Abstract
Snoring may be a risk factor for cardiovascular disease independent of other co-morbidities. However, most prior studies have relied on subjective, self-report, snoring evaluation. This study assessed snoring prevalence objectively over multiple months using in-home monitoring technology, and its association with hypertension prevalence. In this study, 12,287 participants were monitored nightly for approximately six months using under-the-mattress sensor technology to estimate the average percentage of sleep time spent snoring per night and the estimated apnea-hypopnea index (eAHI). Blood pressure cuff measurements from multiple daytime assessments were averaged to define uncontrolled hypertension based on mean systolic blood pressure≥140 mmHg and/or a mean diastolic blood pressure ≥90 mmHg. Associations between snoring and uncontrolled hypertension were examined using logistic regressions controlled for age, body mass index, sex, and eAHI. Participants were middle-aged (mean ± SD; 50 ± 12 y) and most were male (88%). There were 2467 cases (20%) with uncontrolled hypertension. Approximately 29, 14 and 7% of the study population snored for an average of >10, 20, and 30% per night, respectively. A higher proportion of time spent snoring (75th vs. 5th; 12% vs. 0.04%) was associated with a ~1.9-fold increase (OR [95%CI]; 1.87 [1.63, 2.15]) in uncontrolled hypertension independent of sleep apnea. Multi-night objective snoring assessments and repeat daytime blood pressure recordings in a large global consumer sample, indicate that snoring is common and positively associated with hypertension. These findings highlight the potential clinical utility of simple, objective, and noninvasive methods to detect snoring and its potential adverse health consequences.
Similar content being viewed by others
Introduction
Snoring is common and reflects narrowing and tissue vibration of upper airway soft tissues and structures1. The prevalence of habitual loud snoring in the general population remains unclear and is challenging to define in the absence of standardized approaches for snoring assessments, knowledge regarding clinical impacts of snoring itself, and reliance, in most existing epidemiological studies, on self- or bed partner-reports of snoring frequency and/or intensity. For example, a meta-regression of 63 studies reported a snoring prevalence between 2 and 83% in men and between 1 and 71% in women2. Snoring prevalence in clinical populations is also unclear. Snoring is a major feature of sleep-disordered breathing, including hypopnea events, which reflect partial airway obstruction typically with snoring. Consequently, most, but not all, individuals with obstructive sleep apnea (OSA) frequently snore loudly on most nights. OSA severity is usually categorized using clinical cut-offs3 (<5 events/h sleep = no OSA, \(\ge\)5 and <15 = mild, \(\ge\)15 and <30 = moderate and \(\ge\)30 events/h sleep = severe OSA); and more severe OSA is typically associated with more snoring during the night4. However, based on self-report snoring assessments in one of the largest epidemiological studies of sleep-disordered breathing effects on health, the Sleep Heart Health Study (SHHS), one-third of participants with OSA did not report any snoring while one-third of participants who were snorers did not meet criteria for OSA5. Thus, self-reported snoring assessments may not be sufficiently reliable to evaluate potential snoring impacts on cardiovascular health outcomes.
Physiological consequences of partial upper airway obstruction and snoring include substantial negative intrathoracic pressure swings that increase transmural pressure pre- and after-loads on the heart and blood pressure surges associated with brief awakenings from sleep (neuro-cortical arousals). Tissue vibrations may also injure the upper airway and surrounding tissues, including the carotid arteries6,7.
Snoring has been associated with multiple sub-clinical markers of cardiovascular pathology, including elevated blood pressure8,9,10, increased carotid-intima-media thickness11, stenosis12, and atherosclerosis13. These effects could partly reflect mechanical stress imposed by snoring vibrations on the upper airway in combination with a range of shared risk factors for OSA and cardiovascular disease, such as obesity and a sedentary lifestyle. In addition, snoring sound pressure levels are often substantially higher than indoor nighttime noise levels recommended by the World Health Organization14, and comparable to levels associated with poor cardiovascular health, including high blood pressure14,15. Thus, loud snoring may interfere with recuperative sleep and contribute to hypertension risk and other adverse outcomes in snorers and their bed partners through noise disturbance effects. Snoring could, therefore, be an important risk factor for hypertension and incident cardiovascular events, as recently shown with incident stroke in the Sleep Apnea Cardiovascular Endpoints (SAVE) trial16. However, in other studies, snoring was not associated with all-cause mortality or incident cardiovascular event or stroke4,17. Thus, the current evidence is conflicting, which may reflect the shortcomings of prior attempts to quantify snoring and the need for systematic and objective assessment of snoring.
Furthermore, large night-to-night variability in markers of OSA severity, such as the apnea-hypopnea-index, is now well established18,19,20,21. While no studies have examined night-to-night variability in objective snoring assessments, given the mechanistic overlap between hypopneic events and snoring, single-night snoring assessments may also not reliably reflect typical snoring and cumulative exposure risks over time.
Therefore, the existing evidence for associations between snoring and cardiovascular health outcomes remains limited and is primarily based on self-reported snoring assessments or clinical/epidemiological samples with single-night objective snoring assessments in relatively small sample sizes. This study, therefore, aimed to determine the prevalence of snoring and its association with hypertension prevalence using multi-night objective assessment of snoring and multiple daytime blood pressure assessments in a large sample population.
Results
Participant characteristics
The characteristics of the 12,287 participants included in this study are summarized in Table 1. Participants were middle-aged (~50 years), generally overweight (BMI ~ 28 kg/m2) and predominantly male (88%). Each participant had a median [IQR] of 29 [12, 81] repeat blood pressure recordings. Each participant also had an average of 181 ± 69 (mean ± SD) replicate recordings of sleep and snoring throughout the study period. The vast majority of sleep and snoring data were acquired from consecutive nights (~6 nights per week on average, see Table 1). Approximately 45, 29, 14, and 7% of the study population snored for more than 5, 10, 20, and 30% of the night, respectively. For men, these rates were 46, 30, 15, and 8%, whereas for women the equivalent rates were 36, 22, 9, and 4%.
The Spearman correlation coefficient between snoring duration and mean eAHI was 0.42 (p < 0.001). Participants with moderate and severe OSA exhibited substantially higher proportions of sleep time with snoring (Table 2) compared to participants with mild OSA, who were quite evenly represented in each snoring quartile. Only 9% of participants with severe OSA did not snore (lowest quartile of snoring). Participants without OSA had the lowest proportion of sleep time with snoring.
Snoring and blood pressure
Increased snoring duration was associated with both increased systolic and diastolic blood pressure, even after adjusting for averaged eAHI, age, BMI, and sex (Fig. 1). The association between snoring duration and blood pressure modelled without interactions is depicted in Fig. 1. These data show ~3 and ~4 mmHg increases in systolic and diastolic blood pressure for frequent regular snoring versus infrequent snoring independent of age, BMI, sex, and mean eAHI, respectively. While there were significant interactions between obese and nonobese, ≤50 years old vs. >50 years old (median age) in the association between snoring duration and systolic and diastolic blood pressure, the effect size of the interaction was relatively small, with only a ~1 to 2 mmHg difference between BMI and age categories (Supplementary Fig. 1 and Supplementary Table 1).
Similar results were observed when OSA severity was categorized, and snoring duration was divided into quartiles (Table 3). In this model, severe OSA with no snoring was associated with 3.6 mmHg and 3.5 mmHg higher systolic and diastolic blood pressure compared to no snoring or OSA. Furthermore, participants with no OSA but high snoring (quartile 4) had a 3.8 mmHg and 4.5 mmHg higher systolic and diastolic blood pressure compared to participants with no sleep apnea or no snoring. Hence, the association between severe OSA-alone and blood pressure had a similar effect size to the association between snoring-alone and blood pressure.
Snoring and uncontrolled hypertension
Snoring duration was significantly associated with uncontrolled hypertension. The interaction between snoring duration and sex was not significant (p = 0.34). However, there were significant interactions between snoring and age (p = 0.003) and snoring and BMI (p < 0.001), with stronger associations in participants ≤ 50 years (Fig. 2) compared to those > 50 years and in participants with a BMI < 30 compared to ≥ 30 kg/m². In all instances, snoring was significantly associated with uncontrolled hypertension, from a 20% increased likelihood of uncontrolled hypertension in those aged >50 years and obese to a 98% increase in those aged ≤ 50 years with body weight in the normal BMI range.
Models use 3 knots restricted cubic spline and interaction with age categories (median split in years) and BMI categories (kg/m2). ORs (95%CI) represents the difference between the 5% and the 75% percent of the snoring duration distribution. Note that the 5% and 75% percent were determined separately for each BMI category; hence, slightly different x-axis.
Without considering the interactions, snoring duration was associated with an 87% increase in uncontrolled hypertension likelihood (75th vs. 5th percentile; 12% vs. 0.04%; OR [95%CI]; 1.87 [1.63, 2.15]). The association was nonlinear and is shown in Supplementary Fig. 2, with a reference point using the median snoring duration.
Similar results were also observed when OSA severity was categorized, and snoring duration was divided into quartiles (Table 4). The association between severe OSA-alone and uncontrolled hypertension showed a similar effect size to the association between the highest snoring duration-alone and uncontrolled hypertension (2.56-fold versus 2.73-fold increase).
Primary snoring and uncontrolled hypertension
To test for the potential effects of primary snoring on uncontrolled hypertension, a similar analysis was applied to all participants with eAHI <5 events/h. After excluding participants with an eAHI ≥ 5 events/h, 4,529 (37%) participants remained, within which there were 607 (13.4%) cases of uncontrolled hypertension. The association between snoring duration and uncontrolled hypertension remained significant in this group. Participants who snored 5% (75th) of the night vs. those who did not (5th percentiles; < 0.1%), had an 89% higher prevalence of uncontrolled hypertension (OR [95%CI], 1.89 [1.44, 2.46]), independent of age, sex, and BMI. The association between snoring and uncontrolled hypertension was nonlinear, as shown in Supplementary Fig. 3.
Sensitivity analyses
Further adjusting for total sleep time did not change the main findings, and snoring duration in this model was associated with an 88% increase in uncontrolled hypertension (12% vs. 0.04%; OR [95%CI]; 1.88 [1.63, 2.16]). Similar results were also observed when OSA severity was categorized, and in models further adjusted for total sleep time (Supplementary Table 2). In the primary snoring analysis, further adjusting for total sleep time also did not change the main findings, and snoring duration in this model was associated with a 73% increase in uncontrolled hypertension (OR [95%CI]; 1.73 [1.38, 2.18]).
After excluding blood pressure entries not taken during the morning, 5383 (43.7%) participants remained for the sensitivity analysis. Of these, 1170 (21.7%) cases of hypertension were observed. In this model, snoring duration was associated with a 95% increase in uncontrolled hypertension (13% vs. 0.06%; OR [95%CI]; 1.95 [1.59, 2.40]). The main results regarding snoring and OSA severity category (Supplementary Table 2) and the results in primary snorers (data not shown), also remained the same.
Discussion
This study indicates that 15% of the studied population, which mostly comprised overweight men, snore on average for more than 20% of the night and that ~10% of participants without sleep apnea snore more than 12% of the night. The current findings also demonstrate that regular nightly snoring is associated with elevated blood pressure and uncontrolled hypertension, independent of OSA presence or severity. These findings provide important insight into the potential consequences of snoring on hypertension risk and highlight the need to consider snoring as part of clinical care and management of sleep problems, particularly in the context of hypertension management.
There is considerable variability in night-to-night measures of OSA severity18,19,20. Thus, repeated measures over multiple months, as performed in the current investigation, provides substantially greater precision around estimates of OSA severity and snoring than previous studies that have relied on single night recordings22. Moreover, the large-scale dataset surpasses all previous studies that have sought to investigate potential relationships between snoring and hypertension. Objective data were also collected in the naturalistic home environment using simple, low-cost consumer devices with established validation data. Multi-night measures in the home setting are more directly relevant to real-world risk exposure compared to data collected in sleep laboratory settings. Indeed, laboratory testing is typically derived from a single night in an unfamiliar environment, which may confound both sleep and blood pressure measures.
Nonetheless, there are several limitations of note. Firstly, there was a lack of assessment for clinical covariates that may confound the association between snoring duration and blood pressure, such as diet, exercise, alcohol, caffeine, tobacco use, and medications23,24. For example, smoking and alcohol consumption are associated with greater incidence and intensity of snoring and may have influenced the magnitude of the detected association between snoring and hypertension25,26. There was also no information on potential OSA treatment, such as continuous positive airway pressure or mandibular advancement therapy. Furthermore, estimated AHI from the under-mattress sensor includes fewer input variables from which detect respiratory events compared to conventional polysomnography. Although the estimated AHI by the WSA has good agreement with polysomnography18,27,28, the interdependence with snoring, one of the key inputs, may have influenced, at least to some extent, the detected association between snoring and blood pressure. The relative contribution of central vs. obstructive events to the prevalence of snoring and to the interaction between OSA severity and snoring in the association with hypertension also remains unclear and warrants further investigation. Validation of the device-estimated total sleep time also suggests that the device overestimate total sleep time by ~30 mins on average29, similar to other sleep wearables30,31. Given that snoring was expressed as a % of total sleep time in our study, this may have led to an under-estimation of the association between of snoring on blood pressure.
Participants were also predominantly male, which limits generalization and raises the need for caution when interpreting sex difference comparisons. Participants were also self-selected via their decision to purchase and regularly use the under-the-mattress sleep sensor and blood pressure monitor devices from which these data were derived. Consumer choices could reflect concerns about sleep and blood pressure, potentially contributing to a bias towards overestimating snoring prevalence. On the other hand, consumer engagement could potentially be biased towards those who are more engaged in their own health. The data was also collected during the COVID-19 pandemic, and COVID-19 infections may have influenced some of the observed effects.
Without any agreed standardized approaches as to how snoring should be assessed, meaningful comparisons of snoring prevalence across studies are problematic. For example, in our study, ~15% of the population snored on average more than 20% of their estimated sleep time. In the Busselton study, 22% of the studied population snored more than 50% of the total sleep time4. Notwithstanding this limitation, the estimated snoring prevalence across OSA severity category is similar to the literature5,32. Standardized algorithms and methods for snoring assessment are clearly needed for meaningful comparisons of snoring prevalence across studies. Importantly, snoring algorithm design and cut-off choices should be informed by the associated adverse health impacts of snoring, such as hypertension as reported in the current investigation.
It is also unclear how effectively the under-the-mattress sensors detect snoring from the participant and not a bed partner. This may have led to imprecise detection of snoring and influenced the estimated risk for uncontrolled hypertension associated with snoring. Finally, the under-the-mattress sleep sensor does not evaluate snoring intensity, which was shown to be associated with increased next-morning blood pressure8. Thus, the evaluation of snoring loudness is also likely to be beneficial. Similarly, recent evidence suggests that snoring acoustic characteristics may be useful markers to detect the site(s) of upper airway collapse in people with OSA33. Analysis of snoring acoustic characteristics and intensity from under-the-mattress sensors, or potentially bed-side devices, therefore, warrants further investigation in OSA and in snorers.
Identifying associations between blood pressure and uncontrolled hypertension with objective, multi-night snoring recordings is consistent with previous much smaller studies and those reliant on subjective snoring assessments8,9,34. The moderating effect of age and BMI on the associations between snoring duration and hypertension is also consistent with prior OSA literature. Previous studies suggest that downstream effects of OSA per se on adverse health outcomes may be stronger in younger participants and potentially influenced by protective hypoxic pre-conditioning effects in older people with OSA34,35. Bixler and colleagues35 found that the association between OSA severity (measured using the AHI) and hypertension was stronger in younger and non-obese participants. Similarly, we found moderating effects of age and BMI, whereby snoring duration was more strongly associated with hypertension in younger and non-obese participants. This group, which does not present with the usual co-morbidities associated with snoring and OSA, may therefore be a sensible group to target with therapeutic interventions in future studies to reduce snoring and potentially blood pressure.
We have previously shown that AHI and inter-night variability of AHI estimated using the same under-the-mattress device are independently associated with hypertension risk36. Another recent study has shown that increased respiratory effort detected by mandibular jaw movement, some of which likely translated into snoring, measured using a device placed on the chin was independently and strongly associated with prevalent hypertension in 1127 patients37. Together, these findings indicate that non-invasive in-home monitoring over multi-nights using novel technologies may be clinically useful to better detect snoring and OSA, their potential adverse cardiovascular consequences, and to inform therapeutic decision making including in primary care.
In our study, the association between severe OSA-alone and blood pressure had a similar effect size to the association between snoring-alone and blood pressure. This finding supports that snoring may be an important mechanism that contributes to hypertension. Furthermore, the observed effect sizes were similar to prior literature. For example, in the Sleep Heart Health Study, severe OSA (AHI > 30) vs. no-OSA (AHI < 1.5 events/h) was associated with a 3.4 mmHg increase in diastolic blood pressure, independent of age, sex, and BMI23. In this study, regular nightly snoring was associated with up to 4.5 mmHg higher diastolic blood pressure after adjustment for age, sex, BMI, and OSA severity. While uncontrolled factors may explain some of this effect (e.g., smoking or alcohol), snoring could also represent an independent mechanistic pathway via which chronically obstructed breathing during sleep contributes to elevated blood pressure. For example, via intrathoracic pressure effects on baroreceptor mediated control of systemic (and potentially pulmonary) blood pressure or potentially via carotid baroreceptor effects from mechanical vibration emanating from the pharyngeal airway8,9,11,12,13. Observational epidemiological studies have shown a strong and linear association between blood pressure and the risk of cardiovascular disease and cardiovascular death without any evidence of a blood pressure threshold24. The Prospective Studies Collaboration38 estimated that an increase in systolic blood pressure of 20 mmHg, or diastolic blood pressure of 10 mmHg, was associated with a more than twofold increase in the rate of cardiovascular death. Therefore, the increase in blood pressure associated with sleep apnoea, snoring, and their combination (~6 mmHg increases in systolic and diastolic blood pressure compared to no snoring or OSA) has important clinical relevance.
The elevated hypertension prevalence in people with primary snoring (eAHI< 5 events/h) was comparable to other OSA severity categories. This finding further suggests that hypertension risks associated with snoring may be independent of OSA and potentially more strongly related to chronically obstructed breathing rather than blood gas or sleep disturbance effects. These results are concordant with previous studies where associations between snoring loudness and elevated morning blood pressure in patients with an eAHI< 15 events/h were detected8,9. Low-level continuous positive airway pressure has been shown to significantly reduce snoring in participants without OSA39. Whether a reduction in snoring is associated with a decrease in blood pressure and other cardiovascular risk factors clearly warrants further investigation.
In summary, long-term nightly snoring assessments indicate that snoring is highly prevalent in the adult community and is associated with a ~20 to 80% increase in hypertension prevalence, independent of OSA severity. High hypertension prevalence was also observed for people with a high proportion of the night spent snoring, even without sleep apnea (eAHI<5 events/h). These findings provide important insight into the potential consequences of snoring to hypertension. Thus, further investigation is warranted to determine whether therapeutic interventions directed toward snoring can reduce hypertension, one of the leading risk factors for cardiovascular disease and mortality.
Methods
Participants
This study analyzed data from 12,287 participants who registered to use both an under-the-mattress sleep sensor (Withings Sleep Analyzer: WSA) and an FDA-registered home-blood pressure monitor between July 2020 and April 2021. Further inclusion criteria were ≥28 nights of sleep and snoring recordings and at least five separate blood pressure measurements over the recording period for assessment of uncontrolled hypertension outcomes. All participants provided written consent through the Withings app for their deidentified data to be used for research purposes when signing up for a Withings account, and the current study was approved by the Flinders University Human Research Ethics Committee (Project number: 4291).
Snoring and blood pressure assessments
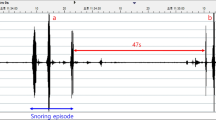
The WSA is a nonwearable sleep monitoring device placed under-the-mattress that detects snoring and estimates the apnea-hypopnea index (estimated AHI; eAHI) and sleep stages. This is achieved via automated proprietary algorithms from a built in microphone and ballistographic assessment of movement, heart rate, and respiratory motion from a pressure sensor28. The estimated AHI has good agreement with in-laboratory polysomnography-derived AHI with high predictive performance to classify mild (89% sensitivity and 75% specificity), moderate-to-severe (88% sensitivity and 88% specificity), and severe OSA (86% sensitivity and 91% specificity)18,27,28. The estimated AHI also has minimal bias with in-laboratory polysomnography derived AHI when the AHI is considered as a continuous variable28. Features characteristic of the power spectrum of snoring sound are calculated, and snoring is scored over 1-minute epochs. To be classified as a snoring epoch, ≥50% of the epoch must contain snoring. A post-processing window over 3 consecutive 1 min epochs is then applied to define snoring. This has the advantage of limiting the number of false positives due to extraneous noise. Snoring duration is then expressed as a percentage of total sleep time by dividing snoring time by the estimated total sleep time (hereafter termed “snoring duration”).
Blood pressure values were obtained via Withings blood pressure monitor measurements undertaken by each participant. The home-blood pressure monitor comes with instructions that outline how to take a blood pressure measurement. Specifically, the user is instructed to 1) rest for ≥5 minutes before taking a measurement, 2) be seated in a comfortable position and in a quiet area with legs uncrossed, feet flat on the floor, and back/arm supported, 3) not speak during the measurements and 4) perform the measurement on their left arm. Uncontrolled hypertension was defined as a mean systolic blood pressure ≥140 mmHg or mean diastolic blood pressure ≥90 mmHg40 averaged across all available blood pressure measurements over the monitoring period.
Statistical analysis
The primary exposure variables were mean eAHI and mean snoring duration across all available nights of data. OSA severity categories were defined using standard clinical cut-offs3 (<5 events/h sleep = no OSA, \(\ge\)5 and <15 = mild, \(\ge\)15 and <30 = moderate and ≥30 events/h sleep = severe OSA). Snoring was categorized using quartiles and tertiles where necessary for interpretation but was otherwise kept as a continuous variable in the analyses.
The prevalence of snoring and mean snoring duration were examined separately by sex. Linear regression models controlling for age, sex, BMI, and mean eAHI were used to investigate the potential association between snoring duration and systolic and diastolic blood pressure. Odds ratio (ORs) and 95% confidence interval (CIs) were determined using logistic regression models to assess the association between mean snoring duration and uncontrolled hypertension adjusted for age, sex, BMI, and average eAHI.
eAHI and mean snoring duration were modelled using restricted cubic splines to account for potential non-linear associations. Interactions between snoring duration with age, sex and BMI were also investigated. When interactions were significant, BMI was categorized as normal (<25 kg/m²), overweight (BMI between 25 and 30 kg/m²), and obese (BMI ≥ 30 kg/m²). When interactions with age were observed, age was categorized using a median split. As a secondary analysis, snoring duration was categorized using tertiles, and the contribution of snoring in each OSA category (from mean eAHI) was examined using logistic regression adjusted for available confounders. Logistic and linear regression were performed in the R programming language.
Sensitivity analyses
To further validate our findings, we conducted two additional sensitivity analyses. In the first sensitivity analysis, given the association between total sleep time and hypertension29, we additionally adjusted our models for total sleep time. Given that blood pressure may vary during the day, we performed an additional sensitivity analysis where only morning blood pressure entries, measured between 6am and 12 pm, were included.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Deidentified data that support the findings of this study, including individual data, are available from the corresponding author upon request subject to ethical and data custodian (Withings) approval.
Code availability
Code for the analysis is available upon reasonable request from the corresponding author.
References
Pevernagie, D., Aarts, R. M. & De Meyer, M. The acoustics of snoring. Sleep. Med. Rev. 14, 131–144, https://doi.org/10.1016/j.smrv.2009.06.002 (2010).
Chan, C. H., Wong, B. M., Tang, J. L. & Ng, D. K. Gender difference in snoring and how it changes with age: systematic review and meta-regression. Sleep. Breath. 16, 977–986, https://doi.org/10.1007/s11325-011-0596-8 (2012).
Epstein, L. J. et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep. Med. 5, 263–276 (2009).
Marshall, N. S., Wong, K. K., Cullen, S. R., Knuiman, M. W. & Grunstein, R. R. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the Busselton Health Study. Sleep 35, 1235–1240 (2012).
Young, T. et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch. Intern Med. 162, 893–900 (2002).
Cho, J. G. et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep 34, 751–757, https://doi.org/10.5665/SLEEP.1042 (2011).
Amatoury, J., Howitt, L., Wheatley, J. R., Avolio, A. P. & Amis, T. C. Snoring-related energy transmission to the carotid artery in rabbits. J. Appl Physiol. (1985) 100, 1547–1553, https://doi.org/10.1152/japplphysiol.01439.2005 (2006).
Furukawa, T., Nakano, H., Yoshihara, K. & Sudo, N. The Relationship between Snoring Sound Intensity and Morning Blood Pressure in Workers. J. Clin. Sleep. Med. 12, 1601–1606, https://doi.org/10.5664/jcsm.6340 (2016).
Furukawa, T. et al. Relationship between snoring sound intensity and daytime blood pressure. Sleep. Biol. Rhythms 8, 245–253, https://doi.org/10.1111/j.1479-8425.2010.00455.x (2010).
Khazaie, H. et al. Among middle-aged adults, snoring predicted hypertension independently of sleep apnoea. J. Int. Med. Res. 46, 1187–1196, https://doi.org/10.1177/0300060517738426 (2018).
Kim, J. et al. Objective snoring time and carotid intima-media thickness in non-apneic female snorers. J. Sleep. Res. 26, 147–150, https://doi.org/10.1111/jsr.12471 (2017).
Deeb, R. et al. Snoring and carotid artery disease: A new risk factor emerges. Laryngoscope 129, 265–268, https://doi.org/10.1002/lary.27314 (2019).
Lee, S. A. et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep 31, 1207–1213 (2008).
Sowho, M., Sgambati, F., Guzman, M., Schneider, H. & Schwartz, A. Snoring: a source of noise pollution and sleep apnea predictor. Sleep 43, https://doi.org/10.1093/sleep/zsz305 (2020).
Munzel, T. et al. Environmental Noise and the Cardiovascular System. J. Am. Coll. Cardiol. 71, 688–697, https://doi.org/10.1016/j.jacc.2017.12.015 (2018).
Li, J. et al. Self-reported Snoring Patterns Predict Stroke Events in High-Risk Patients With OSA: Post Hoc Analyses of the SAVE Study. Chest, https://doi.org/10.1016/j.chest.2020.05.615 (2020).
Marin, J. M., Carrizo, S. J., Vicente, E. & Agusti, A. G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365, 1046–1053 (2005).
Lechat, B. et al. Multinight Prevalence, Variability, and Diagnostic Misclassification of Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 205, 563–569, https://doi.org/10.1164/rccm.202107-1761OC (2022).
Punjabi, N. M., Patil, S., Crainiceanu, C. & Aurora, R. N. Variability and Misclassification of Sleep Apnea Severity Based on Multi-Night Testing. Chest 158, 365–373, https://doi.org/10.1016/j.chest.2020.01.039 (2020).
Roeder, M. et al. Night-to-night variability of respiratory events in obstructive sleep apnoea: a systematic review and meta-analysis. Thorax 75, 1095–1102, https://doi.org/10.1136/thoraxjnl-2020-214544 (2020).
Lechat, B. et al. Multi-night measurement for diagnosis and simplified monitoring of obstructive sleep apnoea. Sleep. Med. Rev. 72, 101843, https://doi.org/10.1016/j.smrv.2023.101843 (2023).
Lechat, B. et al. Single-Night Diagnosis of Sleep Apnea Contributes to Inconsistent Cardiovascular Outcome Findings. Chest 164, 231–240, https://doi.org/10.1016/j.chest.2023.01.027 (2023).
Nieto, F. J. et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283, 1829–1836, https://doi.org/10.1001/jama.283.14.1829 (2000).
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237, https://doi.org/10.1038/s41581-019-0244-2 (2020).
Franklin, K. A. et al. The influence of active and passive smoking on habitual snoring. Am. J. Respir. Crit. Care Med. 170, 799–803, https://doi.org/10.1164/rccm.200404-474OC (2004).
Riemann, R., Volk, R., Muller, A. & Herzog, M. The influence of nocturnal alcohol ingestion on snoring. Eur. Arch. Otorhinolaryngol. 267, 1147–1156, https://doi.org/10.1007/s00405-009-1163-9 (2010).
Yang, R. Y., Bendjoudi, A., Buard, N. & Boutouyrie, P. Pneumatic sensor for cardiorespiratory monitoring during sleep. Biomedical Physics & Engineering Express 5, https://doi.org/10.1088/2057-1976/ab3ac9 (2019).
Edouard, P. et al. Validation of the Withings Sleep Analyzer, an under-the-mattress device for the detection of moderate-severe sleep apnea syndrome. J. Clin. Sleep. Med 17, 1217–1227, https://doi.org/10.5664/jcsm.9168 (2021).
Scott, H. et al. Sleep Irregularity Is Associated With Hypertension: Findings From Over 2 Million Nights With a Large Global Population Sample. Hypertension 80, 1117–1126, https://doi.org/10.1161/Hypertensionaha.122.20513 (2023).
Berryhill, S. et al. Effect of wearables on sleep in healthy individuals: a randomized crossover trial and validation study. J. Clin. Sleep. Med. 16, 775–783, https://doi.org/10.5664/jcsm.8356 (2020).
Lee, X. K. et al. Validation of a Consumer Sleep Wearable Device With Actigraphy and Polysomnography in Adolescents Across Sleep Opportunity Manipulations. J. Clin. Sleep. Med. 15, 1337–1346, https://doi.org/10.5664/jcsm.7932 (2019).
Bearpark, H. et al. Snoring and sleep apnea. A population study in Australian men. Am. J. Respir. Crit. Care Med 151, 1459–1465, https://doi.org/10.1164/ajrccm.151.5.7735600 (1995).
Sebastian, A., Cistulli, P. A., Cohen, G. & de Chazal, P. Association of snoring characteristics with predominant site of collapse of upper airway in obstructive sleep apnea patients. Sleep 44, https://doi.org/10.1093/sleep/zsab176 (2021).
Vgontzas, A. N. et al. Mild-to-moderate sleep apnea is associated with incident hypertension: age effect. Sleep 42, https://doi.org/10.1093/sleep/zsy265 (2019).
Bixler, E. O. et al. Association of hypertension and sleep-disordered breathing. Arch. Intern Med. 160, 2289–2295, https://doi.org/10.1001/archinte.160.15.2289 (2000).
Lechat, B. et al. High night-to-night variability in sleep apnea severity is associated with uncontrolled hypertension. Nat.: Digit Med 6, 57, https://doi.org/10.1038/s41746-023-00801-2 (2023).
Martinot, J. B., Le-Dong, N. N., Malhotra, A. & Pepin, J. L. Respiratory effort during sleep and prevalent hypertension in obstructive sleep apnoea. Eur. Respir. J. 61, https://doi.org/10.1183/13993003.01486-2022 (2023).
Lewington, S. et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913, https://doi.org/10.1016/s0140-6736(02)11911-8 (2002).
Guzman, M. A. et al. The Efficacy of Low-Level Continuous Positive Airway Pressure for the Treatment of Snoring. J. Clin. Sleep. Med. 13, 703–711, https://doi.org/10.5664/jcsm.6588 (2017).
Chobanian, A. V. et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289, 2560–2572, https://doi.org/10.1001/jama.289.19.2560 (2003).
Acknowledgements
DJE is supported by a National Health and Medical Research Council (NHMRC) of Australia Leadership Fellowship (1196261). BL is supported by a National Health and Medical Research Council (NHMRC) of Australia Emerging Leadership Fellowship (2025886).
Author information
Authors and Affiliations
Contributions
D.J.E., B.L., G.N., S.A. and P.C. developed the study concepts and aims. B.L., and G.N. performed the data extraction. B.L., D.J.E. and G.N. performed the data analysis. D.J.E., B.L., G.N., H.S. and J.M. drafted the manuscript. All authors provided important insight on data analysis, interpretation and contributed to the final version of the manuscript. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
Outside the submitted work, DJE has had research grants from Bayer, Apnimed, Takeda, Invicta Medical and Eli Lilly. DJE currently serves as a scientific advisor/consultant for Apnimed, Invicta Medical, Bayer and Mosanna. PE serves as a consultant for Withings. Withings provided sleep analysers for a validation trial. None of the other authors have any potential conflicts to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lechat, B., Naik, G., Appleton, S. et al. Regular snoring is associated with uncontrolled hypertension. npj Digit. Med. 7, 38 (2024). https://doi.org/10.1038/s41746-024-01026-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-024-01026-7