Abstract
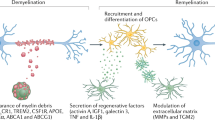
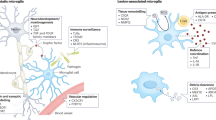
Mononuclear phagocytes are key regulators of both tissue damage and repair in neuroinflammatory conditions such as multiple sclerosis. To examine divergent phagocyte phenotypes in the inflamed CNS, we introduce an in vivo imaging approach that allows us to temporally and spatially resolve the evolution of phagocyte polarization in a murine model of multiple sclerosis. We show that the initial proinflammatory polarization of phagocytes is established after spinal cord entry and critically depends on the compartment they enter. Guided by signals from the CNS environment, individual phagocytes then switch their phenotype as lesions move from expansion to resolution. Our study thus provides a real-time analysis of the temporospatial determinants and regulatory principles of phagocyte specification in the inflamed CNS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Mishra, M. K. & Yong, V. W. Myeloid cells - targets of medication in multiple sclerosis. Nat. Rev. Neurol. 12, 539–551 (2016).
Henderson, A. P., Barnett, M. H., Parratt, J. D. & Prineas, J. W. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann. Neurol. 66, 739–753 (2009).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Brück, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Huitinga, I., van Rooijen, N., de Groot, C. J., Uitdehaag, B. M. & Dijkstra, C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J. Exp. Med. 172, 1025–1033 (1990).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Ryu, J. K. et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat. Commun. 6, 8164 (2015).
Spath, S. et al. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity 46, 245–260 (2017).
Rapalino, O. et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 4, 814–821 (1998).
Weber, M. S. et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 13, 935–943 (2007).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Hu, X. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64 (2015).
Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017).
Kroner, A. et al. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116 (2014).
Shechter, R. et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569 (2013).
Galván-Peña, S. & O’Neill, L. A. Metabolic reprograming in macrophage polarization. Front. Immunol. 5, 420 (2014).
Ransohoff, R. M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 (2016).
Béchade, C., Colasse, S., Diana, M. A., Rouault, M. & Bessis, A. NOS2 expression is restricted to neurons in the healthy brain but is triggered in microglia upon inflammation. Glia 62, 956–963 (2014).
Reese, T. A. et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447, 92–96 (2007).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Vogl, T. et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Invest. 128, 1852–1866 (2018). 10/1172/JCI89867.
Rhee, S. G. & Kil, I. S. Multiple functions and regulation of mammalian peroxiredoxins. Annu. Rev. Biochem. 86, 749–775 (2017).
Rantakari, P. et al. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc. Natl. Acad. Sci. USA 113, 9298–9303 (2016).
Xu, J., Xiao, N. & Xia, J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 13, 22–24 (2010).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Iqbal, A. J. et al. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124, e33–e44 (2014).
Schläger, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Kolodziejski, P. J., Koo, J. S. & Eissa, N. T. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc. Natl. Acad. Sci. USA 101, 18141–18146 (2004).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131 (2017).
Louveau, A. et al. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219 (2017).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Van den Bossche, J. et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17, 684–696 (2016).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e13 (2016).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Ponomarev, E. D., Maresz, K., Tan, Y. & Dittel, B. N. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J. Neurosci. 27, 10714–10721 (2007).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Liddiard, K. & Taylor, P. R. Understanding local macrophage phenotypes in disease: shape-shifting macrophages. Nat. Med. 21, 119–120 (2015).
Kigerl, K. A. et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 (2009).
Mikita, J. et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult. Scler. 17, 2–15 (2011).
Giles, D. A. et al. Myeloid cell plasticity in the evolution of central nervous system autoimmunity. Ann. Neurol. 83, 131–141 (2018).
Auffray, C., Sieweke, M. H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Misharin, A. V. et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep 9, 591–604 (2014).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Lu, G. et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 6, 6676 (2015).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
R Development Core Team. R: a Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2008).
SEQC/MAQC-III Consortium. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat. Biotechnol. 32, 903–914 (2014).
Hanbo, C. VennDiagram: generate high-resolution Venn and Euler plots. The Comprehensive R Archive Network https://CRAN.R-project.org/package=VennDiagram (2016).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 (2014).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Nikić, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Romanelli, E. et al. Cellular, subcellular and functional in vivo labeling of the spinal cord using vital dyes. Nat. Protoc. 8, 481–490 (2013).
Acknowledgements
We thank A. Schmalz, L. Schödel, and B. Fiedler for excellent technical assistance; D. Matzek and B. Stahr for animal husbandry; D. Kofink for help with statistical analysis; and E. Beltran for help with the flow cytometry analysis. We thank R. Hohlfeld and H. Wekerle for critical reading of the manuscript. Work in M.K.’s laboratory is financed through grants from the Deutsche Forschungsgemeinschaft (DFG; Transregio 128, Project B10), the European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013; ERC Grant Agreement n. 310932), the German Multiple Sclerosis Society (DMSG), the “Verein Therapieforschung für MS-Kranke e.V.”, and the Munich Center for Systems Neurology (SyNergy; EXC 1010). M.P. is supported by the Sobek-Stiftung, the DFG (SFB 992, SFB1140, SFB/TRR167, Reinhart-Koselleck-Grant), and the Sonderlinie Hochschulmedizin Baden Württemberg, project “neuroinflammation in neurodegeneration”. M.K. and M.P. are further supported by the German Federal Ministry of Research and Education (BMBF; Competence Network Multiple Sclerosis), and M.K. and M.S. are supported by a common grant from the DFG (Transregio 128, Project B13). G.L. was supported by fellowships from EMBO and Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
G.L., D.T., M.P., and M.K. conceived and designed the experiments. G.L. and D.T. performed FACS experiments, imaging experiments, and image analysis. G.L., D.T., A.K., L.C.-C., M.S., and A.D. established and performed in situ and in vitro analyses of phagocyte polarization. G.L., M.J.C.J., O.S., and M.P. performed and analyzed RNAseq experiments; K.P. and F.M. performed and analyzed proteomics experiments; and A.B. generated and characterized the iNOS-tdTomato-Cre mouse line. G.L., D.T., and M.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 In vitro analysis of phagocyte polarization in iNOS-tdTomato-Cre and Arginase-YFP mice.
(a) Confocal images of tdTomato-specific immunostaining (above, red) and quantification (mean indicated as a grey line) of fluorescence intensities of tdTomato-, MHC-II-, iNOS- and p22phox-immunostaining (below) of bone marrow-derived macrophages isolated from iNOS-tdTomato-cre mice and cultured for 48hrs with mCSF only (Ø), or with mCSF supplemented with LPS + IFNγ or with IL4 + IL13, n = 5 mice. Total number of cells analyzed for tdTomato: MØ = 1,381, M(LPS + IFNγ) = 2,082, M(IL4 + IL13) = 1,415; for MHC-II: MØ = 822, M(LPS + IFNγ) = 451, M(IL4 + IL13) = 607; for iNOS: MØ = 639, M(LPS + IFNγ) = 1,154, M(IL4 + IL13) = 583; for p22phox: MØ = 742, M(LPS + IFNγ) = 928, M(IL4 + IL13) = 832. Scale bar 20 µm. ***, P < 0.001, Kruskal-Wallis with Dunn’s post-hoc comparisons (p values for the Kruskal-Wallis tests and for the post hoc comparisons are p < 0.0001 for MHCII, p < 0.0001 for Tomato, p < 0.0001 for iNOS, p < 0.0001 for p22phox). (b) Confocal images of YFP-specific immunostaining (above, green) and quantifications (mean indicated as a grey line) of fluorescence intensity of YFP-, YM1-, iNOS- and CD206-specific staining (below) of bone marrow-derived macrophages isolated from Arginase-YFP mice and cultured for 48hrs with mCSF only (Ø), or with mCSF supplemented with LPS + IFNγ or with IL4 + IL13, n = 5 mice. Total number of cells analyzed for YFP: MØ = 948, M(LPS + IFNγ) = 702, M(IL4 + IL13) = 1,141; for YM1: MØ = 203, M(LPS + IFNγ) = 176, M(IL4 + IL13) = 342; for iNOS: MØ = 237, M(LPS + IFNγ) = 236, M(IL4 + IL13) = 241; for CD206: MØ = 280, M(LPS + IFNγ) = 129, M(IL4 + IL13) = 309. Scale bar 20 µm. ***, P < 0.001, Kruskal-Wallis with Dunn’s post-hoc comparisons (p values for the Kruskal-Wallis tests and for the post hoc comparisons are p < 0.0001 for iNOS, p < 0.0001 for EYFP, p < 0.0001 for CD206, p < 0.0001 for YM1 (MØ vs. LPS + IFNγ p = 0.0003, MØ vs. IL4 + IL13 p < 0.0001, LPS + IFNγ vs. IL4 + IL13 p < 0.0001).
Supplementary Figure 2 Absence of reporter expression in the healthy CNS.
(a) In vivo image of the spinal cord of a healthy iNOS-tdTomato-cre x Arginase-YFP mouse (tdTomato, red; YFP, green; vasculature revealed by Dextran-AF647 injection, cyan). Scale bar 200 µm. (b) Confocal image of the spinal cord of a healthy iNOS-tdTomato-cre x Arginase-YFP mouse (tdTomato, red; YFP, green; Iba-1-immunostaining, gray). Scale bar 20 µm. (c) Flow cytometric analysis of cells isolated from the CNS of healthy iNOS-tdTomato x Arginase-YFP mice. Microglia were gated based on expression of CD45 and CD11b. Average percentages of polarized cells ( ± s.e.m.) are displayed, n = 4 mice.
Supplementary Figure 3 Cellular co-localization of reporter protein expression in the CNS and correlation with iNOS and arginase-1 expression in situ.
(a-d) Confocal images of the spinal cord of iNOS-tdTomato-cre (upper row; tdTomato, red) and (e-h) Arginase-YFP (lower row; YFP, green) mice perfused at peak of EAE and counterstained for marker proteins of phagocytes (a,e; Iba-1, gray), T cells (b,f; CD3, gray), oligodendrocytes (c,g; Olig2, gray) and astrocytes (d,h; GFAP, gray). Images are representative of 3 independent expeirments. Average percentage of co-localisation between tdTomato and Iba-1 ( ± s.e.m.) was 93.7 ± 2.5 (n = 3 mice and 447 cells analyzed); between YFP and Iba-1 ( ± s.e.m.) was 95.3 ± 1.5 (n = 3 mice and 601 cells analyzed). Scale bar 20 µm. (i) Confocal image of the spinal cord of an iNOS-tdTomato-cre mouse at weight loss (tdTomato, red; iNOS, gray). Scale bar, 20 µm. (j) tdTomato- and iNOS-fluorescence intensities in phagocytes analyzed in spinal EAE lesions of iNOS-tdTomato-cre mice at weight loss. iNOS was expressed in 98.6 ± 1.2% of all tdTomato+ cells (average ± s.e.m.; n = 5 mice and 732 cells analyzed). (k) Confocal image of the spinal cord of an Arginase-YFP mouse at remission (YFP, green; arginase-1, gray). Scale bar 20 µm. (l) YFP- and arginase-1-fluorescence intensities in phagocytes analyzed in spinal EAE lesions of Arginase-YFP mice at remission. Arginase-1 was expressed in 99.8 ± 0.2% of all YFP+ cells (average ± s.e.m.; n = 5 mice and 658 cells analyzed).
Supplementary Figure 4 Evolution of phagocyte phenotypes in brain EAE lesions.
(a,b) Confocal images of midbrain lesions (a, scale bar 20 µm) and quantitative analysis (b) of MiNOS, MiNOS/Arginase and MArginase cells at the indicated EAE timepoints in iNOS-tdTomato-cre x Arginase-YFP mice (n = 2 mice at weight loss, n = 3 at onset, n = 2 at peak). Data are shown as average ± s.e.m. for Onset. No statistical analysis was performed due to the low number of mice with brain lesions.
Supplementary Figure 5 List of regulated RNA transcripts in polarized CNS phagocytes.
List of transcripts that are significantly regulated in MiNOS or MArginase phagocytes based on the Venn diagram illustrated in Fig. 2b, n = 4 animals. Transcripts are color-coded and ordered based on the relative fold-change of MiNOS cells (left) and MArginase cells (right) compared to the unpolarized phagocyte population. Unknown transcripts are not displayed. Asterisks indicated statistical significance p < 0.05. Fastq files were quality controlled and evaluated as detailed in the Online Method section.
Supplementary Figure 6 Expression of selected RNA transcripts in different phagocyte populations at peak disease.
Count per million (c.p.m.) expression of selected genes from isolated CD45high-CD11bhigh-CD64positive MiNOS, MiNOS/Arginase, MArginase and unpolarized phagocytes (MUnp) isolated from the CNS of iNOS-tdTomato-cre x Arginase-YFP mice at peak disease, n = 5 mice. Data are presented as box-and-whiskers (min to max values) plots, *, P < 0.05, **, P < 0.01 ***; P < 0.001, CD86, IL1Ra, Mmp14, Cx3cr1, Trem2, C1qa were analyzed with Kruskal-Wallis with Dunn’s post-hoc comparisons, the other RNA transcripts using 1-way-ANOVA with Bonferroni post-hoc correction. P-values for the 1-way-Anova or Kruskal-Wallis test are: CD40, p = 0.0018 (MiNOS vs MUnp 0.0277, MiNOS/Arginase vs MUnp 0.0014, MArginase vs MUnp 0.0395), Ciita, p = 0.0004 (MiNOS/Arginase vs MUnp 0.0019, MArginase vs MUnp 0.0006), CD86, p = 0.0051 (MiNOS vs MUnp 0.0139, MiNOS/Arginase vs MUnp 0.0139), CD80, p = 0.0028 (MiNOS/Arginase vs MUnp 0.0018), IL1a, p = 0.6915, IL1b, p < 0.0001(MiNOS vs MUnp 0.0011, MiNOS/Arginase vs MUnp < 0.0001, MArginase vs MUnp 0.0096), IL1Ra, p = 0.0018 (MiNOS/Arginase vs MUnp 0.0009), TGFb, p = 0.0035 (MiNOS vs MUnp 0.0227, MiNOS/Arginase vs MUnp 0.0149, MArginase vs MUnp 0.0057), Mmp12, p < 0.0001 (all significant comparisons, p < 0.0001), Mmp14, p = 0.5952, Ccl5, p = 0.4381, Cx3cr1, p = 0.0074 (MiNOS vs MUnp 0.0327, MiNOS/Arginase vs MUnp 0.0166), CD206, p = 0.0071 (MiNOS vs MArginase 0.0338, MUnp vs MArginase 0.0187), C1qa, p = 0.0251 (MiNOS vs MArginase 0.0234), C1qc, p = 0.0273 (MUnp vs MArginase 0.0306), Trem2, p = 0.0139 (MUnp vs MArginase 0.0277, MiNOS/Arginase vs MUnp 0.0385), Tpi1, p = 0.0010 (MiNOS vs MArginase 0.0047, MiNOS vs MUnp 0.002), Gpi1, p = 0.0228, Aldoa, p < 0.0001 (MiNOS vs MUnp < 0.0001, MiNOS vs MArginase 0.0017, MiNOS/Arginase vs MUnp 0.0049), Pfkl, p = 0.0082 (MiNOS vs MArginase 0.044, MiNOS/Arginase vs MArginase 0.0464).
Supplementary Figure 7 Homogeneous distribution of phagocyte phenotypes in parenchymal lesions at onset of EAE.
(a) Confocal images of the spinal cord of iNOS-tdTomato-cre x Arginase-YFP mice perfused at EAE onset (tdTomato, red; YFP, green) illustrating spatial distribution of the polarized cells within the lesion based either on 20µm–thick bands starting from pia surface (left panel), concentric squares starting from lesion center (middle panel), or bands orientated in parallel to a central blood vessel (laminin in cyan; right panel). Scale bar 20 µm. (b) Quantitative analysis of the proportion of MiNOS, MiNOS/Arginase and MArginase cells in lesions subdivided as indicated in (a). Shown is average ± s.e.m.; n = 7 mice and 1,869 cells assessed for the analysis starting from the pial surface (left panel), n = 7 mice and 1,894 cells assessed for the analysis from the center of the lesion (middle panel), n = 5 mice and 436 cells assessed for the analysis starting from the blood vessel (right panel). No significant differences were observed (2-way-ANOVA with Bonferroni post-hoc correction).
Supplementary Figure 8 Absence of reporter protein expression in phagocytes isolated from blood and lymph nodes of iNOS-tdTomato-Cre × Arginase-YFP mice, iNOS-tdTomato-Cre × Rosa26-Stp-fl-YFP and Rosa26-Stp-fl-YFP mice.
(a) Flow cytometric analysis of blood isolated from iNOS-tdTomato-cre x Arginase-YFP mice at peak of EAE. Populations were gated based on expression of CD45 and CD11b. Average percentage of polarized cells ( ± s.e.m.) are displayed, n = 5 mice. (b) Flow cytometric analysis of lymph node preparation from iNOS-tdTomato-cre x Arginase-YFP mice at peak of EAE. Populations were gated based on their expression of CD45 and CD11b. Average percentage of YFP+ and/or tdTomato+ cells ( ± s.e.m.) are displayed. Analysis based on 3 independent experiments with a total of 8 mice. (c,d) Flow cytometric analysis of cells isolated from the blood of iNOS-tdTomato-cre x Rosa26-Stp-fl-YFP (c, n = 5 mice) and control Rosa26-Stp-fl-YFP (d, n = 8 mice) at peak of EAE. Populations were gated based on expression of CD45 and CD11b. Average percentage of YFP+ and/or tdTomato+ cells ( ± s.e.m.) are displayed.
Supplementary Figure 9 Location of phagocytes undergoing a phenotype switch at different stages of EAE.
(a) Quantitative representation of the localization of those phagocytes that switch their phenotype from MiNOS to MiNOS/Arginase over the course of 6hrs at the indicated timepoints of EAE per mouse. Indicated is average and s.e.m with single data points (number of animals = 8 at weight loss, 5 at onset, 4 at peak, 2 at remission). **, P < 0.01, Kruskal-Wallis with Dunn’s post-hoc comparisons (p-value for the Kruskal-Wallis test = 0.0004; UM WL vs Par WL 0.0029). Significant differences are only shown for the comparison of different compartments within a given timepoint. No statistical analysis was performed on the Remission timepoint. Mice in which no change over 6hrs was observed are not represented. (b) Quantitative representation of the localization of those phagocytes that switch their phenotype from MiNOS/Arginase to MArginase over the course of 6hrs at the indicated timepoints of EAE per mouse. Indicated is average and s.e.m. with single data points (number of animals = 2 at weight loss, 2 at onset, 4 at peak, 4 at remission), 1-way-Anova followed by Bonferroni post-hoc multiple comparison test (p-value for the 1-way-Anova test = 0.0359). Significant differences are only shown for the comparison of different compartments within a given timepoint. No statistical analysis was performed on the WL and Onset timepoints. Mice in which no change over 6hrs was observed are not represented.
Supplementary Figure 10 Evolution of phagocyte phenotypes following spinal cord injury.
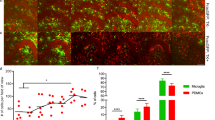
(a,b,d,e) Confocal images of the spinal cord of C57BL/6 mice perfused at (a,b) 24 hours and (d,e) 72 hours after spinal cord injury (iNOS, red; Arginase, green; DAPI, gray, n = 4 mice). Scale bar, 40 µm. (c,f) Quantifications of the density of iNOS- or Arginase-expressing cells at the injury site at (c) 24 hours and (f) 72 hours after spinal cord injury (n = 4 mice). ***, p < 0.001, unpaired t-test two-sided. p = 0.108 in (c), p = 0.0001 in (f).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10
Supplementary Video 1 - In vivo imaging of phagocyte phenotypes in different spinal cord compartments.
In vivo image of the spinal cord of iNOS-tdTomato-cre x Arginase-YFP mice at EAE onset, before and after surgical removal of upper meninges (tdTomato, red; YFP, green). Maximum intensity projections are shown in Figure 4a. Video is representative of imaging in 6 mice.
Supplementary Video 2 - In vivo imaging of initial MiNOS phagocyte polarization in the spinal cord.
In vivo image of the spinal cord of iNOS-tdTomato-cre x CD68-GFP mice at the onset of EAE (tdTomato, red; GFP, gray; vasculature as revealed by Dextran-AF647 injection, cyan). Maximum intensity projection is shown in Figure 5a. Video is representative of imaging in 7 mice.
Supplementary Video 3 - In vivo time-lapse imaging of phagocyte phenotypes in a spinal EAE lesion.
In vivo confocal recordings of a spinal cord lesion in iNOS-tdTomato-cre x Arginase-YFP mice at EAE onset, at timepoint 0 and after 6 hours (tdTomato, red; YFP, green). Shown are maximum intensity projections, followed by image sequences of the confocal stack of the lesion (from top of the dura to deep parenchyma). Through the stack, analyzed cells are marked by a Region of Interest (ROI) and a number. Comparison of tdTomato and YFP fluorescence intensities in cells between the 0 and 6 hours timepoints indicate that, among the 11 MiNOS cells analyzed at timepoint 0 hour, 2 cells (highlighted at the end of the movie) have started expressing YFP after 6 hours. Video is representative of imaging in 7 mice.
Rights and permissions
About this article
Cite this article
Locatelli, G., Theodorou, D., Kendirli, A. et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci 21, 1196–1208 (2018). https://doi.org/10.1038/s41593-018-0212-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0212-3
This article is cited by
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
Diverse functions of myeloid-derived suppressor cells in autoimmune diseases
Immunologic Research (2024)
-
Inducible nitric oxide synthase deficiency promotes murine-β-coronavirus induced demyelination
Virology Journal (2023)
-
Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation
Acta Neuropathologica Communications (2023)
-
ETV3 and ETV6 enable monocyte differentiation into dendritic cells by repressing macrophage fate commitment
Nature Immunology (2023)