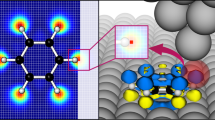
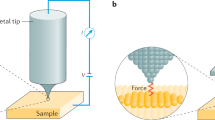
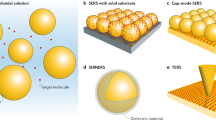
Abstract
Confocal and surface-enhanced Raman spectroscopy (SERS) are powerful techniques for molecular characterization; however, they suffer from the drawback of diffraction-limited spatial resolution. Tip-enhanced Raman spectroscopy (TERS) overcomes this limitation and provides chemical information at length scales in the tens of nanometers. In contrast to alternative approaches to nanoscale chemical analysis, TERS is label free, is non-destructive, and can be performed in both air and liquid environments, allowing its use in a diverse range of applications. Atomic force microscopy (AFM)-based TERS is especially versatile, as it can be applied to a broad range of samples on various substrates. Despite its advantages, widespread uptake of this technique for nanoscale chemical imaging has been inhibited by various experimental challenges, such as limited lifetime, and the low stability and yield of TERS probes. This protocol details procedures that will enable researchers to reliably perform TERS imaging using a transmission-mode AFM-TERS configuration on both biological and non-biological samples. The procedure consists of four stages: (i) preparation of plasmonically active TERS probes; (ii) alignment of the TERS system; (iii) experimental procedures for nanoscale imaging using TERS; and (iv) TERS data processing. We provide procedures and example data for a range of different sample types, including polymer thin films, self-assembled monolayers (SAMs) of organic molecules, photocatalyst surfaces, small molecules within biological cells, single-layer graphene and single-walled carbon nanotubes in both air and water. With this protocol, TERS probes can be prepared within ~23 h, and each subsequent TERS experimental procedure requires 3–5 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kumar, N., Mignuzzi, S., Su, W. & Roy, D. Tip-enhanced Raman spectroscopy: principles and applications. EPJ Tech. Instrum. 2, 9 (2015).
Verma, P. Tip-enhanced Raman spectroscopy: technique and recent advances. Chem. Rev. 117, 6447–6466 (2017).
Deckert-Gaudig, T., Taguchi, A., Kawata, S. & Deckert, V. Tip-enhanced Raman spectroscopy–from early developments to recent advances. Chem. Soc. Rev. 46, 4077–4110 (2017).
Wang, X. et al. Tip-enhanced Raman spectroscopy for surfaces and interfaces. Chem. Soc. Rev. 46, 4020–4041 (2017).
Hartschuh, A. Tip‐enhanced near‐field optical microscopy. Angew. Chem. Int. Ed. Engl. 47, 8178–8191 (2008).
Kawata, S. & Shalaev, V. M. Tip Enhancement (Elsevier, Amsterdam, 2007).
Morton, S. M., Silverstein, D. W. & Jensen, L. Theoretical studies of plasmonics using electronic structure methods. Chem. Rev. 111, 3962–3994 (2011).
Weckhuysen, B. M. In-situ Spectroscopy of Catalysts (American Scientific Publishers, Stevenson Ranch, 2004).
Mestl, G. In situ Raman spectroscopy—a valuable tool to understand operating catalysts. J. Mol. Catal. A Chem. 158, 45–65 (2000).
Colthup, N. Introduction to Infrared and Raman Spectroscopy (Elsevier, New York, 2012).
Perkampus, H.-H. UV-VIS Spectroscopy and Its Applications (Springer Science & Business Media, New York, 2013).
Kneipp, K., Kneipp, H., Itzkan, I., Dasari, R. R. & Feld, M. S. Ultrasensitive chemical analysis by Raman spectroscopy. Chem. Rev. 99, 2957–2976 (1999).
Kim, H., Kosuda, K. M., Van Duyne, R. P. & Stair, P. C. Resonance Raman and surface- and tip-enhanced Raman spectroscopy methods to study solid catalysts and heterogeneous catalytic reactions. Chem. Soc. Rev. 39, 4820–4844 (2010).
Cheng, J.-X. & Xie, X. S. Vibrational spectroscopic imaging of living systems: an emerging platform for biology and medicine. Science 350, aaa8870 (2015).
Fu, D. et al. Nanoscale infrared imaging of zeolites using photoinduced force microscopy. Chem. Commun. 53, 13012–13014 (2017).
Wu, C.-Y. et al. High spatial-resolution mapping of catalytic reactions on single particles. Nature 541, 511–515 (2017).
Zhang, R. et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 498, 82–86 (2013).
Zheng, N., Tsai, H. N., Zhang, X., Shedden, K. & Rosania, G. R. The subcellular distribution of small molecules: a meta-analysis. Mol. Pharm. 8, 1611–1618 (2011).
Huang, B., Bates, M. & Zhuang, X. Super resolution fluorescence microscopy. Annu. Rev. Biochem. 78, 993–1016 (2009).
Hollander, J. M. & Jolly, W. L. X-ray photoelectron spectroscopy. Acc. Chem. Res. 3, 193–200 (1970).
Vogt, S. & Lanzirotti, A. Trends in X-ray fluorescence microscopy. Synchrotron. Radiat. News 26, 32–38 (2013).
De Jonge, N. & Ross, F. M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 6, 695–704 (2011).
Nuñez, J., Renslow, R., Cliff, J. B. III & Anderton, C. R. NanoSIMS for biological applications: current practices and analyses. Biointerphases 13, 03B301 (2018).
Kelly, T. F. & Miller, M. K. Atom probe tomography. Rev. Sci. Instrum. 78, 031101 (2007).
Schmid, T., Yeo, B. S., Leong, G., Stadler, J. & Zenobi, R. Performing tip-enhanced Raman spectroscopy in liquids. J. Raman Spectrosc. 40, 1392–1399 (2009).
Nakata, A., Nomoto, T., Toyota, T. & Fujinami, M. Tip-enhanced Raman spectroscopy of lipid bilayers in water with an alumina-and silver-coated tungsten tip. Anal. Sci. 29, 865–869 (2013).
Scherger, J. D. & Foster, M. D. Tunable, liquid resistant tip-enhanced Raman spectroscopy probes: toward label-free nano-resolved imaging of biological systems. Langmuir 33, 7818–7825 (2017).
Martín Sabanés, N., Driessen, L. M. & Domke, K. F. Versatile side-illumination geometry for tip-enhanced Raman spectroscopy at solid/liquid interfaces. Anal. Chem. 88, 7108–7114 (2016).
Kumar, N. et al. Nanoscale chemical imaging of solid-liquid interfaces using tip-enhanced Raman spectroscopy. Nanoscale 10, 1815–1824 (2018).
Touzalin, T., Dauphin, A. L., Joiret, S., Lucas, I. T. & Maisonhaute, E. Tip-enhanced Raman spectroscopy imaging of opaque samples in organic liquid. Phys. Chem. Chem. Phys. 18, 15510–15513 (2016).
Zeng, Z.-C. et al. Electrochemical tip-enhanced Raman spectroscopy. J. Am. Chem. Soc. 137, 11928–11931 (2015).
Kurouski, D., Mattei, M. & Van Duyne, R. P. Probing redox reactions at the nanoscale with electrochemical tip-enhanced Raman spectroscopy. Nano Lett. 15, 7956–7962 (2015).
Wang, X. et al. Revealing intermolecular interaction and surface restructuring of an aromatic thiol assembling on Au (111) by tip-enhanced Raman spectroscopy. Anal. Chem. 88, 915–921 (2015).
Touzalin, T., Joiret, S., Maisonhaute, E. & Lucas, I. T. Complex electron transfer pathway at a microelectrode captured by in situ nanospectroscopy. Anal. Chem. 89, 8974–8980 (2017).
Mattei, M. et al. Tip-enhanced Raman voltammetry: coverage dependence and quantitative modeling. Nano Lett. 17, 590–596 (2016).
Kumar, N. et al. Simultaneous topographical, electrical and optical microscopy of optoelectronic devices at the nanoscale. Nanoscale 9, 2723–2731 (2017).
Liu, Z. et al. Revealing the molecular structure of single-molecule junctions in different conductance states by fishing-mode tip-enhanced Raman spectroscopy. Nat. Commun. 2, 305 (2011).
Su, W., Kumar, N., Mignuzzi, S., Crain, J. & Roy, D. Nanoscale mapping of excitonic processes in single-layer MoS2 using tip-enhanced photoluminescence microscopy. Nanoscale 8, 10564–10569 (2016).
Beversluis, M. R., Bouhelier, A. & Novotny, L. Continuum generation from single gold nanostructures through near-field mediated intraband transitions. Phys. Rev. B 68, 115433 (2003).
Kumar, N., Kalirai, S., Wain, A. J. & Weckhuysen, B. M. Nanoscale chemical imaging of a single catalyst particle with tip-enhanced fluorescence microscopy. ChemCatChem 11, 417–423 (2019).
Hu, D., Micic, M., Klymyshyn, N., Suh, Y. D. & Lu, H. P. Correlated topographic and spectroscopic imaging beyond diffraction limit by atomic force microscopy metallic tip-enhanced near-field fluorescence lifetime microscopy. Rev. Sci. Instrum. 74, 3347–3355 (2003).
Neugebauer, U. et al. On the way to nanometer-sized information of the bacterial surface by tip-enhanced Raman spectroscopy. ChemPhysChem 7, 1428–1430 (2006).
Cialla, D. et al. Raman to the limit: tip-enhanced Raman spectroscopic investigations of a single tobacco mosaic virus. J. Raman Spectrosc. 40, 240–243 (2009).
Najjar, S. et al. Tip-enhanced Raman spectroscopy of combed double-stranded DNA bundles. J. Phys. Chem. C 118, 1174–1181 (2014).
Bailo, E. & Deckert, V. Tip-enhanced Raman spectroscopy of single RNA strands: towards a novel direct-sequencing method. Angew. Chem. Int. Ed. Engl. 47, 1658–1661 (2008).
Bohme, R. et al. Towards a specific characterisation of components on a cell surface—combined TERS-investigations of lipids and human cells. J. Raman Spectrosc. 40, 1452–1457 (2009).
Deckert-Gaudig, T. & Deckert, V. Tip-enhanced Raman scattering studies of histidine on novel silver substrates. J. Raman Spectrosc. 40, 1446–1451 (2009).
Blum, C. et al. Understanding tip-enhanced Raman spectra of biological molecules: a combined Raman, SERS and TERS study. J. Raman Spectrosc. 43, 1895–1904 (2012).
Yeo, B.-S., Mädler, S., Schmid, T., Zhang, W. & Zenobi, R. Tip-enhanced Raman spectroscopy can see more: the case of cytochrome c. J. Phys. Chem. C 112, 4867–4873 (2008).
Davies, H. S. et al. Secondary structure and glycosylation of mucus glycoproteins by Raman spectroscopies. Anal. Chem. 88, 11609–11615 (2016).
Lipiec, E., Perez-Guaita, D., Kaderli, J., Wood, B. R. & Zenobi, R. Direct nanospectroscopic verification of the amyloid aggregation pathway. Angew. Chem. Int. Ed. Engl. 57, 8519–8524 (2018).
Kumar, N., Drozdz, M. M., Jiang, H., Santos, D. M. & Vaux, D. J. Nanoscale mapping of newly-synthesised phospholipid molecules in a biological cell using tip-enhanced Raman spectroscopy. Chem. Commun. 53, 2451–2454 (2017).
Okuno, Y., Saito, Y., Kawata, S. & Verma, P. Tip-enhanced Raman investigation of extremely localized semiconductor-to-metal transition of a carbon nanotube. Phys. Rev. Lett. 111, 216101 (2013).
Yano, T.-a et al. Tip-enhanced nano-Raman analytical imaging of locally induced strain distribution in carbon nanotubes. Nat. Commun. 4, 2592 (2013).
Yano, T.-A., Verma, P., Saito, Y., Ichimura, T. & Kawata, S. Pressure-assisted tip-enhanced Raman imaging at a resolution of a few nanometres. Nat. Photonics 3, 473–477 (2009).
Marquestaut, N. et al. Imaging of single GaN nanowires by tip-enhanced Raman spectroscopy. J. Raman Spectrosc. 40, 1441–1445 (2009).
Boehmler, M., Wang, Z., Myalitsin, A., Mews, A. & Hartschuh, A. Optical imaging of CdSe nanowires with nanoscale resolution. Angew. Chem. Int. Ed. Engl. 50, 11536–11538 (2011).
Ogawa, Y., Yuasa, Y., Minami, F. & Oda, S. Tip-enhanced Raman mapping of a single Ge nanowire. Appl. Phys. Lett. 99, 053112 (2011).
Mignuzzi, S. et al. Probing individual point defects in graphene via near-field Raman scattering. Nanoscale 7, 19413–19418 (2015).
Su, W., Kumar, N., Dai, N. & Roy, D. Nanoscale mapping of intrinsic defects in single-layer graphene using tip-enhanced Raman spectroscopy. Chem. Commun. 52, 8227–8230 (2016).
Pollard, A. J. et al. Nanoscale optical spectroscopy: an emerging tool for the characterization of graphene and related 2-D materials. J. Mater. Nanosci. 1, 39–49 (2014).
Su, W., Kumar, N., Spencer, S. J., Dai, N. & Roy, D. Transforming bilayer MoS2 into single-layer with strong photoluminescence using UV-ozone oxidation. Nano Res. 8, 3878–3886 (2015).
Smithe, K. K. H. et al. Nanoscale heterogeneities in monolayer MoSe2 revealed by correlated scanning probe microscopy and tip-enhanced Raman spectroscopy. ACS Appl. Nano Mater. 1, 572–579 (2018).
Park, K.-D. et al. Hybrid tip-enhanced nanospectroscopy and nanoimaging of monolayer WSe2 with local strain control. Nano Lett. 16, 2621–2627 (2016).
Feng, S., Vivian, M., Yao, Z., Dieter, S. A. & Renato, Z. Nanoscale chemical imaging of interfacial monolayers by tip‐enhanced Raman spectroscopy. Angew. Chem. Int. Ed. Engl. 56, 9361–9366 (2017).
Vivian, M. et al. Structural characterization of a covalent monolayer sheet obtained by two‐dimensional polymerization at an air/water interface. Angew. Chem. Int. Ed. Engl. 56, 15262–15266 (2017).
van Schrojenstein Lantman, E. M., Deckert-Gaudig, T., Mank, A. J. G., Deckert, V. & Weckhuysen, B. M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 7, 583–586 (2012).
Kumar, N., Stephanidis, B., Zenobi, R., Wain, A. & Roy, D. Nanoscale mapping of catalytic activity using tip-enhanced Raman spectroscopy. Nanoscale 7, 7133–7137 (2015).
Domke, K. F. & Pettinger, B. In situ discrimination between axially complexed and ligand-free co porphyrin on Au(111) with tip-enhanced Raman spectroscopy. ChemPhysChem 10, 1794–1798 (2009).
Zhong, J.-H. et al. Probing the electronic and catalytic properties of a bimetallic surface with 3 nm resolution. Nat. Nanotechnol. 12, 132–136 (2017).
Hartman, T., Wondergem, C. S., Kumar, N., van den Berg, A. & Weckhuysen, B. M. Surface-and tip-enhanced Raman spectroscopy in catalysis. J. Phys. Chem. Lett. 7, 1570–1584 (2016).
Szczerbiński, J., Gyr, L., Kaeslin, J. & Zenobi, R. Plasmon-driven photocatalysis leads to products known from E-beam and X-ray-induced surface chemistry. Nano Lett. 18, 6740–6749 (2018).
Wang, X. et al. High-resolution spectroscopic mapping of the chemical contrast from nanometer domains in P3HT:PCBM organic blend films for solar-cell applications. Adv. Funct. Mater. 20, 492–499 (2010).
Kumar, N., Rae, A. & Roy, D. Accurate measurement of enhancement factor in tip-enhanced Raman spectroscopy through elimination of far-field artefacts. Appl. Phys. Lett. 104, 123106 (2014).
Yeo, B. S., Amstad, E., Schmid, T., Stadler, J. & Zenobi, R. Nanoscale probing of a polymer-blend thin film with tip-enhanced Raman spectroscopy. Small 5, 952–960 (2009).
Xue, L et al. High Resolution Tip Enhanced Raman Mapping on Polymer Thin Films 305 (Wiley-VCH, Weinheim, Germany, 2011). .
Saito, Y., Motohashi, M., Hayazawa, N., Iyoki, M. & Kawata, S. Nanoscale characterization of strained silicon by tip-enhanced Raman spectroscope in reflection mode. Appl. Phys. Lett. 88, 143109 (2006).
Berweger, S. et al. Optical nanocrystallography with tip-enhanced phonon Raman spectroscopy. Nat. Nanotechnol. 4, 496–499 (2009).
Martín Sabanés, N., Ohto, T., Andrienko, D., Nagata, Y. & Domke, K. F. Electrochemical TERS elucidates potential-induced molecular reorientation of adenine/Au(111). Angew. Chem. Int. Ed. Engl. 56, 9796–9801 (2017).
Ren, B., Picardi, G., Pettinger, B., Schuster, R. & Ertl, G. Tip-enhanced Raman spectroscopy of benzenethiol adsorbed on Au and Pt single-crystal surfaces. Angew. Chem. Int. Ed. Engl. 44, 139–142 (2005).
Blum, C. et al. Tip-enhanced Raman spectroscopy—an interlaboratory reproducibility and comparison study. J. Raman Spectrosc. 45, 22–31 (2014).
Wang, X. et al. Revealing intermolecular interaction and surface restructuring of an aromatic thiol assembling on Au(111) by tip-enhanced Raman spectroscopy. Anal. Chem. 88, 915–921 (2016).
Huang, T.-X. et al. Tip-enhanced Raman spectroscopy: tip-related issues. Anal. Bioanal. Chem. 407, 8177–8195 (2015).
Demming, A. L., Festy, F. & Richards, D. Plasmon resonances on metal tips: understanding tip-enhanced Raman scattering. J. Chem. Phys. 122, 184716 (2005).
Yang, Z., Aizpurua, J. & Xu, H. Electromagnetic field enhancement in TERS configurations. J. Raman Spectrosc. 40, 1343–1348 (2009).
Yang, L.-K. et al. Rational fabrication of a gold-coated AFM TERS tip by pulsed electrodeposition. Nanoscale 7, 18225–18231 (2015).
Meng, L. et al. Gold-coated AFM tips for tip-enhanced Raman spectroscopy: theoretical calculation and experimental demonstration. Opt. Express 23, 13804–13813 (2015).
Yeo, B. S., Stadler, J., Schmid, T., Zenobi, R. & Zhang, W. H. Tip-enhanced Raman spectroscopy—its status, challenges and future directions. Chem. Phys. Lett. 472, 1–13 (2009).
Kumar, N. et al. Extending the plasmonic lifetime of tip-enhanced Raman spectroscopy probes. Phys. Chem. Chem. Phys. 18, 13710–13716 (2016).
Health and Safety Executive. Using nanomaterials at work. in Health and Safety Executive UK, HSG272 (Health and Safety Executive, Bootle, UK, 2013).
Yeo, B. S., Schmid, T., Zhang, W. & Zenobi, R. Towards rapid nanoscale chemical analysis using tip-enhanced Raman spectroscopy with Ag-coated dielectric tips. Anal. Bioanal. Chem. 387, 2655–2662 (2007).
Cui, X. et al. Tuning the resonance frequency of Ag-coated dielectric tips. Opt. Express 15, 8309–8316 (2007).
Taguchi, A. et al. Controlling the plasmon resonance wavelength in metal-coated probe using refractive index modification. Opt. Express 17, 6509–6518 (2009).
Hayazawa, N., Yano, T. & Kawata, S. Highly reproducible tip-enhanced Raman scattering using an oxidized and metallized silicon cantilever tip as a tool for everyone. J. Raman Spectrosc. 43, 1177–1182 (2012).
Mahan, J. E. Physical Vapor Deposition of Thin Films (Wiley-VCH, New York, 2000).
Abbe, E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. Mikrosk. Anat. 9, 413–418 (1873).
Opilik, L. et al. Chemical production of thin protective coatings on optical nanotips for tip-enhanced Raman spectroscopy. J. Phys. Chem. C 120, 20828–20832 (2016).
Huang, Y.-F. et al. When the signal is not from the original molecule to be detected: chemical transformation of para-aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 132, 9244–9246 (2010).
Zhang, Z., Merk, V., Hermanns, A., Unger, W. E. S. & Kneipp, J. Role of metal cations in plasmon-catalyzed oxidation: a case study of p-aminothiophenol dimerization. ACS Catal. 7, 7803–7809 (2017).
Buurmans, I. L. C. & Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 4, 873–886 (2012).
Roy, D., Wang, J. & Williams, C. Novel methodology for estimating the enhancement factor for tip-enhanced Raman spectroscopy. J. Appl. Phys. 105, 013530 (2009).
Acknowledgements
N.K., A.J.W. and A.J.P. acknowledge funding from the National Measurement System of the Department of Business, Energy & Industry Strategy (BEIS), UK. B.M.W. acknowledges support from the Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), an NWO Gravitation programme funded by the Ministry of Education, Culture and Science of the Government of The Netherlands.
Author information
Authors and Affiliations
Contributions
N.K., B.M.W., A.J.W. and A.J.P. conceived and designed the experiments. N.K. collected and analyzed the data. All authors contributed to the discussion and presentation of results and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Kumar, N. et al. Nanoscale 10, 1815–1824 (2018): https://doi.org/10.1039/c7nr08257f
van Schrojenstein Lantman, E. M., Deckert-Gaudig, T., Mank, A. J. G., Deckert, V. & Weckhuysen, B. M. Nat. Nanotechnol. 7, 583–586 (2012): https://doi.org/10.1038/nnano.2012.131
Kumar, N., Drozdz, M. M., Jiang, H., Santos, D. M. & Vaux, D. J. Chem. Commun. 53, 2451–2454 (2017): https://doi.org/10.1039/C6CC10226C
Mignuzzi, S. et al. Nanoscale 7, 19413–19418 (2015): https://doi.org/10.1039/C5NR04664E
Integrated supplementary information
Supplementary Figure 1 Nanoscale imaging of a single-layer graphene flake using TERS.
a, Overlay image of confocal Raman maps (60 × 60 pixels) obtained using the intensity of 2D (red), D (green) and G (blue) Raman bands measured from a single-layer graphene flake. Integration time: 1 s. Pixel size: 25 nm. Scale bar: 300 nm. b, Overlay image of TERS maps (1.5 µm × 1.5 µm) obtained using the intensity of the 2D (red), D (green) and G (blue) bands from the single-layer graphene area shown in a. Integration time: 1 s. Pixel size: 30 nm. Scale bar: 300 nm. In the TERS image in b, a localized D band signal is observed at the edge of the graphene flake, clearly delineating the boundary of the single-layer flake. Furthermore, a defect signal from the D-peak is observed within the single-layer flake, which cannot be clearly distinguished in the confocal Raman image due to the lower sensitivity and spatial resolution. Adapted with permission from Su et al.60, Royal Society of Chemistry.
Supplementary Figure 2 TERS measurement of a PEDOT:PSS thin film on glass.
Tip-in (TERS) and tip-out (far-field) Raman spectra measured on a PEDOT:PSS thin film sample. Integration time: 60 s. Laser power: 50 µW. TERS contrast of 1444 cm-1 PEDOT Raman band is calculated to be 1.4 ± 0.1. Spectra are vertically shifted for easier visualisation.
Supplementary Figure 3 TERS measurement of a SAM of BPT on Au.
Tip-in (TERS) and tip-out (far-field) Raman spectra measured on a BPT SAM sample, where the BPT Raman peak is observed only for the TERS measurement. Integration time: 1 s. Laser power: 110 µW. Spectra are vertically shifted for easier visualisation.
Supplementary Figure 4 Monitoring of pNTP → DMAB photocatalytic process using TERS.
Time-dependent TERS spectra monitored before and after pNTP → DMAB reaction. Before starting the reaction, the TERS signal of a pNTP SAM was monitored for at least 1 min using 633 nm excitation (5 s integration time, 380 µW) as shown in part a. pNTP → DMAB was induced by switching to a 532 nm excitation laser (700 µW) for 30 s as shown in part b. Finally, the TERS signal of DMAB was monitored by switching back to the 633 nm laser excitation as shown in part c, where the characteristic Raman bands of DMAB appear at 1142 cm-1, 1390 cm-1 and 1437 cm-1. Reproduced from van Schrojenstein Lantman et al.67, Springer Nature.
Supplementary Figure 5 Nanoscale imaging of pATP → DMAB photocatalytic reaction using TERS.
a, AFM topography image of a nanostructured Ag substrate. Scale bar: 200 nm. b, TERS image of the dashed rectangle region marked in a, showing the locations of pATP → DMAB via variation of the 1142 cm−1 DMAB Raman band intensity. Pixel size: 12.5 nm. Scale bar: 50 nm. c, TERS spectra measured at the positions marked in b. Characteristic Raman bands of DMAB are visible at 1142 cm-1, 1390 cm-1 and 1437 cm-1 in the TERS spectra measured at pATP→ DMAB reaction hotspot locations 1–3, whereas these bands are absent in the TERS spectra from locations 4–6. Integration time: 0.5 s. Adapted from Kumar et al.68, Royal Society of Chemistry.
Supplementary Figure 6 Imaging of newly synthesized phospholipid molecules in biological cells using TERS.
a, TERS image of C–D band intensity of a 1 µm × 1 µm cellular region containing newly-synthesised phospholipids (NSPs). Pixel size: 50 nm. Scale bar: 200 nm. b, TERS image of NSPs (obtained using C–D band intensity) in the 100 nm × 200 nm area marked by a dashed rectangle in a. Pixel size: 13 nm. Scale bar: 50 nm. c, TERS spectra measured at the positions marked in b. The spectra are vertically shifted for easier visualisation. TERS spectra measured at locations 1–3 clearly show the presence of a C-D band at 2100 cm-1, whereas the C-D band is absent in the TERS spectra measured at locations 4–6. Integration time: 10 s. Laser power at the sample: 350 µW. Adapted from Kumar et al.52 under a Creative Commons Attribution 3.0 license (https://creativecommons.org/licenses/by/3.0/legalcode).
Supplementary Figure 7 Nanoscale chemical imaging of SWCNTs in air and water using TERS.
a, AFM topography image showing distribution of SWCNTs over a glass substrate. Scale bar: 500 nm. b, Confocal Raman spectrum of a SWCNT showing characteristic Raman bands. Integration time: 10 s. c, TERS image of a SWCNT in water, obtained using the 1591 cm-1 Raman band intensity. Scale bar: 50 nm. Integration time for TERS imaging: 1 s; pixel size: 10 nm; laser power at the sample: 170 µW. d, Intensity profile along the line marked in c with Gaussian curve fits corresponding to the near-field (green) and far-field (red) contributions. Spatial resolution of the TERS image in c is estimated from the FWHM of the green Gaussian curve to be ≈28 nm. Adapted with permission from Kumar et al.29, Royal Society of Chemistry.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7
Rights and permissions
About this article
Cite this article
Kumar, N., Weckhuysen, B.M., Wain, A.J. et al. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy. Nat Protoc 14, 1169–1193 (2019). https://doi.org/10.1038/s41596-019-0132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0132-z
This article is cited by
-
Crystallization of molecular layers produced under confinement onto a surface
Nature Communications (2024)
-
Structure sensitivity in gas sorption and conversion on metal-organic frameworks
Nature Communications (2023)
-
Accessible hotspots for single-protein SERS in DNA-origami assembled gold nanorod dimers with tip-to-tip alignment
Nature Communications (2023)
-
Origin and effect of surface oxygen-containing species on electrochemical CO or CO2 reduction reactions
Science China Chemistry (2023)
-
Plasmonic phenomena in molecular junctions: principles and applications
Nature Reviews Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.