Abstract
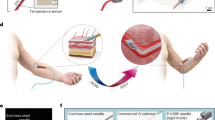
The precision of the delivery of therapeutics to the desired injection site by syringes and hollow needles typically depends on the operator. Here, we introduce a highly sensitive, completely mechanical and cost-effective injector for targeting tissue reliably and precisely. As the operator pushes the syringe plunger, the injector senses the loss-of-resistance on encountering a softer tissue or a cavity, stops advancing the needle and delivers the payload. We demonstrate that the injector can reliably deliver liquids to the suprachoroidal space—a challenging injection site that provides access to the back of the eye—for a wide range of eye sizes, scleral thicknesses and intraocular pressures, and target sites relevant for epidural injections, subcutaneous injections and intraperitoneal access. The design of this simple and effective injector can be adapted for a broad variety of clinical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information.
References
Tsukuda, Y. Venipuncture nerve injuries in the upper extremity from more than 1 million procedures. J. Patient Saf. https://doi.org/10.1097/PTS.0000000000000264 (2016).
Oven, S. & Johnson, J. Radial nerve injury after venipuncture. J. Hand Microsurg. 9, 43–44 (2017).
Aders, A. & Aders, H. Anaesthetic adverse incident reports: an Australian study of 1,231 outcomes. Anaesth. Intens. Care 33, 336–344 (2005).
Magrina, J. F. Complications of laparoscopic surgery. Clin. Obstet. Gynecol. 45, 469–480 (2002).
Benzon, H. T., Huntoon, M. A. & Rathmell, J. P. Improving the safety of epidural steroid injections. J. Am. Med. Assoc. 313, 1713 (2015).
Wu, T., Zhao, W., Dong, Y., Song, H. & Li, J. Effectiveness of ultrasound-guided versus fluoroscopy or computed tomography scanning guidance in lumbar facet joint injections in adults with facet joint syndrome: a meta-analysis of controlled trials. Arch. Phys. Med. Rehabil. 97, 1558–1563 (2016).
Evansa, I., Logina, I., Vanags, I. & Borgeat, A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases. Eur. J. Anaesthesiol. 32, 262–268 (2015).
Bui, J. & Bogduk, N. A systematic review of the effectiveness of CT-guided, lumbar transforaminal injection of steroids. Pain Med. 14, 1860–1865 (2013).
Whipple, T. L. in Sports Injuries 2335–2342 (Springer Berlin, 2015).
D’Agostino, M. A. & Schmidt, W. A. Ultrasound-guided injections in rheumatology: actual knowledge on efficacy and procedures. Best Pract. Res. Clin. Rheumatol. 27, 283–294 (2013).
Palmer, R. Instrumentation et technique de la coelioscopie gynécologique. Gynecol. Obstet. 46, 420–431 (1947).
Bassett, E. K. et al. Design of a mechanical clutch-based needle-insertion device. Proc. Natl Acad. Sci. USA 106, 5540–5545 (2009).
Begg, N. D. M. Blind Transmembrane Puncture Access: Design and Development of a Novel Laparoscopic Trocar and Blade Retraction Mechanism. MSc Thesis, Massachusetts Institute of Technology (2011).
Anderson, T. A., Kang, J. W., Gubin, T., Dasari, R. R. & So, P. T. C. Raman spectroscopy differentiates each tissue from the skin to the spinal cord. Anesthesiology 125, 793–804 (2016).
Riley, E. T. & Carvalho, B. The Episure syringe: a novel loss of resistance syringe for locating the epidural space. Anesth. Analg. 105, 1164–1166 (2007).
Vilos, G. & Vilos, A. The Veress needle causes most laparoscopic injuries and should be abandoned: against: evidence indicates that the primary trocar, not the Veress, causes most serious complications. BJOG 122, 142 (2015).
Vindal, A. & Lal, P. The Veress needle causes most laparoscopic injuries and should be abandoned: for: the open technique is safer and saves time. BJOG 122, 141 (2015).
Cornette, B. & Berrevoet, F. Trocar injuries in laparoscopy: techniques, tools, and means for prevention. A systematic review of the literature. World J. Surg. 40, 2331–2341 (2016).
Gonenc, B., Taylor, R. H., Iordachita, I., Gehlbach, P. & Handa, J. Force-sensing microneedle for assisted retinal vein cannulation. In Proc. IEEE Sensors 2014 698–701 (IEEE, 2014).
Moisseiev, E., Loewenstein, A. & Yiu, G. The suprachoroidal space: from potential space to a space with potential. Clin. Ophthalmol. 10, 173–178 (2016).
Olsen, T. W. et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am. J. Ophthalmol. 142, 777–787 (2006).
Patel, S. R., Lin, A. S. P., Edelhauser, H. F. & Prausnitz, M. R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 28, 166–176 (2011).
Patel, S. R. et al. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investig. Ophthalmol. Vis. Sci. 53, 4433–4441 (2012).
Abarca, E. M., Salmon, J. H. & Gilger, B. C. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J. Ocul. Pharmacol. Ther. 29, 715–722 (2013).
Kadam, R. S., Williams, J., Tyagi, P., Edelhauser, H. F. & Kompella, U. B. Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties, regional differences in delivery, and comparison with intravitreal and intracameral routes. Mol. Vis. 19, 1198–1210 (2013).
Gilger, B. C., Abarca, E. M., Salmon, J. H. & Patel, S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Investig. Ophthalmol. Vis. Sci. 54, 2483–2492 (2013).
Chen, M., Li, X., Liu, J., Han, Y. & Cheng, L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J. Control. Release 203, 109–117 (2015).
Chiang, B. et al. Thickness and closure kinetics of the suprachoroidal space following microneedle injection of liquid formulations. Investig. Opthalmol. Vis. Sci. 58, 555–564 (2017).
Chiang, B., Venugopal, N., Edelhauser, H. F. & Prausnitz, M. R. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp. Eye Res. 153, 101–109 (2016).
Gu, B. et al. Real-time monitoring of suprachoroidal space (SCS) following SCS injection using ultra-high resolution optical coherence tomography in guinea pig eyes. Invest. Ophthalmol. Vis. Sci. 56, 3623–3634 (2015).
Chen, K., Rowley, A. P., Weiland, J. D. & Humayun, M. S. Elastic properties of human posterior eye. J. Biomed. Mater. Res. A 102, 2001–2007 (2014).
Peden, M. C. et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS ONE 6, e17140 (2011).
Goldstein, D. A. Achieving drug delivery via the suprachoroidal space. Retina Today July/August, 82–84 (2014).
Zhang, L. et al. Long-term therapeutic effects of mesenchymal stem cells compared to dexamethasone on recurrent experimental autoimmune uveitis of rats. Invest. Ophth. Vis. Sci. 55, 5561–5571 (2014).
Cao, J. et al. Human umbilical tissue-derived cells rescue retinal pigment epithelium dysfunction in retinal degeneration. Stem Cells 34, 367–379 (2016).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye. Res. 29, 144–168 (2010).
Raju, J. R. & Weinberg, D. V. Accuracy and precision of intraocular injection volume. Am. J. Ophthalmol. 133, 564–566 (2002).
Muffly, M. K. et al. Small-volume injections: evaluation of volume administration deviation from intended injection volumes. Anesth. Analg. 125, 1192–1199 (2017).
Strehle, E.-M. Making the invisible visible: near-infrared spectroscopy and phlebotomy in children. Telemed. J. E. Health 16, 889–893 (2010).
VGStudio Max 2.2 (Volume Graphics GmbH); https://www.volumegraphics.com.
Computer-aided three-dimensional interactive application (CATIA) v.5 (Dassault Systèmes, 2018); https://www.3ds.com/products-services/catia/.
Acknowledgements
Funding for this research was provided by grant no. R01HL095722 to J.M.K. Animal experiments were funded through the Boston-KPro research fund to M.G.A. and A.C. We thank Y. Jung and C. P. Lin for use of the intravital microscope, H. G. Lin and Harvard’s Center for Nanoscale Systems for use of the microCT system, and K. Cormier and the Hope Babette Tang Histology Facility at the Koch Institute at MIT for performing histology sections. We also thank the Schepens Eye Research Institute’s Animal Facility, especially J. Hoadley, M. Ortega and C. Beiler for their help. We appreciate the feedback on performing SCS injections with an I2T2 from D. Vavvas, V. Satitpitakul and K. Suri, from the Massachusetts Eye and Ear Infirmary.
Author information
Authors and Affiliations
Contributions
G.D.C. M.K.S.V. and J.M.K. conceptualized and iterated the principle and design of the I2T2. G.D.C., M.K.S.V. and J.L. were responsible for study design, guided by J.M.K. G.D.C. and J.L. developed the analytical model, improved the design and performed detailed experimental studies with focus on ocular applications. G.D.C., M.G.-A., A.C. and J.L. designed and performed the in vivo experiments. B.E.M., A.S., N.L.-B., Z.T., K.M. and K.Y. provided experimental support for the experiments with cell injections in the SCS. G.D.C., M.K.S.V. and A.D. tested the device for applications other than eye. G.D.C. and J.L. were responsible for conceptual drawings and data representation. B.E.M. captured all the photographs under the supervision of G.D.C. and J.L. G.D.C. wrote the manuscript with constructive feedback and editing from J.M.K., J.L., B.E.M. and P.A.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and video captions.
Supplementary Video 1
Ventricular access through the heart wall by using i2T2 (ex vivo model).
Supplementary Video 2
Accessing the peritoneal cavity through the abdominal wall by using i2T2 (ex vivo model).
Rights and permissions
About this article
Cite this article
Chitnis, G.D., Verma, M.K.S., Lamazouade, J. et al. A resistance-sensing mechanical injector for the precise delivery of liquids to target tissue. Nat Biomed Eng 3, 621–631 (2019). https://doi.org/10.1038/s41551-019-0350-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0350-2
This article is cited by
-
A wireless battery-free eye modulation patch for high myopia therapy
Nature Communications (2024)
-
A temperature-responsive intravenous needle that irreversibly softens on insertion
Nature Biomedical Engineering (2023)
-
Current State and Opportunities with Long-acting Injectables: Industry Perspectives from the Innovation and Quality Consortium “Long-Acting Injectables” Working Group
Pharmaceutical Research (2023)
-
Site- and Zone-Dependent Changes in Proteoglycan Content and Biomechanical Properties of Bluntly and Sharply Grooved Equine Articular Cartilage
Annals of Biomedical Engineering (2022)
-
Measuring the Hyperelastic Response of Porcine Liver Tissues In-Vitro Using Controlled Cavitation Rheology
Experimental Mechanics (2021)