3.9).
CD severity score
CD severity score was calculated as follows: degree of hypercortisolemia (24 h UFC normal = 0; 24 h UFC up to 3× normal = 1; 24 h UFC > 3× normal = 2), impaired glucose tolerance (fasting plasma glucose (FPG) < 100 = 0; FGP ≥ 100 = 1; FPG ≥ 126 or diabetes mellitus diagnosis = 2), hypertension (systolic blood pressure (SBP) and diastolic blood pressure (DBP) Z-score both <2 = 0; SBP or DBP Z-score ≥ 2 = 1; HTN-Tx = 2), height Z-score (>−0.5 = 0; ≤−0.5 = 1), BMI Z-score (<2 = 0; ≥2 = 1), time to diagnosis (<3 years = 0; ≥3 = 1) and tumor characteristics (adenoma < 5 mm = 0; adenoma ≥ 5 mm and or CS invasion = 1). Pre-TSS GlycA, TRLP (medium and very small sizes), LDLP (large size), HDLP (medium size), HDLC, and ApoA1 showed statistically significant individual correlations with one or more of the following CD severity score variables: BMI Z-score, 24-h urinary cortisol concentrations, midnight serum cortisol levels, early morning serum cortisol levels, and age at surgery.
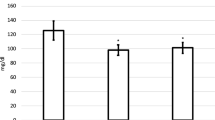
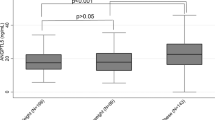
Table 4 summarizes the correlations of individual NMR lipid variables with CD severity score variables that reached statistical significance and carry potential importance. Total HDLP, ApoA1 (surrogate of HDL) and HDL-cholesterol are all considered to exert a protective cardiovascular effect and all showed a reverse correlation with pre-TSS morning serum cortisol levels (r = −0.36, r = −0.45, and r = −0.50, respectively), indicating that the higher the degree of hypercortisolemia, the worse the patient’s HDL lipid profile (Fig. 1a, b, d). Also, the very small TRLPs (VSTRLP), which act by inhibiting the protective mechanism of lipolysis, showed a positive correlation with the morning cortisol values (r = 0.37) and also exhibited a robust post-TSS decrease by −27.1 (p = 0.0162) (Table 3). Thus, a higher cortisol level, which indicates more severe CD, is clinically correlated with a higher VSTRLP value (Fig. 1c). Finally, GlycA, a novel inflammatory biomarker, which showed a robust post-op decrease by −69.0 (p = 0.0050), also exhibited a positive correlation with BMI Z-score (r = 0.47). The higher the BMI, the worse the CD, which clinically correlates with a higher GlycA level (Fig. 2).
Clinically relevant correlations between lipoprotein variables and morning cortisol levels before transsphenoidal surgery. a–d high-density lipoprotein (HDL)-cholesterol, HDL particle, very small triglyceride-rich particles, and apolipoprotein A1 showed a significant correlation with morning serum cortisol levels (r = −0.50, r = −0.36, r = 0.37, and r = −0.45, respectively)
Discussion
This is the first study to our knowledge to report NMR spectroscopy data in pediatric patients with CD in order to assess their cardiovascular risk. We hypothesized that normalization of cortisol levels post-TSS would improve the patient’s lipoprotein levels and thus have a positive effect on their atherogenic profile. In contrast to regular lipid profile that only measures lipoprotein concentrations, NMR has the ability to measure lipoprotein particle number, size, and concentration.12
Clinical interpretation of the individual NMR lipid variables is not yet well established in the literature. The total LDLP is approved by the FDA for clinical use in adults. Matyus et al.10 examined the analytical performance of the NMR-based LDLP assay as per clinical laboratory standards institute (CLSI) guidelines and report mean LDLP value at 1193 ± 472 nmol/l (95% CI 457–2282). In our pediatric population, the mean pre-TSS value was 1751.1 ± 578.5 and decreased to 1479.5 ± 506.2 post surgery (p = 0.0225), which represents a significant post-cure decrease; however, it did not reach the normal adult reference range of <1200 nmol/l. Twenty one percent of our patients continued to have abnormal LDLP levels at 1-year follow-up, implying that some lipid abnormalities may persist even after CD is cured. A recent study reported total LDLP values, as part of the NMR lipid profile, using the 400-MHz proton NMR analyzer at Liposcience, in 232 lean, 228 obese, and 214 type 2 diabetic adolescents and young adults at 849.7 ± 248, 1102.6 ± 326.8, and 1257 ± 437.9 nmol/l, respectively.16 Shah et al.17 measured NMR lipoprotein data, using the LipoProfile-3 algorithm by LipoScience, in 118 obese normoglycemic and 96 obese pre-diabetic youth; mean total LDLP levels were1037 (834, 1266) and 1098 (898, 1353) nmol/l respectively. As compared to both these studies, keeping in mind that coefficient of variations between machines may be a consideration, our patient population had much higher LDLP values even after CD was cured, implying that the dyslipidemia associated with CD might be more severe than the degree of dyslipidemia associated with obesity or type 2 diabetes mellitus.
All LDL particles decreased post cure; however, only the total and large LDL particles were statistically significant. Furthermore, very small TRLP, a very proatherogenic form of TRLPs, showed a significant post-TSS decrease by −27.1 (p = 0.0162). The GlycA NMR signal is a novel inflammatory biomarker that reflects the aggregated levels of several glycosylated acute-phase proteins and is associated with increased cardiovascular disease risk in adults.18 In our study, GlycA levels decreased post cure, indicating an improvement in the patients’ atherogenic profile after cortisol normalization. Importantly, BMI Z-score showed a positive correlation with GlycA levels (r = 0.47); however, we interpret this result with caution as obesity itself may contribute to the abnormal GlycA regardless of the CD. In our study, the mean BMI Z-score improved from 1.88 ± 1.00 pre-surgery to 1.61 ± 0.78 post surgery, indicating that obesity was still a consideration despite cortisol normalization. Jago et al.19 recently analyzed by NMR spectroscopy GlycA levels in 1664 adolescents and showed that GlycA was significantly higher in higher BMI groups.
While most of the lipid markers (TRLP, LDLP, TC, LDL, ApoB) in our study changed in a cardiovascular advantageous direction in the post-op samples, this did not hold true for the HDLPs and ApoA1, which both decreased post cure of CD. We do not have a reasonable explanation for the paradoxical worsening of the HDL markers after normalization of the cortisol levels; however, this seems to be in accordance with prior studies reporting a variable effect of CS on HDL cholesterol; different studies have shown both increases and decreases of the HDL and ApoA1 levels.20,21 Of note, the effect of CS on LDL cholesterol and ApoB is consistent among different series; LDL cholesterol is increased in patients with CS, and it often correlates with the severity of the disease.20,21,22
Limitations of our study include the relatively small patient population and the retrospective design of the study that did not allow us to calculate cardiovascular risk and HOMA-IR scores, as we did not routinely get insulin levels and/or waist circumference during CD evaluation. Furthermore, the retrospective nature of the study in combination with the rarity of CD in pediatrics accounted for the lack of consistency at the post-surgery sampling times among our study participants, as most patients were referred to our center by primary hospitals across the country. Study limitations also includes lack of a control or comparator population for variables that may affect lipid measurements over time, such as pubertal change during the study period, race/ethnicity, family history, diet, exercise, body composition, and concomitant and conditions (including genetic associations).
NMR spectroscopy is an emerging technology in the field of lipidiology; thus, its superiority in addressing cardiovascular disease parameters, as compared to traditional lipid concentrations, has not yet been established in the pediatric population. However, recent studies have demonstrated that NMR lipid profiles are more strongly associated with cardiovascular risk factors in adolescents and young adults compared to conventional lipid profiles.16,17 Urbina et al.16 compared NMR and traditional lipid profiles in 214 lean, 228 obese, and 214 T2 diabetic patients (10–24 years old), and found that while LDL concentration was similar in the obese and diabetic group, the LDL size measured by NMR was able to differentiate the three groups (p ≤ 0.0001). Using general linear models, which included patient demographics, risk factors, and traditional or NMR lipid parameters and a composite vascular function score, the authors demonstrated that NMR lipid profiles were better in predicting vascular damage and were more strongly associated to vascular function, as compared to traditional lipid profiles. Shah et al.17 compared NMR and traditional lipid profiles in 96 obese youth with prediabetes and 118 obese normoglycemic controls and report that while conventional lipid profiles were not significantly different between groups, patients with prediabetes had higher levels of small LDLP and HDLP, as measured by NMR.
CD although rare can serve as a model to study the effect of excess cortisol on cardiovascular disease. In our cohort of 33 CD pediatric patients who achieved biochemical remission at 1 year post-TSS, there was a significant post-cure reduction in most of the lipid variables that were measured, with the exception of the HDL and ApoA1 markers, despite the fact that most patients were peri-pubertal, and there is a well-documented tendency of lipid profile worsening in puberty. Our hypothesis is that the improvement in the lipid profile was due to a combination of lowering the cortisol levels and the BMI. Approaching dyslipidemia in patients with CD is challenging; even with normalization of the cortisol levels, lipid markers might remain elevated long after remission was achieved, suggesting that dyslipidemia in this population should be a life-long concern, with appropriate follow-up.
In conclusion, NMR lipoprotein profile may serve as a marker of cardiovascular risk in pediatric patients with CD. Further studies are needed to see if lipoprotein markers are associated with preclinical markers of atherosclerosis in a larger cohort.
References
Gkourogianni, A. et al. Pediatric Cushing disease: disparities in disease severity and outcomes in the Hispanic and African-American populations. Pediatr. Res. 82, 272–277 (2017).
Stratakis, C. A. Cushing syndrome in pediatrics. Endocrinol. Metab. Clin. N. Am. 41, 793–803 (2012).
Holst, J. M. et al. Cushing’s syndrome in children and adolescents: a Danish nationwide population-based cohort study. Eur. J. Endocrinol. 176, 567–574 (2017).
Dekkers, O. M. et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J. Clin. Endocrinol. Metab. 98, 2277–2284 (2013).
Etxabe, J. & Vazquez, J. A. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin. Endocrinol. (Oxf.) 40, 479–484 (1994).
Lupoli, R. et al. Markers of atherosclerosis in patients with Cushing’s syndrome: a meta-analysis of literature studies. Ann. Med. 49, 206–216 (2017).
Faggiano, A. et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J. Clin. Endocrinol. Metab. 88, 2527–2533 (2003).
Lodish, M. B. et al. Blood pressure in pediatric patients with Cushing syndrome. J. Clin. Endocrinol. Metab. 94, 2002–2008 (2009).
Libuit, L. G. et al. A gender-dependent analysis of Cushing’s disease in childhood: pre- and postoperative follow-up. Clin. Endocrinol. (Oxf.) 83, 72–77 (2015).
Matyus, S. P. et al. NMR measurement of LDL particle number using the Vantera® Clinical Analyzer. Clin. Biochem. 47, 203–210 (2014).
Jeyarajah, E. J., Cromwell, W. C. & Otvos, J. D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab Med. 26, 847–870 (2006).
Mora, S. Advanced lipoprotein testing and subfractionation are not (yet) ready for routine clinical use. Circulation 119, 2396–2404 (2009).
Mackey, R. H. et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care 38, 628–636 (2015).
Garvey, W. T. et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 52, 453–462 (2003).
Wang, J. et al. Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish men. J. Intern. Med. 272, 562–572 (2012).
Urbina, E. M. et al. Lipoprotein particle number and size predict vascular structure and function better than traditional lipids in adolescents and young adults. J. Clin. Lipidol. 11, 1023–1031 (2017).
Shah, A. S. et al. Superiority of lipoprotein particle number to detect associations with arterial thickness and stiffness in obese youth with and without prediabetes. J. Clin. Lipidol. 10, 610–618 (2016).
McGarrah, R. W. et al. A novel protein glycan-derived inflammation biomarker independently predicts cardiovascular disease and modifies the association of HDL subclasses with mortality. Clin. Chem. 63, 288–296 (2017).
Jago, R. et al. Novel measures of inflammation and insulin resistance are related to obesity and fitness in a diverse sample of 11–14 year olds: The HEALTHY Study. Int. J. Obes. (Lond.) 40, 1157–1163 (2016).
Feingold, K., Brinton, E. A. & Grunfeld, C. in The Effect of Endocrine Disorders on Lipids and Lipoproteins Endotext [Internet] (eds De Groot, L. J. et al.) (MDText.com Inc., South Dartmouth, 2017).
Arnaldi, G. et al. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology 92, 86–90 (2010).
Mancini, T., Kola, B., Mantero, F., Boscaro, M. & Arnaldi, G. High cardiovascular risk in patients with Cushing’s syndrome according to 1999WHO/ISH guidelines. Clin. Endocrinol. (Oxf.) 61, 768–777 (2004).
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver NICHD, NIH.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: A.M., A.C., N.S., A.T.R., M.S., M.K., E.B., C.L., M.D.L.L.S., C.A.S., M.L. Drafting the article or revising it critically for important intellectual content: A.M., N.S., C.A.S., M.Ldish. Final approval of the version to be published: A.T.R., C.A.S., M.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makri, A., Cheung, A., Sinaii, N. et al. Lipoprotein particles in patients with pediatric Cushing disease and possible cardiovascular risks. Pediatr Res 86, 375–381 (2019). https://doi.org/10.1038/s41390-019-0438-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0438-0
This article is cited by
-
Childhood Obesity and Cardiovascular Disease Risk
Current Atherosclerosis Reports (2023)
-
Differential Effects of Amount, Intensity, and Mode of Exercise Training on Insulin Sensitivity and Glucose Homeostasis: A Narrative Review
Sports Medicine - Open (2022)