Abstract
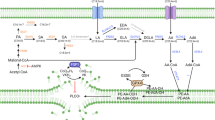
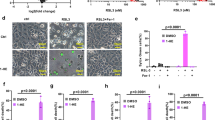
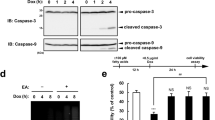
Reactive oxygen species (ROS) are transient, highly reactive intermediates or byproducts produced during oxygen metabolism. However, when innate mechanisms are unable to cope with sequestration of surplus ROS, oxidative stress results, in which excess ROS damage biomolecules. Oxidized phosphatidylserine (PS), a proapoptotic ‘eat me’ signal, is produced in response to elevated ROS, yet little is known regarding its chemical composition and metabolism. Here, we report a small molecule that generates ROS in different mammalian cells. We used this molecule to detect, characterize and study oxidized PS in mammalian cells. We developed a chemical–genetic screen to identify enzymes that regulate oxidized PS in mammalian cells and found that the lipase ABHD12 hydrolyzes oxidized PS. We validated these findings in different physiological settings including primary peritoneal macrophages and brains from Abhd12–/– mice under inflammatory stress, and in the process, we functionally annotated an enzyme regulating oxidized PS in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all the data that support the findings of this study are available in the paper, associated supplementary information files and datasets.
References
Aruoma, O. I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil. Chem. Soc. 75, 199–212 (1998).
Niedzielska, E. et al. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 53, 4094–4125 (2016).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Liou, G. Y. & Storz, P. Reactive oxygen species in cancer. Free. Radic. Res. 44, 479–496 (2010).
Imlay, J. A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 (2013).
Porter, N. A. A perspective on free radical autoxidation: the physical organic chemistry of polyunsaturated fatty acid and sterol peroxidation. J. Org. Chem. 78, 3511–3524 (2013).
Yin, H., Xu, L. & Porter, N. A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011).
Spickett, C. M. & Pitt, A. R. Oxidative lipidomics coming of age: advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox. Signal. 22, 1646–1666 (2015).
Smith, W. L. & Murphy, R. C. Oxidized lipids formed non-enzymatically by reactive oxygen species. J. Biol. Chem. 283, 15513–15514 (2008).
Vance, J. E. & Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554 (2013).
Leventis, P. A. & Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 (2010).
Hazen, S. L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283, 15527–15531 (2008).
Greenberg, M. E. et al. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625 (2006).
Kagan, V. E. et al. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J. Immunol. 169, 487–499 (2002).
Fiskerstrand, T. et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: an inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 87, 410–417 (2010).
Chen, D. H. et al. Two novel mutations in ABHD12: expansion of the mutation spectrum in PHARC and assessment of their functional effects. Hum. Mutat. 34, 1672–1678 (2013).
Dharmaraja, A. T. & Chakrapani, H. A small molecule for controlled generation of reactive oxygen species (ROS). Org. Lett. 16, 398–401 (2014).
Dharmaraja, A. T., Alvala, M., Sriram, D., Yogeeswari, P. & Chakrapani, H. Design, synthesis and evaluation of small molecule reactive oxygen species generators as selective Mycobacterium tuberculosis inhibitors. Chem. Commun. (Camb). 48, 10325–10327 (2012).
Tyagi, P., Dharmaraja, A. T., Bhaskar, A., Chakrapani, H. & Singh, A. Mycobacterium tuberculosis has diminished capacity to counteract redox stress induced by elevated levels of endogenous superoxide. Free Radic. Biol. Med. 84, 344–354 (2015).
Long, J. Z. & Cravatt, B. F. The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem. Rev. 111, 6022–6063 (2011).
Huu, T. P., Marquetty, C., Pasquier, C. & Hakim, J. Luminol assay for microdetermination of superoxide dismutase activity: its application to human fetal blood. Anal. Biochem. 142, 467–472 (1984).
Zhao, H. et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 102, 5727–5732 (2005).
McCormack, D. & McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell Longev. 2013, 575482 (2013).
Kerksick, C. & Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports. Nutr. 2, 38–44 (2005).
Liebeke, M. et al. Depletion of thiol-containing proteins in response to quinones in Bacillus subtilis. Mol. Microbiol. 69, 1513–1529 (2008).
O’Brien, P. J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 80, 1–41 (1991).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Speers, A. E., Adam, G. C. & Cravatt, B. F. Activity-based protein profiling in vivo using a copper(i)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 125, 4686–4687 (2003).
Zschörnig, K. & Schiller, J. A simple method to generate oxidized phosphatidylcholines in amounts close to one milligram. Chem. Phys. Lipids 184, 30–37 (2014).
Pathak, D., Mehendale, N., Singh, S., Mallik, R. & Kamat, S. S. Lipidomics suggests a new role for ceramide synthase in phagocytosis. ACS Chem. Biol. 13, 2280–2287 (2018).
Knittelfelder, O. L. & Kohlwein, S. D. Thin-layer chromatography to separate phospholipids and neutral lipids from yeast. Cold Spring Harb. Protoc. 2017, pdb.prot085456 (2017).
Kamat, S. S. et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 11, 164–171 (2015).
Nomura, D. K. & Casida, J. E. Lipases and their inhibitors in health and disease. Chem. Biol. Interact. 259, 211–222 (2016).
Wu, C., Jin, X., Tsueng, G., Afrasiabi, C. & Su, A. I. BioGPS: building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 44, D313–D316 (2016).
Hoover, H. S., Blankman, J. L., Niessen, S. & Cravatt, B. F. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg. Med. Chem. Lett. 18, 5838–5841 (2008).
Liu, Y., Patricelli, M. P. & Cravatt, B. F. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96, 14694–14699 (1999).
Long, J. Z. et al. Metabolomics annotates ABHD3 as a physiologic regulator of medium-chain phospholipids. Nat. Chem. Biol. 7, 763–765 (2011).
Ramanadham, S. et al. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J. Lipid Res. 56, 1643–1668 (2015).
Blankman, J. L., Long, J. Z., Trauger, S. A., Siuzdak, G. & Cravatt, B. F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 110, 1500–1505 (2013).
Hsu, H. Y. & Wen, M. H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 277, 22131–22139 (2002).
Viader, A. et al. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. eLife 5, e12345 (2016).
You, L. H. et al. Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis. 16, e2676 (2017).
Hou, C. et al. Development of a positron emission tomography radiotracer for imaging elevated levels of superoxide in neuroinflammation. ACS Chem. Neurosci. 9, 578–586 (2018).
Matsura, T. et al. The presence of oxidized phosphatidylserine on Fas-mediated apoptotic cell surface. Biochim. Biophys. Acta 1736, 181–188 (2005).
Joshi, A. et al. Biochemical characterization of the PHARC-associated serine hydrolase ABHD12 reveals its preference for very-long-chain lipids. J. Biol. Chem. 293, 16953–16963 (2018).
Blankman, J. L., Simon, G. M. & Cravatt, B. F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 (2007).
Niphakis, M. J. et al. A global map of lipid-binding proteins and their ligandability in cells. Cell 161, 1668–1680 (2015).
Hulce, J. J., Cognetta, A. B., Niphakis, M. J., Tully, S. E. & Cravatt, B. F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 10, 259–264 (2013).
Rosenson, R. S. & Stafforini, D. M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 53, 1767–1782 (2012).
Shimanaka, Y. et al. Omega-3 fatty acid epoxides are autocrine mediators that control the magnitude of IgE-mediated mast cell activation. Nat. Med. 23, 1287–1297 (2017).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Simon, G. M. & Cravatt, B. F. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J. Biol. Chem. 285, 11051–11055 (2010).
Inloes, J. M. et al. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc. Natl. Acad. Sci. USA 111, 14924–14929 (2014).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics with StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Fahy, E., Sud, M., Cotter, D. & Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 35, W606–W612 (2007).
Hsu, K. L. et al. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 8, 999–1007 (2012).
Nomura, D. K. et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813 (2011).
Acknowledgements
This work was supported by grants from the Wellcome Trust DBT India Alliance (IA/I/15/2/502058 to S.S.K.), DST-SERB (ECR/2016/001261 to S.S.K.; EMR/2015/000668 to H.C.), DBT (BT/PR15848/MED/29/1025/2016 to H.C.) and DST-FIST (Infrastructure Development to IISER Pune Biology Department). B. F. Cravatt (The Scripps Research Institute) is thanked for providing chemical compounds, inhibitors and ABHD12-knockout mice used in this study, and for insightful comments on the manuscript. N. Balasubramanian (IISER Pune) is thanked for access to the EVOS Imaging System for the cellular fluorescence experiments. The National Facility for Gene Function in Health and Disease, IISER Pune, is thanked for maintaining and providing mice for this study. G.R., A.K.S. and A.R. acknowledge research fellowships from the Council for Scientific and Industrial Research (CSIR), Government of India, and N.M. acknowledges a research fellowship from DBT, Government of India.
Author information
Authors and Affiliations
Contributions
D.S.K., N.M., S.S., A.J., A.R. and S.S.K. performed the biochemical experiments and analyzed the data. G.R., A.K.S., A.M. and H.C. synthesized and chemically characterized all the chemical compounds in this study. D.S.K. and S.S.K. performed and analyzed the proteomics data. S.S.K. and H.C. conceived the project and designed the experiments. S.S.K. wrote the paper, to which all authors provided input.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–26
Supplementary Note
Synthetic procedures
Supplementary Dataset 1
LC-MS/MS based chemoproteomic characterization
Supplementary Dataset 2
MRM transitions and quantitation of PS, lyso-PS and oxidized PS lipids
Supplementary Dataset 3
Highly focused library of lipase inhibitors tested in the chemical genetic screen
Supplementary Dataset 4
Complete proteomics data sets from RAW264.7 cells
Rights and permissions
About this article
Cite this article
Kelkar, D.S., Ravikumar, G., Mehendale, N. et al. A chemical–genetic screen identifies ABHD12 as an oxidized-phosphatidylserine lipase. Nat Chem Biol 15, 169–178 (2019). https://doi.org/10.1038/s41589-018-0195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0195-0
This article is cited by
-
Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma
Nature Communications (2022)
-
Genetic architecture and genomic selection of fatty acid composition predicted by Raman spectroscopy in rainbow trout
BMC Genomics (2021)
-
Current Knowledge on the Biology of Lysophosphatidylserine as an Emerging Bioactive Lipid
Cell Biochemistry and Biophysics (2021)
-
The Lysophosphatidylserines—An Emerging Class of Signalling Lysophospholipids
The Journal of Membrane Biology (2020)