Abstract
Triptolide (TP) is the main active ingredient of Tripterygium wilfordii Hook.f, which has attracted great interest due to its promising efficacy for autoimmune diseases and tumors. However, severe adverse reactions, especially hepatotoxicity, have restricted its approval in the market. In the present study we explored the role of hepatic natural killer T (NKT) cells in the pathogenesis of TP-induced liver injury in mice. TP (600 μg/kg/day, i.g.) was administered to female mice for 1, 3, or 5 days. We found that administration of TP dose-dependently induced hepatotoxicity, evidenced by the body weight reduction, elevated serum ALT and AST levels, as well as significant histopathological changes in the livers. However, the mice were resistant to the development of TP-induced liver injury when their NKT cells were depleted by injection of anti-NK1.1 mAb (200 μg, i.p.) on days −2 and −1 before TP administration. We further revealed that TP administration activated NKT cells, dominantly releasing Th1 cytokine IFN-γ, recruiting neutrophils and macrophages, and leading to liver damage. After anti-NK1.1 injection, however, the mice mainly secreted Th2 cytokine IL-4 in the livers and exhibited a significantly lower percentage of hepatic infiltrating neutrophils and macrophages upon TP challenge. The activation of NKT cells was associated with the upregulation of Toll-like receptor (TLR) signaling pathway. Collectively, these results demonstrate a novel role of NKT cells contributing to the mechanisms of TP-induced liver injury. More importantly, the regulation of NKT cells may promote effective measures that control drug-induced liver injury.
Similar content being viewed by others
Introduction
Triptolide (diterpenoid triepoxide (TP)) is purified from the roots and leaves of the plant Tripterygium wilfordii Hook.f. (TWHF), which grows in China, Japan, and Korea. TWHF has been used in Chinese traditional medicine for centuries for the treatment of rheumatoid arthritis, nephritis, and other disorders, including some tumors [1]. TP is a principal active compound of TWHF [2]. Severe adverse reactions, however, especially hepatotoxicity, have greatly restricted its approval in the market. Although several published reports have demonstrated that TP causes liver injury by lipid peroxidation, stress responses, and hepatocyte necrosis [3, 4], the underlying cellular mechanisms of TP-induced liver injury require more detailed investigations. Recently, it has been found that hepatocytes may not be the only target in drug-induced liver injury (DILI) [5]. Immune cells may also play an essential role in DILI. To unravel these phenomena, we investigated the roles of immune cells in TP-induced liver injury.
Natural killer T cells (NKT cells) express both NK cell receptors and semiinvariant T cell receptors, bridging innate and acquired immunity [6]. NKT cells produce Th1 (interferon-γ (IFN-γ)), Th2 (interleukin (IL)-4), and Th17 (IL-17) cytokines and regulate the balance of pro-inflammatory and anti-inflammatory responses [7]. NKT cells are most abundant in liver among all the organs. Activated NKT cells play a crucial role in liver injury. NKT-deficient mice are resistant to the development of ischemic reperfusion injury [8] or high-fat diet [9] or concanavalin A (Con A) [10]-induced liver injury. However, the role of NKT cells in DILI remains largely unknown. Drug-dependent or -independent stimuli may activate NKT cells, including self-lipid “danger signals” released from damaged cells, cytokines, and bacterial or viral antigens [11]. Activated NKT cells thereby recruit leukocytes, release inflammatory cytokines, and kill hepatocytes. In the present study, we investigated the role of NKT cells in TP-induced liver injury.
TP can improve diabetic nephropathy by regulating the balance of T helper cell 1/2 (Th1/Th2) cytokine secretion in the kidney [12]. TP also mediates IL-12/IL-23 expression in antigen-presenting cells [13]. These studies provide some implications for the effect of TP on NKT cells. The production of both Th1 and Th2 cytokines is a hallmark of NKT cell activation. Activation of NKT cells is mediated directly by the recognition of glycolipids or indirectly by TLR ligands and IL-12 secretion [14]. We have previously investigated the hepatotoxicity of TP in association with its immunomodulatory capacity and shown that TP could induce immune-associated liver injury [15]. Therefore, we hypothesized that NKT cells are involved in TP-induced liver injury. A better understanding of TP-induced liver injury will aid investigations and predictions of the potential risks of hepatotoxicity of TWHF drugs.
Materials and methods
Chemicals
TP (CAS: 38748-32-2, batch number: 130401, contents >98%) was bought from the Guilin Sanleng Biotech Co., Ltd (Guilin, China) and was reconstituted in propylene glycol and stored at −20 °C. Then, TP was freshly diluted to the appropriate concentrations with a 0.5 % carboxymethylcellulose solution before the experiments.
Animals and treatment
Female C57BL/6 mice, age of 6–8 weeks and weighing 18–20 g, were purchased from Vital River Experimental Animal Technology, Co., Ltd (Beijing, China). All the mice were housed under pathogen-free conditions and provided with mouse chow and water ad libitum. The animals were maintained at a controlled temperature (22 ± 2 °C) and photoperiod (12 h of light and 12 h of dark). The animals were acclimated to the laboratory for 1 week before the experiments. This study was approved by the Ethical Committee of China Pharmaceutical University and the Laboratory Animal Management Committee of Jiangsu Province (Approval No.: 2110748). All animals received humane care, and the study protocols complied with the institution’s guidelines. The animal experiments were carried out in accordance with the approved guidelines. Female C57BL/6 mice were administered by intragastric gavage (i.g.) with TP at a dose of 600 μg/kg per mouse for 1, 3, or 5 days. Every group contained 8 mice. The acute toxicity study showed that the median lethal dose (LD50) value of i.g. administration of TP was 1280 μg/kg in C57BL6 mice [16]. NKT cells were depleted with 200 µg of anti-NK1.1 monoclonal antibody (mAb; clone PK136, from BioXcell, West Lebanon, NH, USA) injected intraperitoneally (i.p.) on days −2 and −1 before TP administration.
Blood chemistry analysis
The blood was collected in tubes without anticoagulant to obtain serum, which was analyzed for the level of alanine transaminase (ALT) and aspartate transaminase (AST) using the ALT and AST quantification kit (Whitman Biotech, Nanjing, China).
Histopathological evaluations
Sections from the livers were removed and fixed in 10% neutral-buffered formalin. For histopathological examination, all the fixed organs were processed for embedding in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Slides were coded, randomized, and evaluated by pathophysiologists who were unaware of the treatment schedule. The slides were photographed using the 1X81 Olympus confocal laser scanning microscope (Olympus, Japan).
Nonparenchymal cell (NPC) isolation and labeling
Single-cell suspensions were prepared from livers from which blood was eliminated by perfusion of the heart with saline solution. To isolate NPCs, single-cell suspensions were mixed with Percoll and then treated with red blood cell lysis solution (0.15 M NH4Cl and 0.1 mM Na2 EDTA) to eliminate erythrocytes.
NPCs were blocked with anti-CD16/32 and stained with fluorescence-conjugated anti-mouse CD3, CD45, CD49b, CD11b, Ly-6G, Ly-6C, and F4/80 antibodies (Becton Dickinson, San Diego, CA, USA) for surface labeling. NKT (CD3+CD49b+) cells were permeabilized with Cytoperm/Cytofix (Becton Dickinson) according to the manufacturer’s instructions and then incubated with antibodies specific for IFN-γ or IL-4 for intracellular labeling. The cells were then centrifuged, and the pellets were washed to remove unbound antibodies. After surface and intracellular labeling, the cells were analyzed using a Calibrate flow cytometer (Becton Dickinson, Palo Alto, CA, USA), and the data were analyzed using FlowJo version 10 software (FlowJo, Ashland, OR, USA).
Detection of damage-associated molecular patterns (DAMPs), endotoxin, and cytokines by enzyme-linked immunosorbent assay (ELISA)
Serum was used to determine the concentrations of high mobility group box-1 protein (HMGB1, Yanhui Biotech, Shanghai, China), lipopolysaccharides (LPS, Yanhui Biotech), and macrophage inflammatory protein-2 (MIP-2) by ELISA (Fcmacs Biotech, Nanjing, China) in accordance with the manufacturer’s protocol.
RNA extraction and real-time PCR
RNA was isolated from liver sections with TRIzol reagent (Vazyme Biotech, Nanjing, China). The complementary DNA (cDNA) synthesis was performed according to the manufacturer’s instructions using the HiScriptTM Q RT SuperMix for qPCR (+gDNA wiper) kit (Vazyme Biotech). Real-time PCR was performed in a 20 μL system containing 10 μL of 1× SYBR Green Master Mix (Vazyme Biotech), 5 μL of cDNA, 3.5 μL of RNase/DNase-free water, 0.5 μL of ROX Reference Dye1, and 0.5 μL of each primer. The thermal cycler conditions included a hold for 5 min at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C, and then 15 s at 95 °C, 1 min at 60 °C and 15 s at 95 °C. A melting curve analysis was performed for each reaction with a 65–95 °C ramp. The threshold cycle at which the fluorescent signal reached an arbitrarily set threshold near the middle of the log-linear phase of the amplification for each reaction was calculated, and the relative quantity of messenger RNA (mRNA) was determined. The mRNA levels were normalized against the mRNA levels of the housekeeping gene GAPDH. The primer sequences used are shown in the Table 1.
Protein extraction and western blot analyses
The protein was extracted using a membrane, nuclear, and cytoplasmic protein extraction kit (KeyGen Biotech, Nanjing, China) according to the manufacturer’s protocol. The protein concentration was determined using a commercial kit (Beyotime Biotechnology). Protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% separation gels for TLR2 (90 kDa) and MyD88 (33 kDa), transferred to poly-vinylidene difluoride membranes, and then blocked for 1 h at room temperature with 5% bovine serum albumin in Tris-buffered saline and 0.1% Tween-20. Western blot analyses were performed with primary antibodies. Blots were incubated with the appropriate secondary antibodies, and then the protein was detected using the enhanced chemiluminescence kit for horseradish peroxidase.
Statistical analysis
The data are expressed as the mean ± SEM. The groups were evaluated using Student’s t-test between two groups, a one-way analysis of variance, and Dunnett’s t-test among groups. The P values of <0.05 were considered statistically significant.
Results
NKT cell depletion alleviates TP-induced liver injury
We observed that TP induced hepatotoxicity in female C57BL/6 mice. Compared with control mice, continuous TP administration led to significantly increased ALT and AST levels, which began to increase at day 1 and continued to significantly increase until day 5 (Fig. 1a, b). Animals that received TP for 7 days showed significant reductions in body weights compared with the control group. Mice treated with TP and anti-NK1.1 showed insignificant differences in body weight compared with the control group (Fig. 1c). Using anti-NK1.1 to deplete NKT cells, the increasing ALT and AST levels induced by TP were significantly inhibited (Fig. 1d, e), which indicated that NKT cell depletion alleviated TP-induced hepatotoxicity. In addition, administration of anti-NK1.1 alone showed no hepatotoxic effects (data not shown).
NKT cell depletion alleviates TP-induced liver injury. Triptolide (TP; 600 μg/kg; i.g.) was administered to mice, and serum were collected at days 1, 3, and 5 after administration for the assessment of ALT (a) and AST (b). NKT cells were depleted with 200 μg of anti-NK1.1 mAb injected intraperitoneally on days −2 and −1 before TP administration. The mice were killed at 1, 3, and 5 days after the administration of TP. c Alteration in body weight. d ALT and e AST were compared between the TP group and TP+anti-NK1.1 group. All values are the mean ± SEM (n = 8). **P<0.01, ***P<0.001 vs. control. ###P<0.001 vs. TP group
Histopathological changes in livers showed tendencies toward augmented focal necrosis (black arrows), inflammatory cell infiltration (yellow arrows), and bile duct hyperplasia starting from day 1 after TP administration (Fig. 2a). Furthermore, addition of anti-NK1.1 caused protection against TP-induced liver injury. These results were confirmed by the toxicity score analysis (Fig. 2b).
Liver histopathological analysis shows that anti-NK1.1 protects against TP-induced hepatotoxicity. a The liver tissue sections were stained with hematoxylin and eosin (H&E, ×20). Focal necrosis (black arrows), inflammatory cell infiltration (yellow arrows). b Hepatotoxicity score in the absence or presence of anti-NK1.1. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
TP treatment induces NKT cell activation
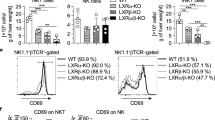
Compared with control mice, hepatic NKT cell frequencies began to decrease at day 1 and continued to decline until day 5 (Fig. 3a, b). In addition, CD3+ cells were first gated. Then, CD49b+CD69+ cells were measured to evaluate the activation of NKT cells. Upregulation of the activation marker CD69 was observed at day 3 after TP administration (Fig. 3c, d), which indicated that NKT cells were highly activated. However, a remarkable decrease in the percentage of NKT cells can be caused by other factors, such as the loss of specific NKT cell surface markers [17], reduced proliferation, increased apoptosis [18] or endoplasmic reticulum stress [19].
Triptolide treatment induces NKT cell activation. The mice were killed at days 1, 3, and 5 after the administration of triptolide (TP, 600 μg/kg; i.g.). a Liver NKT cells. b Analysis of liver NKT cells. c CD69 expression on liver NKT cells. d Analysis of CD69 expression. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
NKT cells produce Th1/Th2 cytokines after TP treatment
CD3+ cells were first gated. Then, CD49b+IFN-γ+ or CD49b+IL-4+ cells were measured to indicate the Th1/Th2 cytokine production by NKT cells. The pro-inflammatory Th1 cytokine IFN-γ produced by NKT cells began to increase at day 1 and remained at a high level until day 5 (Fig. 4a, b). Compared with IFN-γ, the Th2 cytokine IL-4 showed delayed production (Fig. 4d, e). IFN-γ can induce the generation of reactive oxygen species (ROS) and endoplasmic reticulum stress proteins in hepatocytes [20]. NKT cell-initiated injury has been reported to be dependent on the production of IFN-γ [21]. In contrast, the anti-inflammatory IL-4 has been reported to have protective effects on liver injury [22]. In addition, the CD3+CD49b−IFN-γ+ subset, which was produced by T cells, was significantly higher at day 1 than the control (Fig. 4c). The CD3+CD49b−IL-4+ subset, which was produced by T cells, increased dramatically at day 3 (Fig. 4f).
NKT cells secrete Th1/Th2 cytokines after TP treatment. The mice were killed at days 1, 3, and 5 after the administration of triptolide (TP, 600 μg/kg; i.g.). a IFN-γ produced by NKT cells. b Analysis of hepatic IFN-γ produced by NKT cells. c Analysis of hepatic IFN-γ produced by T cells. d IL-4 produced by NKT cells. e Analysis of hepatic IL-4 produced by NKT cells. f Analysis of hepatic IL-4 produced by T cells. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
TP treatment induces leukocyte recruitment
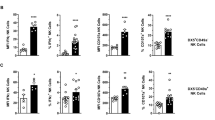
Hepatic neutrophil (CD45+CD11b+Ly-6G+ cells) frequencies began to increase at day 3 and peaked at day 5 compared with the control mice (Fig. 5a, b). The percentage of hepatic macrophages (CD45+CD11b+F4/80+ cells), and not traditional Kupffer cells, started to increase at day 3 and reached the highest level at day 5 compared with the control mice (Fig. 5c, d).
TP treatment induces leukocyte recruitment. The mice were killed at days 1, 3, and 5 after the administration of triptolide (TP, 600 μg/kg; i.g.). a The percentages of hepatic neutrophils. b Analysis of hepatic neutrophils. c The percentages of hepatic macrophages. d Analysis of hepatic macrophages. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
NKT cell depletion alters Th1/Th2 cytokine production and leukocyte infiltration
TP and anti-NK1.1 treatment significantly reduced the production of IFN-γ from day 1 to day 5 and decreased the production of IL-4 on day 1 (Fig. 6a, b). Moreover, the production of IL-4 at day 5 showed a negative feedback upregulation (Fig. 6b). NKT cell depletion also markedly diminished the recruitment of neutrophils and macrophages at days 3 and 5 (Fig. 6c, d). The recruitment of neutrophils is inhibited in CD1d-/- mice [11]. The production of IFN-γ and IL-4 by NKT cells contribute to the accumulation of neutrophils and macrophages [14, 23]. However, whether the diminished production of IFN-γ and IL-4 leads to reduced hepatic neutrophil and macrophages has been rarely reported. The decreased production of IFN-γ and IL-4 might have partly contributed to the inhibition of neutrophil and macrophage recruitment.
NKT cell depletion alters Th1/Th2 cytokine production and leukocyte infiltration. NKT cells were depleted with 200 µg of anti-NK1.1 mAb injected intraperitoneally on days −2 and −1 before TP administration. The mice were killed at days 1, 3, and 5 after the administration of triptolide (TP, 600 μg/kg, i.g.). a Analysis of IFN-γ produced by NKT cells. b Analysis of IL-4 produced by NKT cells. c Analysis of hepatic neutrophils. d Analysis of hepatic macrophages. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. TP group
TP treatment induces the downregulation of CD1d and upregulation of the TLR signaling pathway
The level of CD1d expression correlates with the ability to maintain NKT cell homeostasis [24]. Decreased hepatocytes CD1d expression at day 1 could lead to NKT cell depletion (Fig. 7a). TLR2 mRNA displayed a significant upregulation (Fig. 7b). The MyD88 (myeloid differentiation primary response 88)-dependent pathway is one of the major downstream pathways in TLR signaling, which promotes the production of cytokines and chemokines [25]. Immunoblot results showed that the TLR2–MyD88 pathway was upregulated after TP treatment (Fig. 7c). Both CD1d and TLRs play a key role in the activation of NKT cells [23].
TP treatment induces the downregulation of CD1d and upregulation of the TLR signaling pathway. The mice were killed at days 1, 3, and 5 after the administration of triptolide (TP, 600 μg/kg, i.g.). a Hepatic mRNA expression of CD1d. b Hepatic mRNA expression of TLR2. c Protein was isolated from livers for immunoblot analysis. GAPDH or β-actin was used as a loading control. Representative immunoblots for each protein are shown. d Hepatic mRNA expression of Fas. e Serum HMGB1 levels. f Serum LPS levels. g Serum MIP-2 levels. All values are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
Activated NKT cells could kill hepatocytes directly via the effector of Fas/Fas ligand [23]. Hepatic expression of Fas was rapidly upregulated at day 1, which might kill hepatocytes and NKT cells themselves (Fig. 7d). HMGB1 is recognized as one of the most important DAMPs among liver injuries [26]. HMGB1 increased strikingly at days 3 and 5 compared with control mice (Fig. 7e). Endotoxin LPS can be released into blood when hepatotoxicants destroy the permeability of the intestine [27]. Serum LPS showed moderate increases following TP administration at days 1 and 5 (Fig. 7f). Serum MIP2 increased significantly at day 1 after TP administration, which could not only activate NKT cells but also recruit neutrophils in the liver [28] (Fig. 7g).
Discussion
The liver is the primary reservoir of NKT cells. NKT cells regulate both innate and adaptive immune responses [29]. The number of hepatic NKT cells decreases in various animal models [30], including in leptin-deficient mice [31] or high-fat-diet-induced liver steatosis [32], bacterial liver injury [17], Con A-induced liver injury [10], and hepatotoxic liver injury [19]. However, following hepatic ischemia reperfusion injury [21] or partial hepatectomy [33], the number of NKT cells increases. This change in cell number has been reported to be attributed to the loss of specific NKT cell surface markers [17], activation-induced cell death (AICD) [10], sympathetic activation [31], endoplasmic reticulum stress [19], and reduced proliferation or increased apoptosis [34, 35]. The pathological role of NKT cells in DILI remains largely unknown.
Inhibition of the mitochondrial respiratory chain has been reported in TP-induced liver injury, featured by a depolarized mitochondrial membrane, increase in ROS, and enhanced oxidant stress [4]. Endogenous ROS stimulates Th1 signaling in NKT cells, which promote the development of acute fulminant liver failure [36]. The transcription factor of NKT cells, promyelocytic leukemia zinc finger, also controls ROS levels, which in turn govern the inflammatory function of NKT cells [37]. Therefore, TP may induce the interaction of ROS and Th1/Th2 signaling in NKT cells. The Th17/regulatory T (Treg) cell imbalance has also been reported to play a role in TP-induced liver injury [15, 39, 40]. TP is a natural reactive electrophile containing diterpene diepoxide lactone, with the ability to covalently bind to XPB (xeroderma pigmentosum type B) often linked to its hepatotoxity. The transcription factor XPB subunit is important for initiating mRNA and protein synthesis, which may be the reason for the multiple effects of TP on the immune system [38]. The correlation between NKT cells and Th17/Treg cells remains unclear. However, NKT cells have been considered to initiate a faster immune response than other Th cells [41]. Therefore, the aim of the present study was to investigate the role of NKT cells in TP-induced liver injury.
In TP-induced hepatotoxicity, sex-related differences in the toxicity of TP have received attention in recent years [42]. Female mice exhibit more severe toxicity than male mice [3]. Since sex is a fundamental biological variable that cannot be overlooked, only female mice were used in our toxicological research of TP. NKT cell depletion significantly alleviated TP-induced liver injury and inhibited the increase in ALT and AST. Although anti-NK1.1 was not specific, depleting both NKT cells and NK cells, our data still confirm that NKT cells play a crucial role in TP-induced liver injury.
In the present study, we observed remarkably decreased NKT cells from day 1. The disappearance of NKT cells is thought to be an early sign of NKT cell activation [43]. Activation of NKT cells was not only assessed by their percentage but also by their surface phenotype, such as CD69, an early activation marker. Upregulation of CD69 leads to AICD, and downregulation of CD1d also causes a reduction of NKT cells. This change in NKT cell percentages has also been reported to be attributed to decreased norepinephrine [31], endoplasmic reticulum stress [19], reduced proliferation, or increased apoptosis [18]. Several studies have suggested that tissue-specific decreases in NKT cells promote organ damage [31]. Activated NKT cells also killed themselves and hepatocytes directly via increased Fas expression, which caused liver injury (Fig. 7).
Interestingly, NKT cells could produce both Th1 and Th2 cytokines, making them both pathogenic and protective [7]. When tissue NKT cell populations are decreased, over-production of pro-inflammatory cytokines leads to organ damage. In our study, NKT cells predominantly produced IFN-γ following TP treatment. An overabundance of the pro-inflammatory cytokine IFN-γ not only led to hepatic accumulation of neutrophils and macrophages, but it also sensitized hepatocytes to liver injury [44]. The resulting neutrophil influx can also exacerbate tissue damage. The delayed production of the Th2 cytokine IL-4 was thought to be anti-inflammatory and protective (Fig. 4). Moreover, NKT cells can control pro-inflammatory Th1 cytokine activities by inducing the expression of anti-inflammatory Th2 cytokines.
Furthermore, NKT cell depletion remarkably reduced hepatic neutrophil and macrophage infiltration and induced decreased IFN-γ production from NKT cells. However, hepatic IL-4 production showed a delayed negative-feedback upregulation. It has been reported that IL-4 also facilitates tissue repair and resolves the inflammatory response [45]. These findings suggest that NKT cell depletion might contribute to the promotion of liver repair by regulating Th1/Th2 cytokine expression.
NKT cells can be readily activated by DAMPs released from damaged cells [11]. DAMPs are biomolecules that can initiate and perpetuate inflammatory responses [46]. HMGB1, one of the most important DAMPs, has been reported to induce pro-inflammatory cytokine synthesis, activate macrophages and recruit neutrophils in liver through activation of TLRs [47]. In our study, HMGB1 significantly increased after TP treatment, which could be associated with the NKT cell-induced inflammatory response. (Fig. 7). TLRs constitute the first defense of the immune system. TLRs initiate cytokine secretion and stimulate the immune response of the organism through their signal transduction pathways, such as the cytoplasmic adapter proteins MyD88, TIRAP, TRIF, and TRAM [48]. Two major downstream pathways are involved in TLR signaling: the MyD88-dependent pathway that causes early production of pro-inflammatory cytokines and the TRIF-dependent pathway that causes late-phase activation. In addition to TLR3, the remaining TLRs mediate the MyD88-dependent pathway [49]. Recent studies have highlighted the functional importance and increased expression of TLRs 2, 4, and 9 in liver diseases [50]. Our study showed that the NKT cell-mediated immune response was associated with recognition of the danger signals HMGB1 through the binding of TLR2, leading to the upregulation of the TLR2–MyD88 pathway (Fig. 7). However, elucidation of the specific target of TP on NKT cells requires further study.
In summary, our study demonstrated that NKT cell-depleted mice were resistant to the development of TP-induced liver injury. NKT cell depletion also exhibited significantly lower percentages of infiltrating neutrophils and macrophages in the liver and reduced production of IFN-γ by NKT cells upon TP challenge. This finding strongly suggests that NKT cells play a critical role in the development and progression of TP-induced liver injury. In addition, TLR signaling pathway contributed to the NKT cell-associated immune response in TP-induced liver injury. Collectively, these findings position NKT cells as potential targets for pharmacotherapy of DILI. The novel role of TP in the activation of NKT cells elucidates novel mechanisms of TP-induced liver injury. In addition, regulating Th1/Th2 balance through NKT cell depletion will promote the discovery of effective measures that may control the development of DILI.
References
Li XJY, Jiang ZZ, Zhang LY. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155:67–79.
Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11:377–83.
Jiang Z, Huang X, Huang S, Guo H, Wang L, Li X, et al. Sex-related differences of lipid metabolism induced by triptolide: the possible role of the LXRalpha/SREBP-1 signaling pathway. Front Pharmacol. 2016;7:87.
Fu Q, Huang X, Shu B, Xue M, Zhang P, Wang T, et al. Inhibition of mitochondrial respiratory chain is involved in triptolide-induced liver injury. Fitoterapia. 2011;82:1241–8.
Regev A. Drug-induced liver injury and drug development: industry perspective. Semin Liver Dis. 2014;34:227–39.
Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2011;343:43–55.
Li N, Hua JL. Immune cells in liver regeneration. Oncotarget. 2017;8:3628–39.
Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–55.
Deng ZB, Liu YL, Liu CR, Xiang XY, Wang JH, Cheng ZQ, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–20.
Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–503.
Cheng L, You Q, Yin H, Holt MP, Ju C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem Pharmacol. 2010;80:255–61.
Guo H, Pan C, Chang B, Wu X, Guo J, Zhou Y, et al. Triptolide improves diabetic nephropathy by regulating Th cell balance and macrophage infiltration in rat models of diabetic nephropathy. Exp Clin Endocr Diab. 2016;124:389–98.
Zhang Y, Ma X. Triptolide inhibits IL-12/IL-23 expression in APCs via CCAAT/enhancer-binding protein alpha. J Immunol. 2010;184:3866–77.
Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology. 2013;58:1474–85.
Wang X, Jiang Z, Cao W, Yuan Z, Sun L, Zhang L. Th17/Treg imbalance in triptolide-induced liver injury. Fitoterapia. 2014;93:245–51.
Wang Y, Jia L, Wu CY. Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. ScandJ Immunol. 2008;68:383–90.
Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–15.
Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–8.
Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–94.
Watanabe Y, Suzuki O, Haruyama T, Akaike T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem. 2003;89:244–53.
Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–48.
Zhang NN, Huang NY, Zhou XK, Luo XL, Liu CY, Zhang Y, et al. Protective effects of IL-4 on Bacillus Calmette-Guerin and lipopolysaccharide induced immunological liver injury in mice. Inflamm Res. 2012;61:17–26.
Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–20.
Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–93.
O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64.
Gong QA, Zhang H, Li JH, Duan LH, Zhong S, Kong XL, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med. 2010;88:1289–98.
Kallus SJ, Brandt LJ. The intestinal microbiota and obesity. J Clin Gastroenterol. 2012;46:16–24.
Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–60.
Margalit M, Ilan Y. Induction of immune tolerance: a role for natural killer T lymphocytes? Liver Int. 2005;25:501–4.
Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–52.
Li ZP, Oben JA, Yang SQ, Lin HZ, Stafford EA, Soloski MJ, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–41.
Ma X, Hua J, Li ZP. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–30.
Kato T, Sato Y, Takahashi S, Kawamura H, Hatakeyama K, Abo T. Involvement of natural killer T cells and granulocytes in the inflammation induced by partial hepatectomy. J Hepatol. 2004;40:285–90.
Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, et al. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31:1720–7.
Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, et al. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–15.
Downs I, Liu J, Aw TY, Adegboyega PA, Ajuebor MN. The ROS scavenger, NAC, regulates hepatic Valpha14iNKT cells signaling during Fas mAb-dependent fulminant liver failure. PLoS One. 2012;7:e38051.
Kim YH, Kumar A, Chang CH, Pyaram K. Reactive oxygen species regulate the inflammatory function of NKT cells through promyelocytic leukemia zinc finger. J Immunol. 2017;199:3478–87.
He QL, Titov DV, Li J, Tan M, Ye Z, Zhao Y, et al. Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide. Angew Chem Int Ed. 2015;54:1859–63.
Wang X, Jiang Z, Xing M, Fu J, Su Y, Sun L, et al. Interleukin-17 mediates triptolide-induced liver injury in mice. Food Chem Toxicol. 2014;71:33–41.
Wang X, Sun L, Zhang L, Jiang Z. Effect of adoptive transfer or depletion of regulatory T cells on triptolide-induced liver injury. Front Pharmacol. 2016;7:99.
Subramanian M, Kini R, Madasu M, Ohta A, Nowak M, Exley M, et al. Extracellular adenosine controls NKT-cell-dependent hepatitis induction. Eur J Immunol. 2014;44:1119–29.
Liu L, Jiang Z, Liu J, Huang X, Wang T, Liu J, et al. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology. 2010;271:57–63.
Kawamura T, Takeda K, Kaneda H, Matsumoto H, Hayakawa Y, Raulet DH, et al. NKG2A inhibits invariant NKT cell activation in hepatic injury. J Immunol. 2009;182:250–8.
Shimizu Y, Margenthaler JA, Landeros K, Otomo N, Doherty G, Flye MW. The resistance of P. acnes--primed interferon gamma-deficient mice to low-dose lipopolysaccharide-induced acute liver injury. Hepatology. 2002;35:805–14.
Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–43.
Kwon HJ, Won YS, Park O, Feng DC, Gao B. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology. 2014;59:1094–106.
Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–43.
Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511–9.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Seki E, Park E, Fujimoto J. Toll-like receptor signaling in liver regeneration, fibrosis and carcinogenesis. Hepatol Res. 2011;41:597–610.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (No. 81703626, No. 81773995, No. 81773827, No. 81573514, No. 81673684, No. 81673443, No. 81573690, and No. 81320108029), the Fundamental Research Funds for the Central Universities (2632017PY11), the Natural Science Foundation of Jiangsu Province (BK20151439), and grants from the College Students Innovation Project for the R&D of Novel Drugs (J1310032).
Author contributions
X-zW designed the experiments; X-zW, R-fX, and S-yZ performed the experiments; X-zW, Y-tZ, and L-yZ analyzed and discussed the data; X-zW and Z-zJ wrote the paper. All authors contributed to editing of the paper and scientific discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wang, Xz., Xue, Rf., Zhang, Sy. et al. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacol Sin 39, 1847–1854 (2018). https://doi.org/10.1038/s41401-018-0084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-018-0084-9
Keywords
This article is cited by
-
Screening of major hepatotoxic components of Tripterygium wilfordii based on hepatotoxic injury patterns
BMC Complementary Medicine and Therapies (2023)
-
Activation of cDCs and iNKT cells contributes to triptolide-induced hepatotoxicity via STING signaling pathway and endoplasmic reticulum stress
Cell Biology and Toxicology (2023)
-
Recent advances in aggregation-induced emission luminogens in photoacoustic imaging
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
Combination of metronomic administration and target delivery strategies to improve the anti-angiogenic and anti-tumor effects of triptolide
Drug Delivery and Translational Research (2020)